Beyond MIND and Mediterranean Diets: Designing a Diet to Optimize Parkinson’s Disease Outcomes
Abstract
1. Introduction
2. Materials and Methods
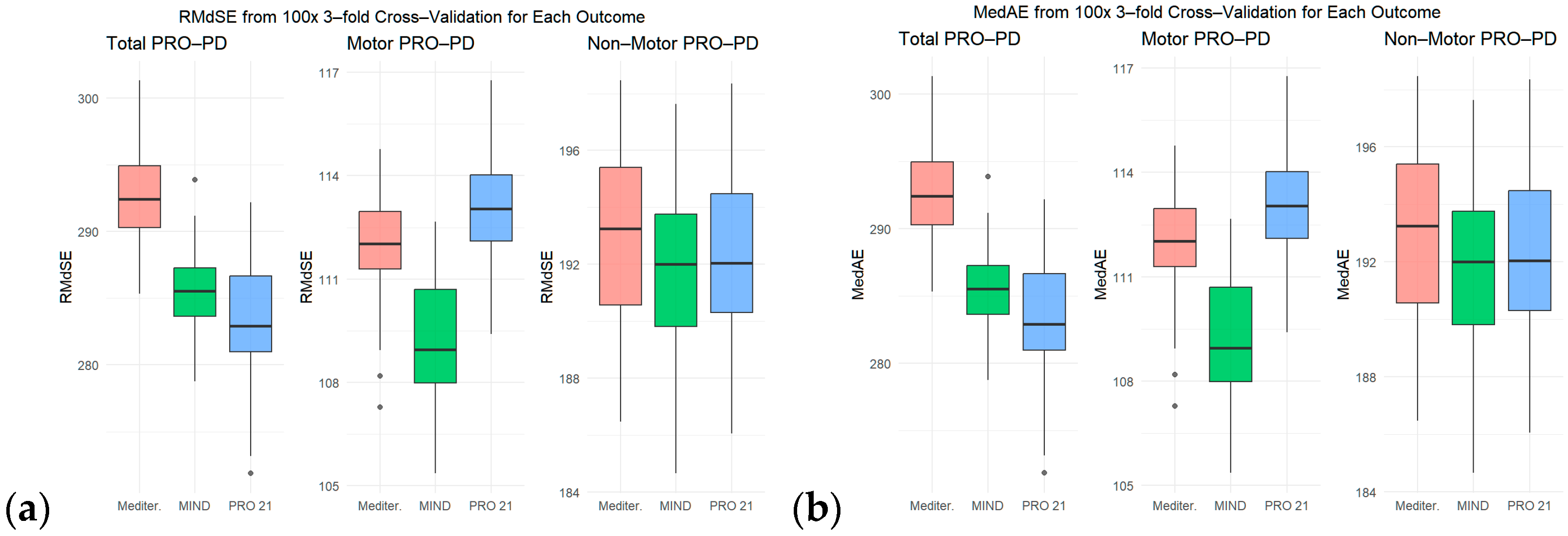
3. Results
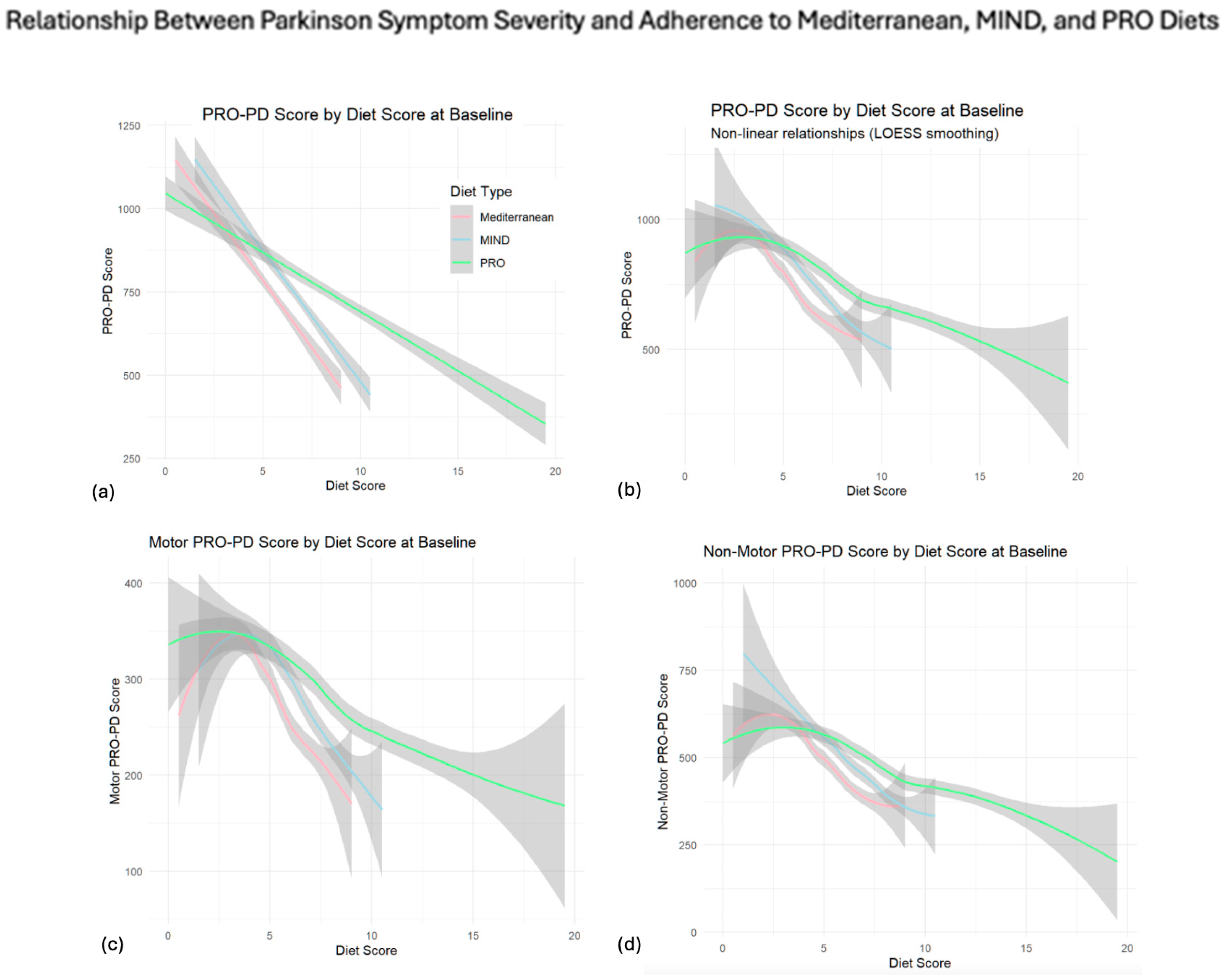
PRO-21 Dietary Adherence and Subsequent Slope of Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.M.; Hernan, M.A.; Willett, W.C.; Ascherio, A. Diet and Parkinson’s disease: A potential role of dairy products in men. Ann. Neurol. 2002, 52, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Tumas, V.; Aureliano, M.J.; Rieder, C.R.M.; Schuh, A.F.S.; Ferraz, H.B.; Borges, V.; Soares, M.C.; Boone, D.L.; Silva, C.C.D.; Costa, M.C.; et al. Modifiable risk factors associated with the risk of developing Parkinson’s disease: A critical review. Arq. Neuropsiquiatr. 2025, 83, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Keramati, M.; Kheirouri, S.; Etemadifar, M. Dietary approach to stop hypertension (DASH), but not Mediterranean and MIND, dietary pattern protects against Parkinson’s disease. Food Sci. Nutr. 2024, 12, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Tosefsky, K.N.; Zhu, J.; Wang, Y.N.; Lam, J.S.T.; Cammalleri, A.; Appel-Cresswell, S. The Role of Diet in Parkinson’s Disease. J. Park. Dis. 2024, 14, S21–S34. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.J.; Park, S.J.; Mischley, L.K. Comparison of Associations between MIND and Mediterranean Diet Scores with Patient-Reported Outcomes in Parkinson’s Disease. Nutrients 2022, 14, 5185. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.; Ryan, J.; Laws, M.; Devine, A. A comprehensive examination of the evidence for whole of diet patterns in Parkinson’s disease: A scoping review. Nutr. Neurosci. 2024, 27, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Coronary heart disease in seven countries. Summary. Circulation 1970, 41, I186–I195. [Google Scholar]
- Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ros, E.; Covas, M.I.; Fiol, M.; Warnberg, J.; Aros, F.; Ruiz-Gutierrez, V.; Lamuela-Raventos, R.M.; et al. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.; Fito, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Peng, Y.; Lin, Z.; Gong, Y. Association between Mediterranean diet adherence and Parkinson’s disease: A systematic review and meta-analysis. J. Nutr. Health Aging 2025, 29, 100451. [Google Scholar] [CrossRef] [PubMed]
