Synergistic Effects of Probiotic and Omega-3 Supplementation with Ultra-Short Race Pace Training on Sprint Swimming Performance
Abstract
1. Introduction
2. Methodology
2.1. Participants
2.2. Sample Size Calculation
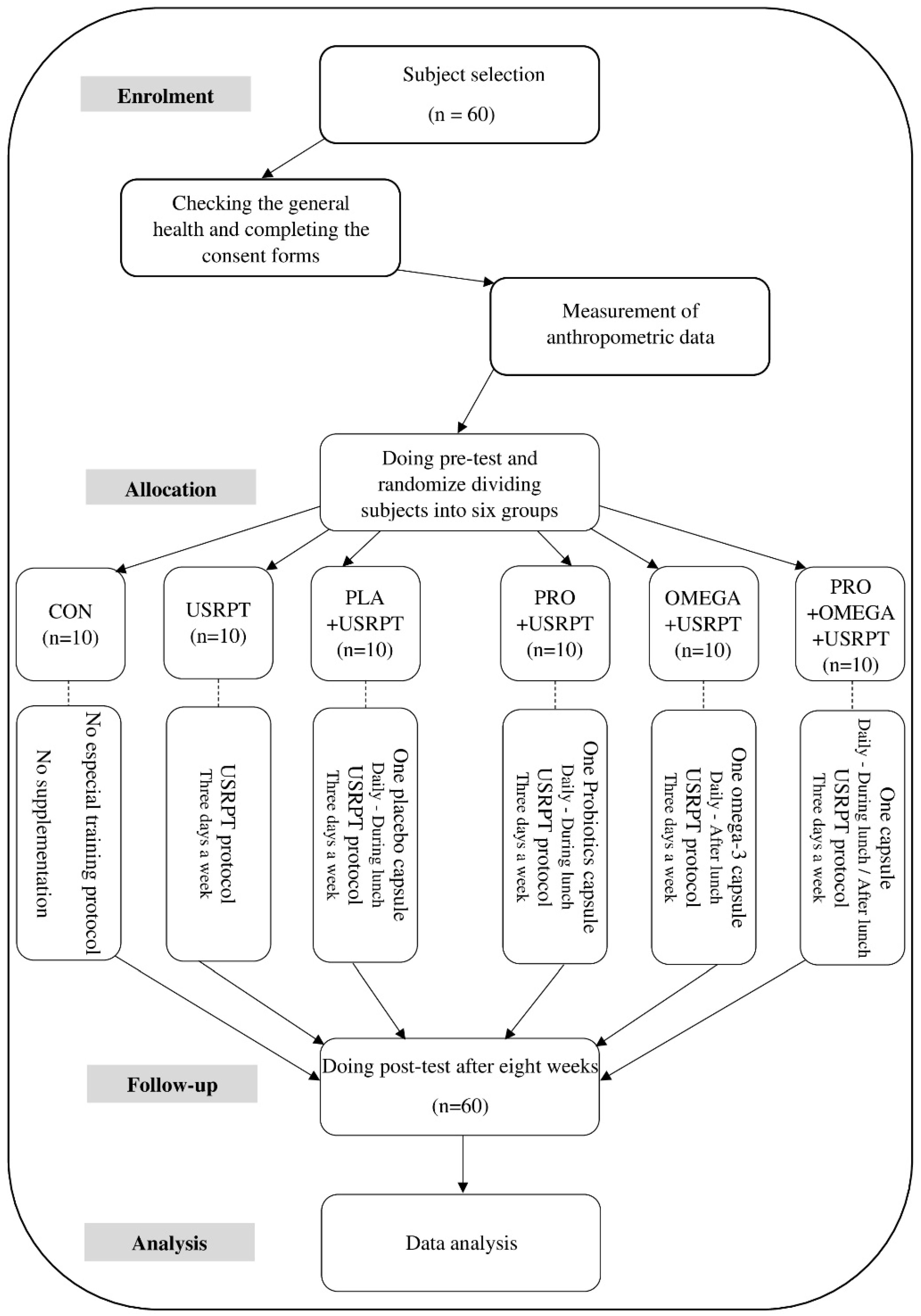
2.3. Study Design
2.4. Blinding Procedure
2.5. Training Protocol
2.6. Supplementation Protocol
2.7. Functional Tests
2.7.1. The 50 m and 100 m Freestyle
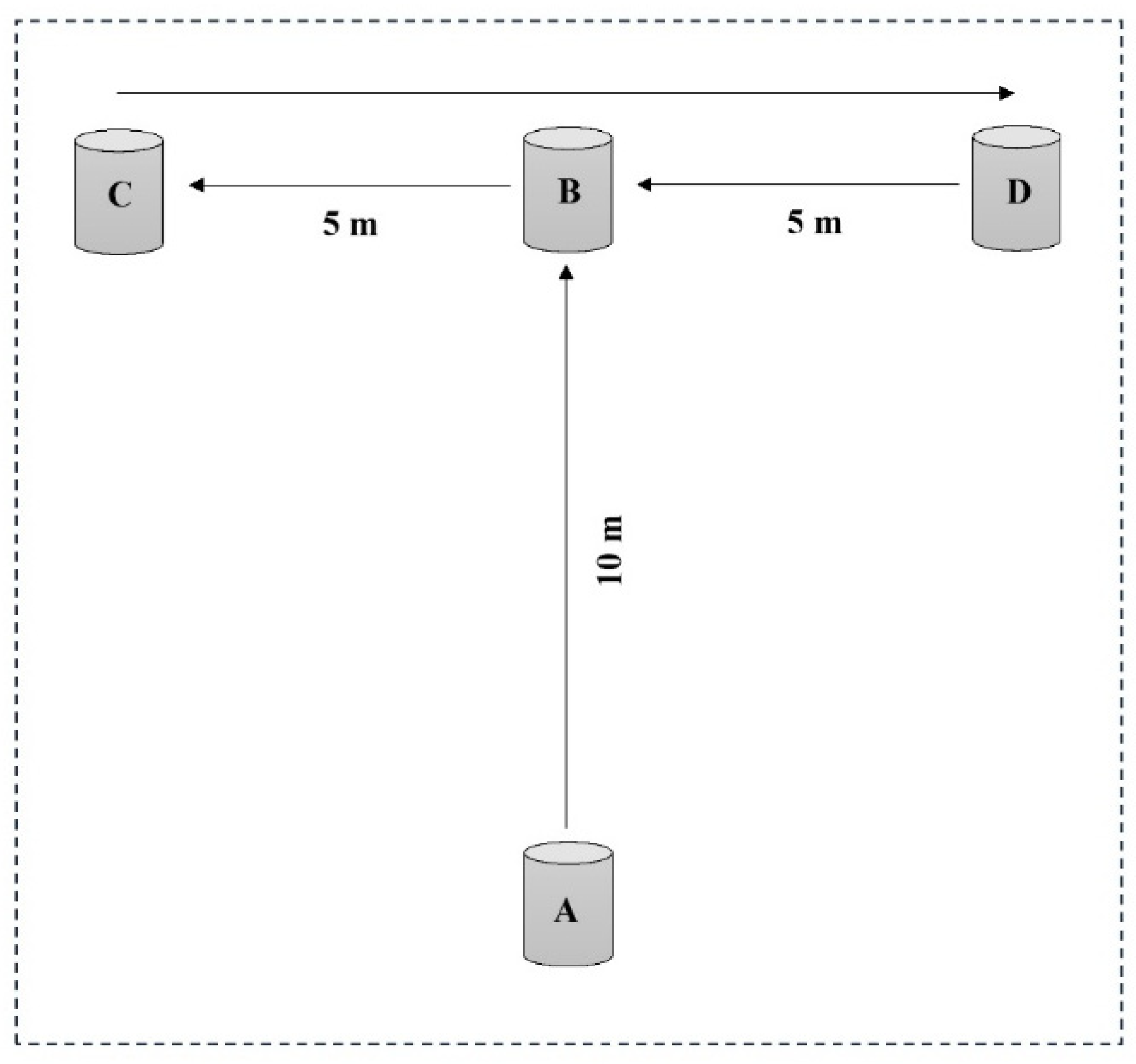
2.7.2. Start Distance (SD)
2.7.3. Push-Off Distance (PD)
2.7.4. In-Water Vertical Jump Test
2.7.5. Repeated Vertical Jump Test in Water (RWVJ)
2.7.6. Anaerobic Capacity Test
2.7.7. Reaction Time (RT)
2.7.8. Agility Test (T-Test)
2.7.9. Medicine Ball Throw Test
2.7.10. Body Composition Analysis
2.7.11. Sargent’s Jump Test
2.8. Statistical Analyses
3. Results
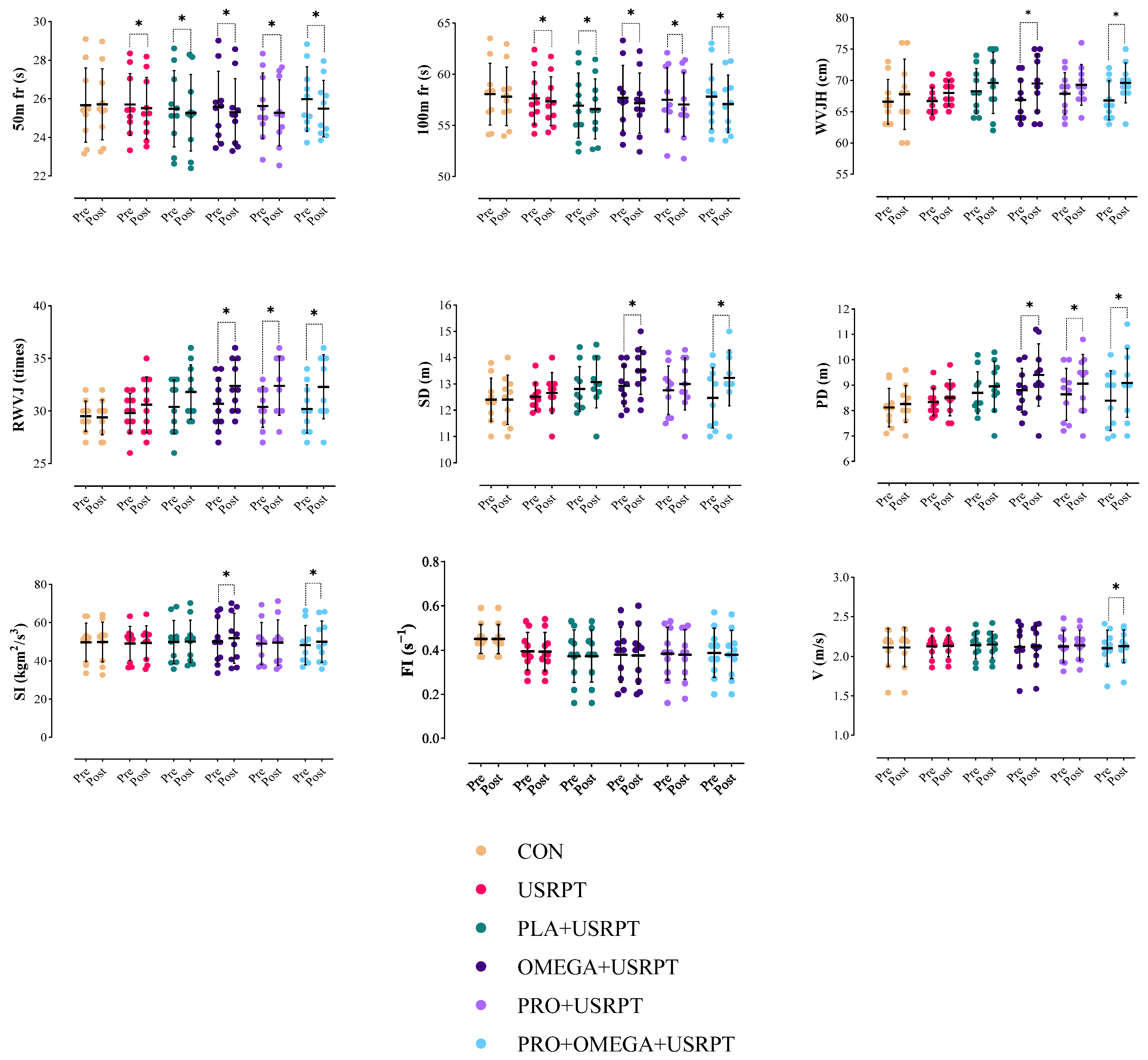
3.1. In-Water Performance Metrics
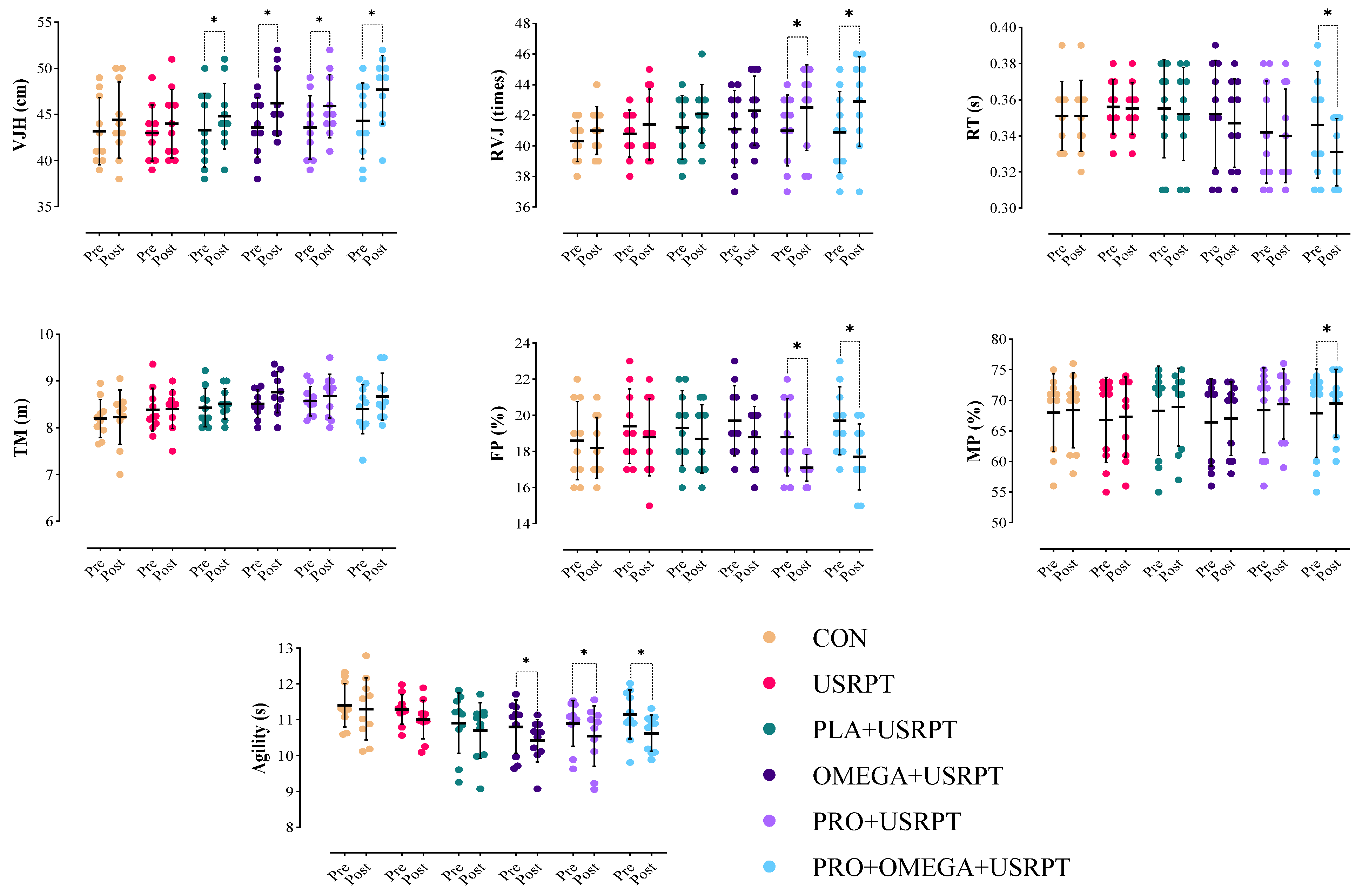
3.2. Dry-Land Performance Metrics
4. Discussion
4.1. Summary of Key Findings
4.2. Potential Mechanisms of Probiotic Effects
4.3. Body Composition and Probiotics’ Role
4.4. Potential Mechanisms of Omega-3 Effects
4.5. Synergistic Effects of Combined Supplementation
4.6. Limitations and Directions for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hołub, M.; Stanula, A.; Baron, J.; Głyk, W.; Rosemann, T.; Knechtle, B. Predicting Breaststroke and Butterfly Stroke Results in Swimming Based on Olympics History. Int. J. Environ. Res. Public Health 2021, 18, 6621. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Jesús-Sánchez-Oliver, A.; Cuenca, E.; Jodra, P.; Da Silva, S.F.; Mata-Ordóñez, F. Nutritional needs in the professional practice of swimming: A review. J. Exerc. Nutr. Biochem. 2017, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Samanipour, M.H.; Mohammadian, S.; Del Coso, J.; Salehian, O.; Jeddi, F.K.; Khosravi, M.; González-Ravé, J.M.; Ceylan, H.I.; Liu, H.; Sawan, S.A.; et al. Body Composition and Dietary Intake Profiles of Elite Iranian Swimmers and Water Polo Athletes. Nutrients 2024, 16, 2393. [Google Scholar] [CrossRef]