- Rusch, C.; Beke, M.; Nieves, C., Jr.; Mai, V.; Stiep, T.; Tholanikunnel, T.; Ramirez-Zamora, A.; Hess, C.W.; Langkamp-Henken, B. Promotion of a Mediterranean Diet Alters Constipation Symptoms and Fecal Calprotectin in People with Parkinson’s Disease: A Randomized Controlled Trial. Nutrients 2024, 16, 2946. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, Z.; Sheklabadi, E.; Derakhshan, Y.; Bagherniya, M.; Chitsaz, A. The effect of the Mediterranean diet on cognitive function in patients with Parkinson’s disease: A randomized clinical controlled trial. Complement. Ther. Med. 2020, 50, 102366. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe-Roach, A.; Yu, A.C.; Golz, E.; Cirstea, M.; Sundvick, K.; Kliger, D.; Foulger, L.H.; Mackenzie, M.; Finlay, B.B.; Appel-Cresswell, S. MIND and Mediterranean Diets Associated with Later Onset of Parkinson’s Disease. Mov. Disord. 2021, 36, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. MIND Diet Associated with Reduced Incidence and Delayed Progression of ParkinsonismA in Old Age. J. Nutr. Health Aging 2018, 22, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxid. Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Farahnik, J.; Mantay, L.; Punzi, J.; Szampruch, K.; Ferguson, T.; Fox, D.J. Parkinson Symptom Severity and Use of Nutraceuticals. Nutrients 2023, 15, 802. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.F.; Subramanian, I.; Farahnik, J.; Grout, L.; Salcido, C.; Kurtzer, J.; Mischley, L.K. Physical Activity, Patient-Reported Outcomes, and Quality of Life in Parkinson’s Disease. J. Geriatr. Psychiatry Neurol. 2025, 8919887251346495. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, I.; McDaniels, B.; Farahnik, J.; Mischley, L.K. Childhood Trauma and Parkinson Disease: Associations of Adverse Childhood Experiences, Disease Severity, and Quality of Life. Neurol. Clin. Pract. 2023, 13, e200124. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Conesa, M.T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, I.; Farahnik, J.; Mischley, L.K. Synergy of pandemics-social isolation is associated with worsened Parkinson severity and quality of life. NPJ Park. Dis. 2020, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Asoudeh, F.; Naeini, F.; Sadeghi, E.; Travica, N.; Mohammadi, H. Association between animal protein sources and risk of neurodegenerative diseases: A systematic review and dose-response meta-analysis. Nutr. Rev. 2023, 81, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Rizzi, L.; Somaa, F. The role of nutrition on Parkinson’s disease: A systematic review. Nutr. Neurosci. 2023, 26, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Kistner, A.; Krack, P. Parkinson’s disease: No milk today? Front. Neurol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Mentis, A.A.; Dardiotis, E.; Efthymiou, V.; Chrousos, G.P. Correction to: Non-genetic risk and protective factors and biomarkers for neurological disorders: A meta-umbrella systematic review of umbrella reviews. BMC Med. 2021, 19, 297. [Google Scholar] [CrossRef] [PubMed]
- Martino, R.; Candundo, H.; Lieshout, P.V.; Shin, S.; Crispo, J.A.G.; Barakat-Haddad, C. Onset and progression factors in Parkinson’s disease: A systematic review. Neurotoxicology 2017, 61, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Page, R.; Truman, P. Smoking, coffee intake, and Parkinson’s disease: Potential protective mechanisms and components. Neurotoxicology 2025, 106, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Asuku, A.O.; Ayinla, M.T.; Olajide, T.S.; Oyerinde, T.O.; Yusuf, J.A.; Bayo-Olugbami, A.A.; Fajemidagba, G.A. Coffee and Parkinson’s disease. Prog. Brain Res. 2024, 289, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Mhd Rodzi, N.A.R. Addressing the Neuroprotective Actions of Coffee in Parkinson’s Disease: An Emerging Nutrigenomic Analysis. Antioxidants 2022, 11, 1587. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Jimenez, F.J.; Alonso-Navarro, H.; Garcia-Martin, E.; Agundez, J.A.G. Alcohol consumption and risk for Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2019, 266, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.; Chohan, H.; Bestwick, J.P.; Noyce, A.J. Alcohol and Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Park. Dis. 2022, 12, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.; Ilieva, N.; Stone, W.J.; De Miranda, B.R. Low-dose inhalation exposure to trichloroethylene induces dopaminergic neurodegeneration in rodents. Toxicol. Sci. 2023, 196, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Park, D.; Lee, Y.C. Neurotoxic Effects of Airborne Particulate Matter: Mechanisms and Health Implications. J. Biochem. Mol. Toxicol. 2025, 39, e70322. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Bloem, B.R. Parkinson’s Disease Is Predominantly an Environmental Disease. J. Park. Dis. 2024, 14, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Conley, K.E.; Shankland, E.G.; Kavanagh, T.J.; Rosenfeld, M.E.; Duda, J.E.; White, C.C.; Wilbur, T.K.; De La Torre, P.U.; Padowski, J.M. Central nervous system uptake of intranasal glutathione in Parkinson’s disease. NPJ Park. Dis. 2016, 2, 16002. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Thompson, A.S.; Bondonno, N.; Jennings, A.; Kuhn, T.; Cassidy, A. Plant-Based Dietary Patterns and Parkinson’s Disease: A Prospective Analysis of the UK Biobank. Mov. Disord. 2023, 38, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Drescher, G.; Trichopoulou, A.; Willett, W.C.; Martinez-Gonzalez, M.A. Three decades of the Mediterranean diet pyramid: A narrative review of its history, evolution, and advances. Am. J. Clin. Nutr. 2025, 122, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K. PRO Diet Coach, Version 1.0. [Conversational GPT AI Tool]; 2025. Available online: https://chatgpt.com/g/g-68419c9084548191ba6850a9ae801e2c-pro-diet-coach (accessed on 7 June 2025).
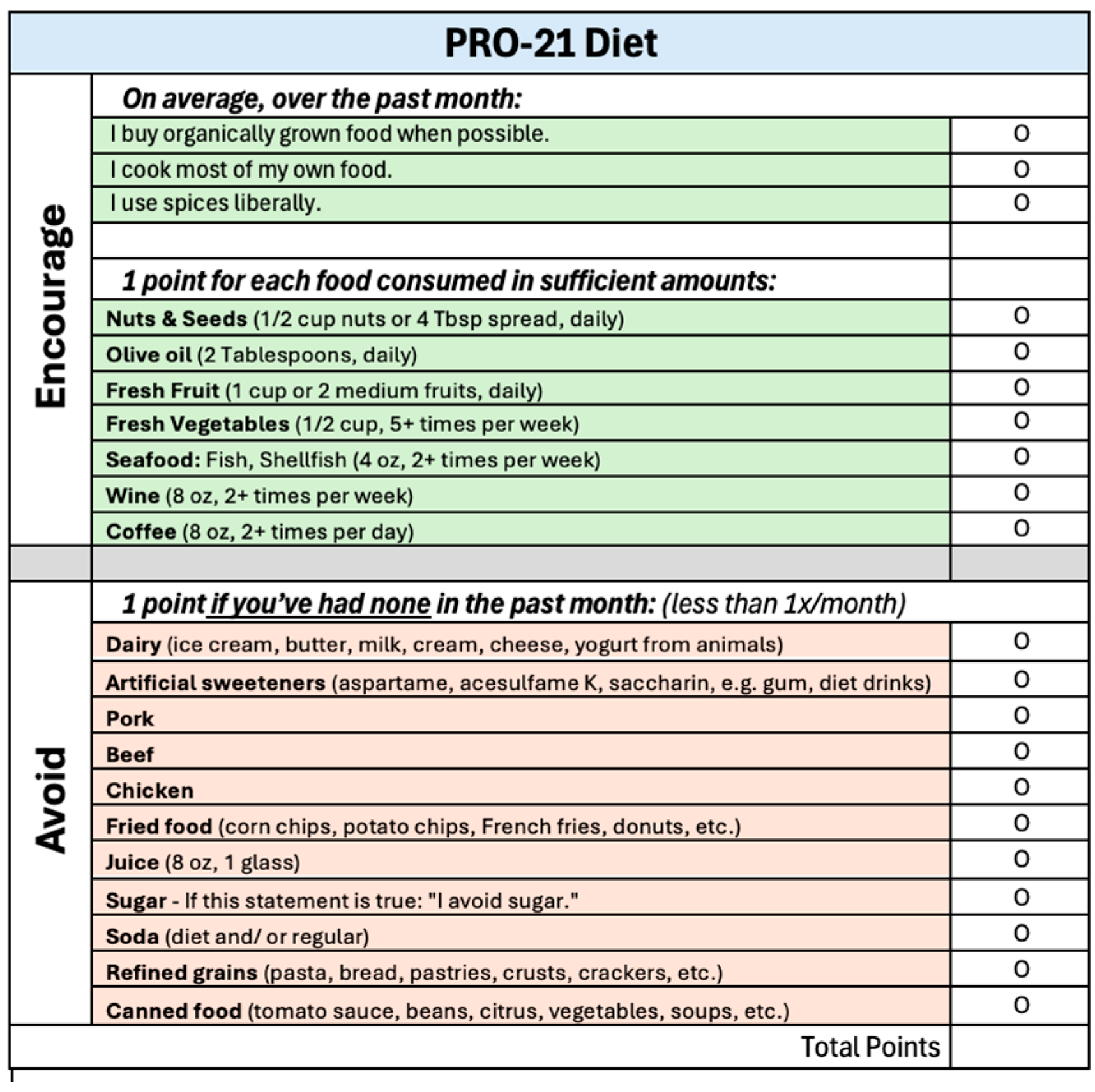


| Number of PRO-21 Points at Baseline | ||||||
|---|---|---|---|---|---|---|
| Participant Characteristics | All Participants | 0–5 Points | 6–9 Points | 10–14 Points | 15+ Points | p.overall |
| N = 2290 | n = 454 | n = 893 | n = 832 | n = 111 | ||
| Age | 64.1 [57.1;69.7] | 63.0 [54.7;69.5] | 64.0 [57.3;69.6] | 64.7 [58.1;69.6] | 63.4 [55.0;70.4] | 0.021 |