- Amawi, A.; AlKasasbeh, W.; Jaradat, M.; Almasri, A.; Alobaidi, S.; Abu Hammad, A.; Bishtawi, T.; Fataftah, B.; Turk, N.; Al Saoud, H.; et al. Athletes’ nutritional demands: A narrative review of nutritional requirements. Front. Nutr. 2024, 10, 1331854. [Google Scholar] [CrossRef]
- Pinos, A.J.; Fernandes, E.M.; Viana, E.; Logan-Sprenger, H.M.; Bentley, D.J. Applicability of Maximal Ergometer Testing and Sprint Performance in Adolescent Endurance and Sprint Trained Swimmers. Sports 2021, 9, 55. [Google Scholar] [CrossRef]
- Acar, K.; Mor, A.; Mor, H.; Kargın, Z.; Alexe, D.I.; Abdioğlu, M.; Karayiğit, R.; Alexe, C.I.; Cojocaru, A.M.; Mocanu, G.D. Caffeine Improves Sprint Time in Simulated Freestyle Swimming Competition but Not the Vertical Jump in Female Swimmers. Nutrients 2024, 16, 1253. [Google Scholar] [CrossRef]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 1–44. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-Muscle Axis Exists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Di Dio, M.; Calella, P.; Pelullo, C.P.; Liguori, F.; Di Onofrio, V.; Gallè, F.; Liguori, G. Effects of Probiotic Supplementation on Sports Performance and Performance-Related Features in Athletes: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 2226. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaczmarczyk, M.; Skonieczna-Żydecka, K.; Palma, J.; Bytowska, Z.K.; Kujach, S.; Kaczor, J.J. Combined probiotics with vitamin D3 supplementation improved aerobic performance and gut microbiome composition in mixed martial arts athletes. Front. Nutr. 2023, 10, 1256226. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Huang, W.-C.; Lin, J.-S.; Chen, Y.-M.; Ho, S.-T.; Huang, C.-C.; Tung, Y.-T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Imanian, B.; Hemmatinafar, M.; Daryanoosh, F.; Koureshfard, N.; Sadeghi, R.; Niknam, A.; Rezaei, R.; Qashqaei, A. The effect of probiotics and casein supplementation on aerobic capacity parameters of male soccer players. J. Int. Soc. Sports Nutr. 2024, 21, 2382165. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n− 3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef] [PubMed]
- Lakhwani, L.; Tongia, S.K.; Pal, V.S.; Agrawal, R.P.; Nyati, P.; Phadnis, P. Omega-3 fatty acids have antidepressant activity in forced swimming test in Wistar rats. Acta Pol. Pharm. 2007, 64, 271–276. [Google Scholar]
- Rossato, L.T.; Schoenfeld, B.J.; de Oliveira, E.P. Is there sufficient evidence to supplement omega-3 fatty acids to increase muscle mass and strength in young and older adults? Clin. Nutr. 2019, 39, 23–32. [Google Scholar] [CrossRef]
- Therdyothin, A.; Phiphopthatsanee, N.; Isanejad, M. The Effect of Omega-3 Fatty Acids on Sarcopenia: Mechanism of Action and Potential Efficacy. Mar. Drugs 2023, 21, 399. [Google Scholar] [CrossRef]
- Corder, K.E.; Newsham, K.R.; McDaniel, J.L.; Ezekiel, U.R.; Weiss, E.P. Effects of Short-Term Docosahexaenoic Acid Supplementation on Markers of Inflammation after Eccentric Strength Exercise in Women. J. Sports Sci. Med. 2016, 15, 176–183. [Google Scholar]
- Gray, P.; Chappell, A.; Jenkinson, A.M.; Thies, F.; Gray, S.R. Fish Oil Supplementation Reduces Markers of Oxidative Stress But Not Muscle Soreness After Eccentric Exercise. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 206–214. [Google Scholar] [CrossRef]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 Fatty Acids Correlate with Gut Microbiome Diversity and Production of N-Carbamylglutamate in Middle Aged and Elderly Women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Kabasakalis, A.; Papadopoulos, A.; Mavridis, G.; Tsalis, G. Comparison of Ultra-Short Race Pace and High-Intensity Interval Training in Age Group Competitive Swimmers. Sports 2023, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.; McCarthy, E.; Ditroilo, M. Acute Physiological Responses to Ultra Short Race-Pace Training in Competitive Swimmers. J. Hum. Kinet. 2020, 75, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Rushall, B. Relevant training effects in pool swimming: Ultra-short race-pace training (Revised). Swim. Sci. Bull. 2013, 40, 11. [Google Scholar]