| Gender (n = 2277) | . | |||||
| Male | 988 (43.4%) | 262 (58.1%) | 391 (44.0%) | 289 (34.9%) | 46 (41.8%) | |
| Female | 1288 (56.6%) | 189 (41.9%) | 498 (56.0%) | 537 (64.9%) | 64 (58.2%) | |
| Non-binary | 1 (0.04%) | 0 (0.00%) | 0 (0.00%) | 1 (0.12%) | 0 (0.00%) | |
| Income (n = 2163) | 0.004 | |||||
| Less than $20,000 | 122 (5.64%) | 28 (6.53%) | 39 (4.63%) | 51 (6.47%) | 4 (3.85%) | |
| Between $20–40,000 | 258 (11.9%) | 62 (14.5%) | 105 (12.5%) | 83 (10.5%) | 8 (7.69%) | |
| Between $40–60,000 | 287 (13.3%) | 68 (15.9%) | 109 (12.9%) | 99 (12.6%) | 11 (10.6%) | |
| Between $60–80,000 | 295 (13.6%) | 60 (14.0%) | 128 (15.2%) | 97 (12.3%) | 10 (9.62%) | |
| Between $80–100,000 | 304 (14.1%) | 65 (15.2%) | 131 (15.6%) | 95 (12.1%) | 13 (12.5%) | |
| Between $100–150,000 | 417 (19.3%) | 68 (15.9%) | 162 (19.2%) | 159 (20.2%) | 28 (26.9%) | |
| More than $150,000 | 480 (22.2%) | 78 (18.2%) | 168 (20.0%) | 204 (25.9%) | 30 (28.8%) | |
| Geographic Location: (n = 1316) | . | |||||
| Africa | 9 (0.68%) | 2 (0.95%) | 4 (0.84%) | 3 (0.55%) | 0 (0.00%) | |
| Asia | 10 (0.76%) | 1 (0.48%) | 1 (0.21%) | 8 (1.46%) | 0 (0.00%) | |
| Europe | 197 (15.0%) | 23 (11.0%) | 61 (12.9%) | 94 (17.2%) | 19 (22.4%) | |
| North America | 1035 (78.6%) | 174 (82.9%) | 380 (80.2%) | 416 (76.1%) | 65 (76.5%) | |
| Oceania | 59 (4.48%) | 7 (3.33%) | 28 (5.91%) | 23 (4.20%) | 1 (1.18%) | |
| South America | 6 (0.46%) | 3 (1.43%) | 0 (0.00%) | 3 (0.55%) | 0 (0.00%) | |
| Estimated Hoehn & Yahr, by patient (n = 2245) | . | |||||
| 1-sided symptoms only, minimal disability | 1266 (56.4%) | 198 (44.9%) | 473 (53.9%) | 524 (64.1%) | 71 (64.5%) | |
| Both sides affected, balance is stable | 395 (17.6%) | 87 (19.7%) | 140 (16.0%) | 147 (18.0%) | 21 (19.1%) | |
| Mild to moderate disability, balance affected | 508 (22.6%) | 130 (29.5%) | 235 (26.8%) | 127 (15.5%) | 16 (14.5%) | |
| Severe disability, able to walk and stand without help | 48 (2.14%) | 17 (3.85%) | 18 (2.05%) | 12 (1.47%) | 1 (0.91%) | |
| Confinement to bed or wheelchair unless aided | 4 (0.18%) | 2 (0.45%) | 1 (0.11%) | 1 (0.12%) | 0 (0.00%) | |
| Don’t know | 24 (1.07%) | 7 (1.59%) | 10 (1.14%) | 6 (0.73%) | 1 (0.91%) | |