- Meckel, Y.; Bishop, D.J.; Rabinovich, M.; Kaufman, L.; Nemet, D.; Eliakim, A. The relationship between short- and long-distance swimming performance and repeated sprint ability. J. Strength Cond. Res. 2012, 26, 3426–3431. [Google Scholar] [CrossRef]
- Nugent, F.; Comyns, T.; Kearney, P.; Warrington, G. Ultra-Short Race-Pace Training (USRPT) In Swimming: Current Perspectives. Open Access J. Sports Med. 2019, 10, 133–144. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Xing, R.; Yang, J.; Wang, R.; Wang, Y. Extracorporeal shock wave therapy for treating primary dysmenorrhea: A randomized controlled trial. Medicine 2021, 100, e23798. [Google Scholar] [CrossRef]
- Pirmohammadi, S.; Hemmatinafar, M.; Nemati, J.; Imanian, B.; Abdollahi, M.H. Early absorption sources of caffeine can be a useful strategy for improving female table tennis players-specific performance. J. Int. Soc. Sports Nutr. 2023, 20, 2282051. [Google Scholar] [CrossRef]
- Schulz, K.F.; Grimes, D.A. Allocation concealment in randomised trials: Defending against deciphering. Lancet 2002, 359, 614–618. [Google Scholar] [CrossRef]
- Rushall, B. Swimming energy training in the 21st century: The justification for radical changes. Swim. Sci Bull 2011, 39, 1–59. [Google Scholar]
- Sadeghi, R.; Hemmatinafar, M.; Eftekhari, F.; Imanian, B.; Koureshfard, N. Pre-sleep casein ingestion with probiotic strains improves anaerobic power and lower-body-specific strength and power performance in soccer players. J. Int. Soc. Sports Nutr. 2025, 22, 2505184. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Fernández, F.; Ruiz-Teba, A.; López-Contreras, G.; Arellano, R. Effects of 2 Types of Activation Protocols Based on Postactivation Potentiation on 50-m Freestyle Performance. J. Strength Cond. Res. 2020, 34, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Barbosa, T.M.; Neiva, H.P.; Moriyama, S.-I.; Silva, A.J.; Morais, J.E. The effect of the start and finish in the 50 m and 100 m freestyle performance in elite male swimmers. Int. J. Perform. Anal. Sport 2021, 21, 1041–1054. [Google Scholar] [CrossRef]
- Matúš, I.; Kandráč, R. Kinematic analysis of the kick start from OSB12. Phys. Act. Rev. 2020, 8, 86–96. [Google Scholar] [CrossRef]
- Barlow, H.; Halaki, M.; Stuelcken, M.; Greene, A.; Sinclair, P.J. The effect of different kick start positions on OMEGA OSB11 blocks on free swimming time to 15m in developmental level swimmers. Hum. Mov. Sci. 2014, 34, 178–186. [Google Scholar] [CrossRef]
- Platanou, T. Simple ‘in-water’vertical jump testing in water polo. Kinesiology 2006, 38, 57–62. [Google Scholar]
- Stemm, J.D.; Jacobson, B.H. Comparison of Land- and Aquatic-Based Plyometric Training on Vertical Jump Performance. J. Strength Cond. Res. 2007, 21, 568–571. [Google Scholar]
- Szczepan, S.; Wróblewska, Z.; Klich, S.; Michalik, K.; Gonjo, T.; Olstad, B.H.; Rejman, M. Reliability of a semi-tethered front crawl sprint performance test in adolescent swimmers. Front. Physiol. 2023, 14, 1260346. [Google Scholar] [CrossRef]
- Papic, C.; Sinclair, P.; Fornusek, C.; Sanders, R. The effect of auditory stimulus training on swimming start reaction time. Sports Biomech. 2018, 18, 378–389. [Google Scholar] [CrossRef]
- Keerthika, N.; Sathish, E.; Kiruthika, V.; Santhakumar, M. (Eds.) A Novel Design for Automatic Measurement of Reaction Time for Audiovisual and Muscular Stimulus. In Proceedings of the 2023 International Conference on Bio Signals, Images, and Instrumentation (ICBSII), Chennai, India, 23–25 March 2023; IEEE: Piscataway, NJ, USA, 2023. [Google Scholar]
- Scanlan, A.T.; Wen, N.; Pyne, D.B.; Stojanović, E.; Milanović, Z.; Conte, D.; Vaquera, A.; Dalbo, V.J. Power-Related Determinants of Modified Agility T-test Performance in Male Adolescent Basketball Players. J. Strength Cond. Res. 2021, 35, 2248–2254. [Google Scholar] [CrossRef]