| Years since diagnosis, at baseline (n = 2211) | 3.21 [1.29;6.48] | 4.08 [1.67;8.05] | 3.21 [1.39;6.63] | 2.93 [1.15;5.54] | 2.47 [0.90;5.22] | <0.001 |
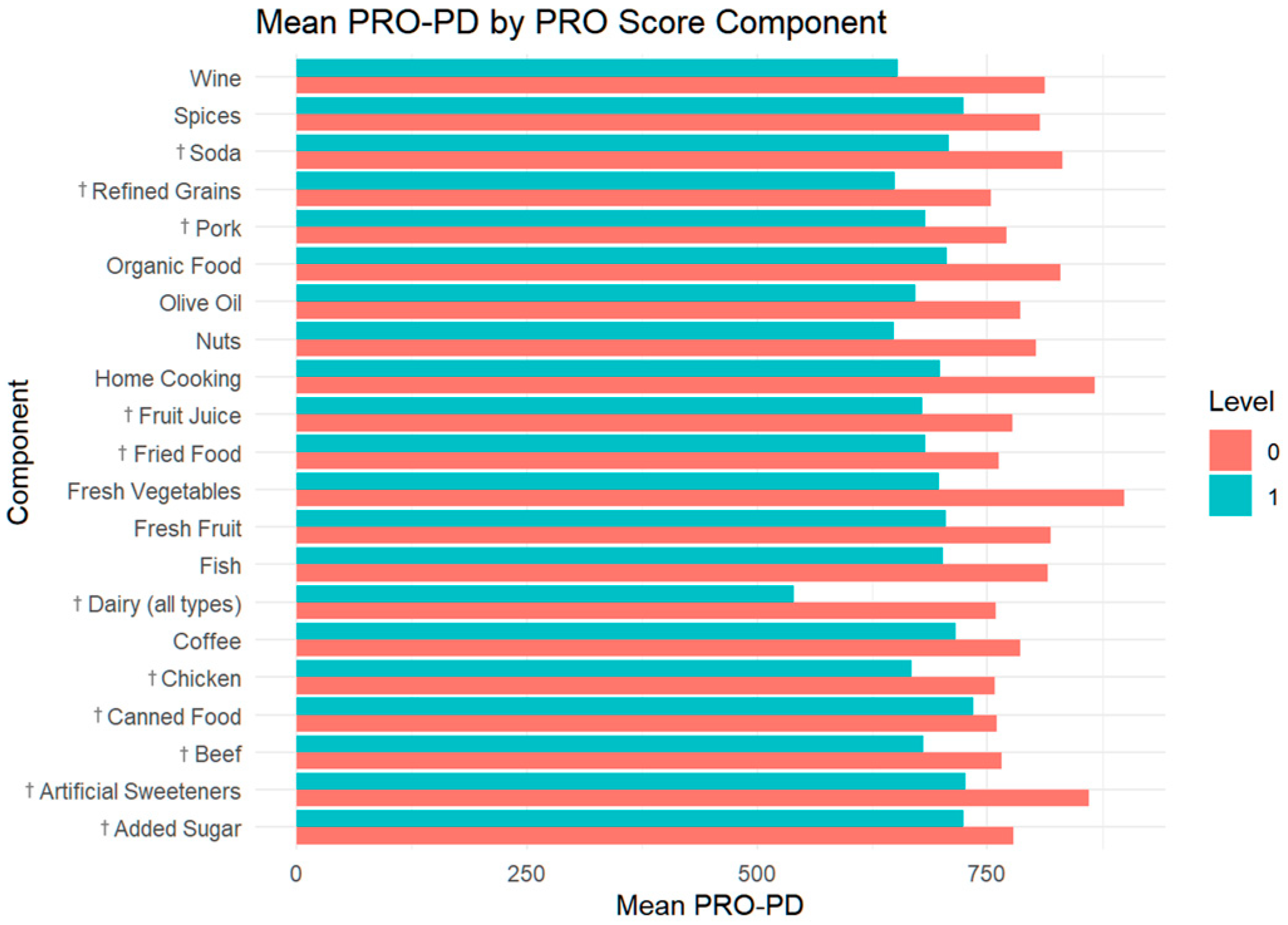
| PRO-PD Symptom severity score, mean (n = 2074) | 647 [374;1050] | 881 [537;1235] | 713 [415;1126] | 534 [330;872] | 396 [257;704] | <0.001 |
| PRO-21 Diet score, mean (n = 2290) | 8.50 [6.00;11.0] | 4.00 [3.00;4.50] | 7.50 [6.50;8.50] | 11.0 [10.0;12.5] | 15.0 [14.5;16.0] | 0 |
| Impact of Dietary Adherence on Patient-Reported Outcomes in Parkinson’s Disease | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Impact on Motor Symptoms | Impact on Non-Motor Symptoms | Impact on Total PRO-PD Score | |||||||
| Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | |
| Mediterranean | −21 | −27, −16 | <0.001 | −44 | −53, −35 | <0.001 | −64 | −78, −51 | <0.001 |
| Age | 0.12 | −0.71, 0.94 | 0.8 | −0.59 | −1.9, 0.76 | 0.4 | −0.14 | −2.2, 1.9 | 0.9 |
| Sex, Female | −19 | −34, −4.3 | 0.012 | −13 | −37, 12 | 0.3 | −33 | −71, 5.2 | 0.091 |
| Income | −15 | −19, −11 | <0.001 | −28 | −34, −21 | <0.001 | −41 | −51, −31 | <0.001 |
| Years since diagnosis | 14 | 13, 16 | <0.001 | 15 | 12, 17 | <0.001 | 29 | 25, 33 | <0.001 |
| MIND | −22 | −27, −17 | <0.001 | −43 | −51, −35 | <0.001 | −64 | −77, −51 | <0.001 |
| Age | 0.34 | −0.48, 1.2 | 0.4 | −0.28 | −1.6, 1.1 | 0.7 | 0.38 | −1.7, 2.4 | 0.7 |
| Sex, Female | −19 | −34, −4.1 | 0.013 | −11 | −36, 13 | 0.4 | −32 | −69, 5.9 | 0.1 |
| Income | −15 | −19, −11 | <0.001 | −28 | −34, −21 | <0.001 | −41 | −51, −31 | <0.001 |
| Years since diagnosis | 14 | 13, 16 | <0.001 | 15 | 12, 17 | <0.001 | 29 | 25, 33 | <0.001 |
| PRO-21 | −10 | −12, −7.9 | <0.001 | −18 | −22, −15 | <0.001 | −29 | −34, −23 | <0.001 |
| Age | 0.29 | −0.54, 1.1 | 0.5 | −0.49 | −1.9, 0.88 | 0.5 | 0.11 | −2.0, 2.2 | >0.9 |
| Sex, Female | −15 | −30, 0.93 | 0.065 | −2.3 | −28, 23 | 0.9 | −18 | −57, 21 | 0.4 |
| Income | −16 | −20, −12 | <0.001 | −30 | −37, −23 | <0.001 | −43 | −53, −33 | <0.001 |
| Years since diagnosis | 14 | 13, 16 | <0.001 | 14 | 12, 17 | <0.001 | 28 | 24, 33 | <0.001 |