- Mayhew, J.L.; Bird, M.; Cole, M.L.; Koch, A.J.; Jacques, J.A.; Ware, J.S.; Buford, B.N.; Fletcher, K.M. Comparison of the Backward Overhead Medicine Ball Throw to Power Production in College Football Players. J. Strength Cond. Res. 2005, 19, 514–518. [Google Scholar]
- Yang, S.-W.; Kim, T.-H.; Choi, H.-M. The reproducibility and validity verification for body composition measuring devices using bioelectrical impedance analysis in Korean adults. J. Exerc. Rehabil. 2018, 14, 621–627. [Google Scholar] [CrossRef] [PubMed]
- do Amaral Vasconcellos, F.V.; Fonseca, R.T.; Dantas, E.H.M. Validity and reproducibility of the sargent jump test in the assessment of explosive strength in soccer players. J. Hum. Kinet. 2012, 33, 115. [Google Scholar]
- Marina, M.; Rodríguez, F. Usefulness and metabolic impli-cations of a 60-second repeated jumps test as a predictor of acrobatic jumping performance in gymnasts. Biol. Sport 2013, 30, 9–16. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Lee, C.-C.; Liao, Y.-C.; Lee, M.-C.; Cheng, Y.-C.; Chiou, S.-Y.; Lin, J.-S.; Huang, C.-C.; Watanabe, K. Different impacts of heat-killed and viable Lactiplantibacillus plantarum TWK10 on exercise performance, fatigue, body composition, and gut microbiota in humans. Microorganisms 2022, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Liao, Y.-C.; Lee, M.-C.; Cheng, Y.-C.; Chiou, S.-Y.; Lin, J.-S.; Huang, C.-C.; Watanabe, K. The Potential Immunomodulatory Effect of Bifidobacterium longum subsp. longum BB536 on Healthy Adults through Plasmacytoid Dendritic Cell Activation in the Peripheral Blood. Nutrients 2023, 16, 42. [Google Scholar] [CrossRef]
- Daneshpour, N. Bifidobacterium and the Immune System: A Key Player Against Gastrointestinal Cancers. J. Microbiota 2024, 1, e154264. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Y.; Wang, M.; Zhang, Y.; Yuan, Y.; Hou, H.; Mao, Y.-H. The Improvement and Related Mechanism of Microecologics on the Sports Performance and Post-Exercise Recovery of Athletes: A Narrative Review. Nutrients 2024, 16, 1602. [Google Scholar] [CrossRef] [PubMed]
- Hasaniani, N.; Ghasemi-Kasman, M.; Halaji, M.; Rostami-Mansoor, S. Bifidobacterium breve Probiotic Compared to Lactobacillus casei Causes a Better Reduction in Demyelination and Oxidative Stress in Cuprizone-Induced Demyelination Model of Rat. Mol. Neurobiol. 2024, 61, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Fukatsu, K.; Takahashi, K.; Noguchi, M.; Watkins, A.; Matsumoto, N.; Murakoshi, S. Influences of a fermented milk with Lactobacillus bulgaricus and Streptococcus thermophiles on gut associated lymphoid tissue, mucosal IgA, and gut flora in mice. Clin. Nutr. Open Sci. 2022, 42, 36–48. [Google Scholar] [CrossRef]
- Lee, M.-C.; Hsu, Y.-J.; Chen, M.-T.; Kuo, Y.-W.; Lin, J.-H.; Hsu, Y.-C.; Huang, Y.-Y.; Li, C.-M.; Tsai, S.-Y.; Hsia, K.-C.; et al. Efficacy of Lactococcus lactis subsp. lactis LY-66 and Lactobacillus plantarum PL-02 in Enhancing Explosive Strength and Endurance: A Randomized, Double-Blinded Clinical Trial. Nutrients 2024, 16, 1921. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef]
- Pugh, J.N.; Wagenmakers, A.J.M.; Doran, D.A.; Fleming, S.C.; Fielding, B.A.; Morton, J.P.; Close, G.L. Probiotic supplementation increases carbohydrate metabolism in trained male cyclists: A randomized, double-blind, placebo-controlled crossover trial. Am. J. Physiol. Metab. 2020, 318, E504–E513. [Google Scholar] [CrossRef]
- Carbuhn, A.F.; Reynolds, S.M.; Campbell, C.W.; Bradford, L.A.; Deckert, J.A.; Kreutzer, A.; Fry, A.C. Effects of Probiotic (Bifidobacterium longum 35624) Supplementation on Exercise Performance, Immune Modulation, and Cognitive Outlook in Division I Female Swimmers. Sports 2018, 6, 116. [Google Scholar] [CrossRef]
- West, N.; Pyne, D.; Peake, J.; Cripps, A.W. Probiotics, Immunity and Exercise: A Review. Exerc. Immunol. Rev. 2009, 15, 107–126. [Google Scholar]
- Salarkia, N.; Ghadamli, L.; Zaeri, F.; Rad, L.S. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: A randomized controlled trial. Med. J. Islam Repub. Iran 2013, 27, 141–146. [Google Scholar]