| Diet | Symptom Type | Slope (β) | Per-Symptom Effect | AIC |
|---|---|---|---|---|
| Mediterranean | Motor | −21.49 | −1.65 | 26,227.41 |
| Non-Motor | −43.84 | −2.19 | 27,980.33 | |
| Total | −64.20 | — | 28,897.24 | |
| MIND | Motor | −21.90 | −1.68 | 26,118.01 |
| Non-Motor | −42.80 | −2.14 | 27,901.36 | |
| Total | −64.04 | — | 28,793.08 | |
| PRO | Motor | −10.09 | −0.77 | 24,861.99 |
| Non-Motor | −18.44 | −0.92 | 26,646.29 | |
| Total | −28.62 | — | 27,500.71 |
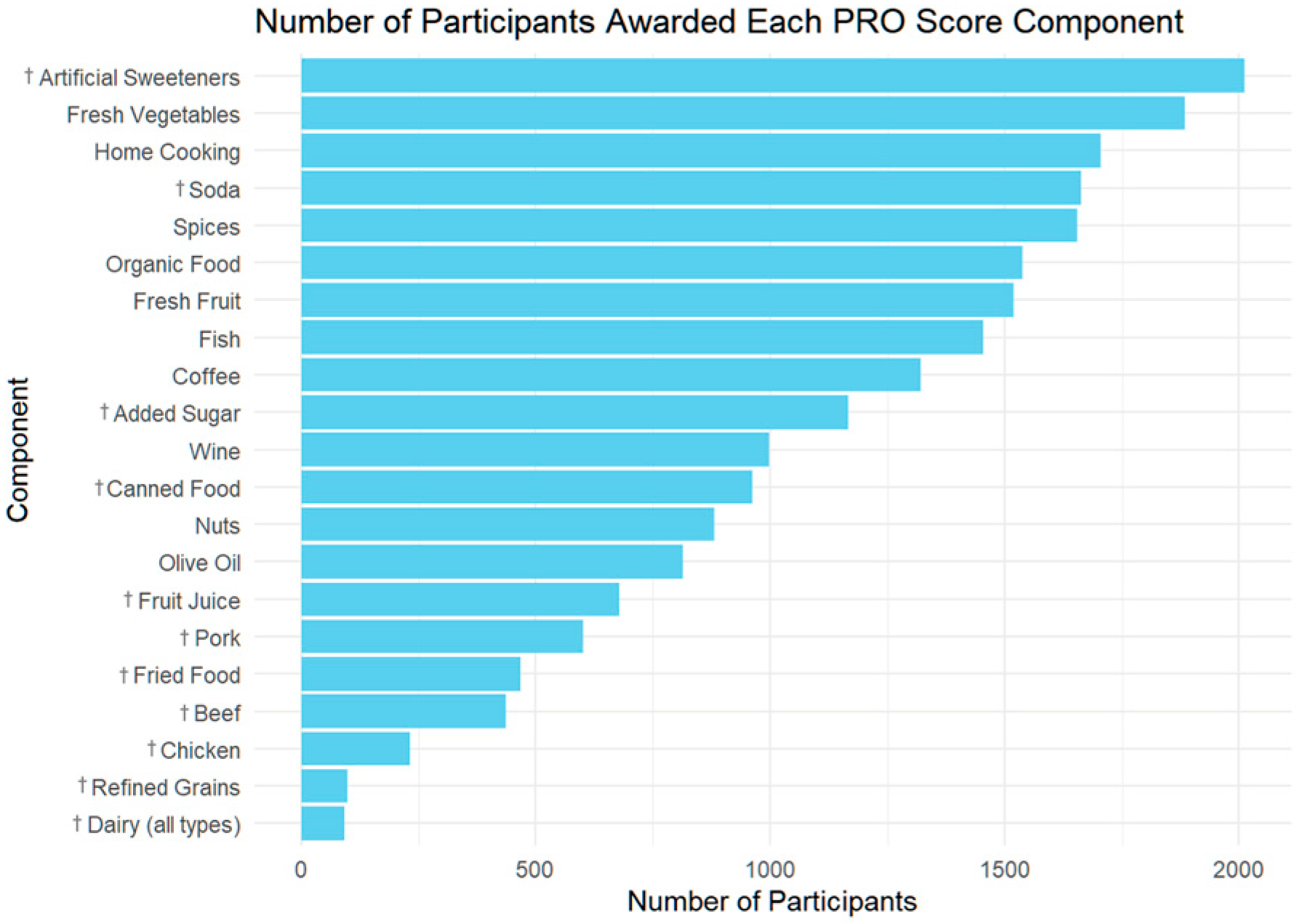
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | StdError | t-Value | p-Value | Estimate | StdError | t-Value | p-Value | |
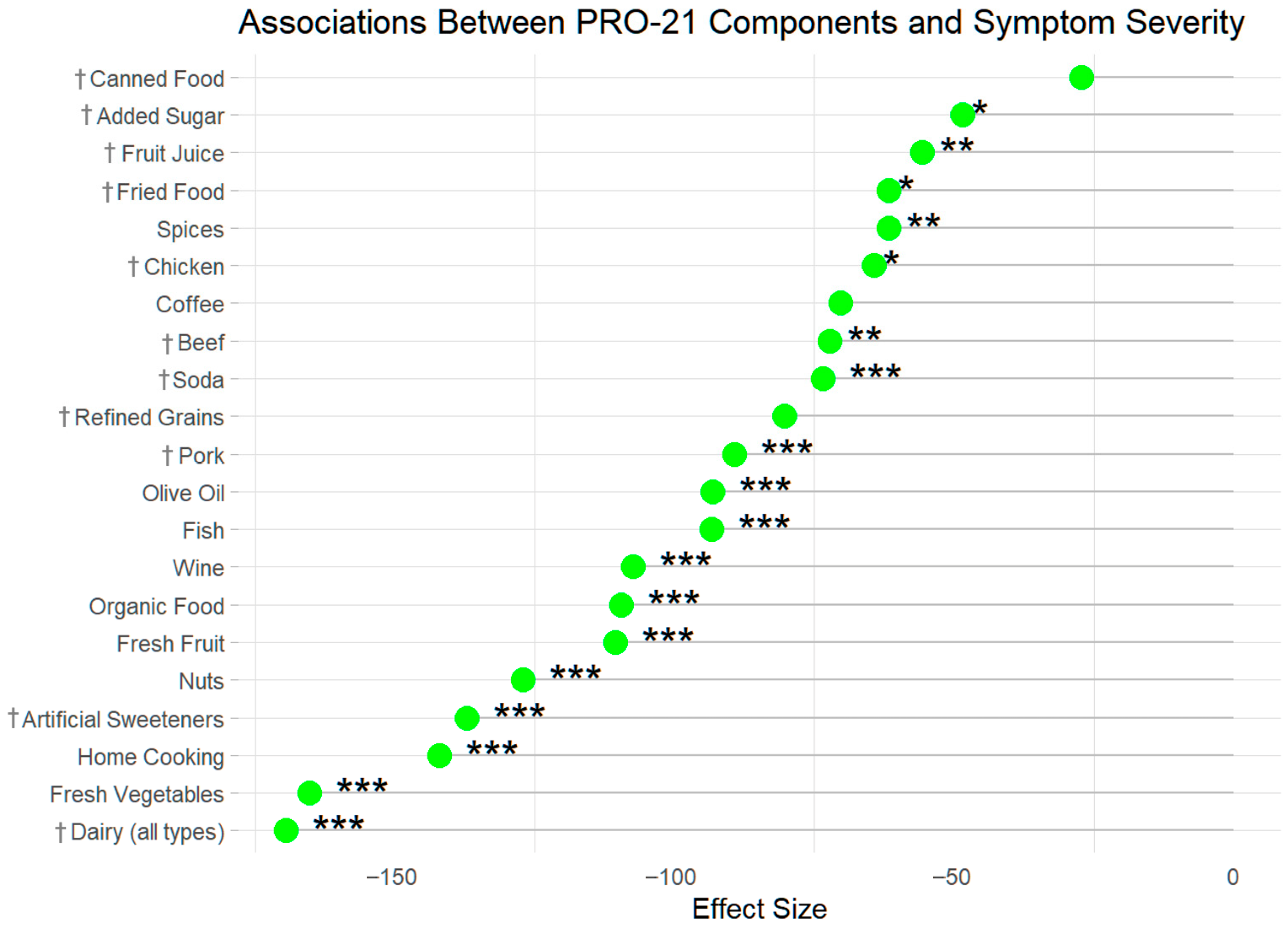
| Dairy–less than 1 serving per month | −219.81 | 49.477 | −4.443 | 0 | −169.612 | 48.609 | −3.489 | 0 |
| Fresh vegetables (1/2 cup, 5+ times per week) | −200.868 | 21.54 | −9.325 | 0 | −165.335 | 21.636 | −7.642 | 0 |
| I cook most of my own food. | −168.009 | 19.903 | −8.442 | 0 | −142.123 | 21.09 | −6.739 | 0 |
| Artificial sweeteners–“I avoid artificial sweeteners.” | −134.451 | 23.283 | −5.775 | 0 | −137.153 | 23.147 | −5.925 | 0 |
| Nuts & seeds (1/2 cup nuts or 4 Tbsp spread, daily) | −153.387 | 19.87 | −7.72 | 0 | −127.209 | 19.729 | −6.448 | 0 |
| Fresh fruit (1 cup or 2 medium fruits, daily) | −114.03 | 19.44 | −5.866 | 0 | −110.626 | 19.411 | −5.699 | 0 |
| I buy organically grown food when possible. | −122.869 | 19.321 | −6.359 | 0 | −109.593 | 19.197 | −5.709 | 0 |
| Wine (8 oz, 2+ times per week) | −159.021 | 19.263 | −8.255 | 0 | −107.394 | 19.49 | −5.51 | 0 |
| Seafood: fish, shellfish (4 oz, 2+ times per week) | −113.378 | 19.267 | −5.885 | 0 | −93.317 | 19.325 | −4.829 | 0 |
| Olive oil (2 Tablespoons, daily) | −113.576 | 20.307 | −5.593 | 0 | −93.201 | 20.154 | −4.624 | 0 |
| Pork–less than 1 serving per month | −89.052 | 22.35 | −3.985 | 0 | −89.336 | 22.172 | −4.029 | 0 |
| Refined grains (pasta, bread)–less than 1 per month | −103.921 | 50.236 | −2.069 | 0.039 | −80.398 | 49.496 | −1.624 | 0.104 |
| Soda (diet and/or regular)–less than 1 serving per month | −124.051 | 20.145 | −6.158 | 0 | −73.538 | 20.415 | −3.602 | 0 |
| Beef–less than 1 serving per month | −84.884 | 24.996 | −3.396 | 0.001 | −72.184 | 24.812 | −2.909 | 0.004 |
| Coffee (8 oz, 2+ times per day) | −140.295 | 38.312 | −3.662 | 0 | −70.401 | 38.186 | −1.844 | 0.065 |
| Chicken–less than 1 serving per month | −90.441 | 32.847 | −2.753 | 0.006 | −64.326 | 32.791 | −1.962 | 0.05 |
| I use spices liberally. | −83.371 | 20.005 | −4.167 | 0 | −61.746 | 20.031 | −3.083 | 0.002 |
| Fried food–less than 1 serving per month | −79.371 | 24.586 | −3.228 | 0.001 | −61.728 | 24.139 | −2.557 | 0.011 |
| Juice (8 oz, 1 glass)–less than 1 serving per month | −98.348 | 21.406 | −4.594 | 0 | −55.721 | 21.502 | −2.591 | 0.01 |
| Sugar–“I avoid sugar.” | −54.92 | 19.106 | −2.875 | 0.004 | −48.578 | 19.005 | −2.556 | 0.011 |
| Canned food–less than 1 serving per month | −24.548 | 19.846 | −1.237 | 0.216 | −27.243 | 19.707 | −1.382 | 0.167 |
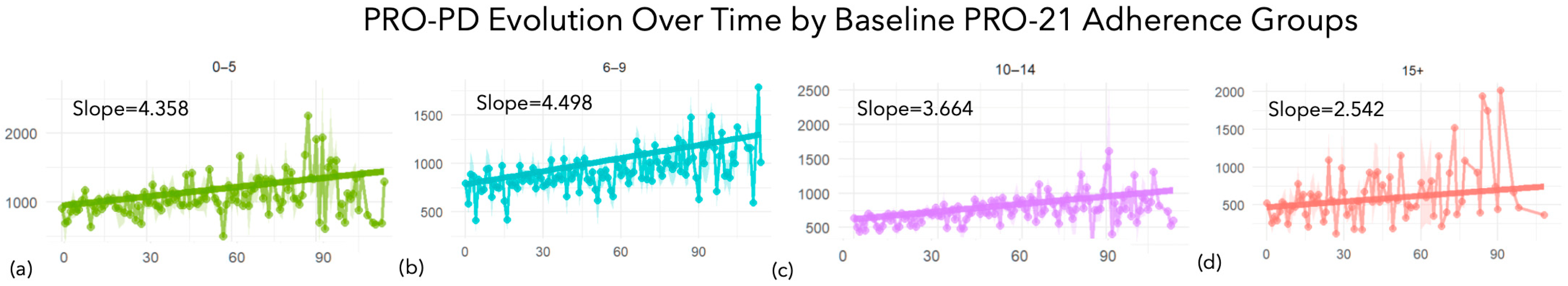
| Linear Unadjusted Mixed Model Results by PRO-21 Group | ||||||
|---|---|---|---|---|---|---|
| PRO-21 Adherence Cohort | Time Slope | Std. Error | t Value | p-Value | Observations | n |
| 0–5 points | 4.451 | 0.23 | 19.38 | 0 | 1795 | 448 |
| 6–9 points | 4.48 | 0.157 | 28.62 | 0 | 3748 | 879 |
| 10–14 points | 3.691 | 0.179 | 20.63 | 0 | 3450 | 818 |
| 15+ points | 2.889 | 0.484 | 5.96 | 0 | 383 | 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mischley, L.K.; Murawska, M. Beyond MIND and Mediterranean Diets: Designing a Diet to Optimize Parkinson’s Disease Outcomes. Nutrients 2025, 17, 2330. https://doi.org/10.3390/nu17142330
Mischley LK, Murawska M. Beyond MIND and Mediterranean Diets: Designing a Diet to Optimize Parkinson’s Disease Outcomes. Nutrients. 2025; 17(14):2330. https://doi.org/10.3390/nu17142330
Chicago/Turabian StyleMischley, Laurie K., and Magdalena Murawska. 2025. "Beyond MIND and Mediterranean Diets: Designing a Diet to Optimize Parkinson’s Disease Outcomes" Nutrients 17, no. 14: 2330. https://doi.org/10.3390/nu17142330
APA StyleMischley, L. K., & Murawska, M. (2025). Beyond MIND and Mediterranean Diets: Designing a Diet to Optimize Parkinson’s Disease Outcomes. Nutrients, 17(14), 2330. https://doi.org/10.3390/nu17142330