- Hegazy, S.K.; El-Bedewy, M.M. Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J. Gastroenterol. 2010, 16, 4145–4151. [Google Scholar] [CrossRef]
- Therdyothin, A.; Prokopidis, K.; Galli, F.; Witard, O.C.; Isanejad, M. The effects of omega-3 polyunsaturated fatty acids on muscle and whole-body protein synthesis: A systematic review and meta-analysis. Nutr. Rev. 2024, 83, e131–e143. [Google Scholar] [CrossRef] [PubMed]
- You, J.-S.; Park, M.-N.; Lee, Y.-S. Dietary fish oil inhibits the early stage of recovery of atrophied soleus muscle in rats via Akt–p70s6k signaling and PGF2α. J. Nutr. Biochem. 2010, 21, 929–934. [Google Scholar] [CrossRef]
- Guzmán, J.F.; Esteve, H.; Pablos, C.; Pablos, A.; Blasco, C.; Villegas, J.A. DHA-Rich Fish Oil Improves Complex Reaction Time in Female Elite Soccer Players. J. Sports Sci. Med. 2011, 10, 301–305. [Google Scholar] [PubMed]
- Lewis, E.J.H.; Stucky, F.; Radonic, P.W.; Metherel, A.H.; Wolever, T.M.S.; Wells, G.D. Neuromuscular adaptations to sprint interval training and the effect of mammalian omega-3 fatty acid supplementation. Eur. J. Appl. Physiol. 2017, 117, 469–482. [Google Scholar] [CrossRef]
- Saboori, S.; Koohdani, F.; Nematipour, E.; Rad, E.Y.; Saboor-Yaraghi, A.; Javanbakht, M.; Eshraghian, M.; Ramezani, A.; Djalali, M. Beneficial effects of omega-3 and vitamin E coadministration on gene expression of SIRT1 and PGC1α and serum antioxidant enzymes in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 489–494. [Google Scholar] [CrossRef]
- Roussel, C.; Guebara, S.A.B.; Plante, P.-L.; Desjardins, Y.; Di Marzo, V.; Silvestri, C. Short-term supplementation with ω-3 polyunsaturated fatty acids modulates primarily mucolytic species from the gut luminal mucin niche in a human fermentation system. Gut Microbes 2022, 14, 2120344. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Lawrence, S.; Rechichi, C.; Bishop, D.; Dawson, B.; Goodman, C. Time–motion analysis of elite field hockey, with special reference to repeated-sprint activity. J. Sports Sci. 2004, 22, 843–850. [Google Scholar] [CrossRef]
- Allen, S.V.; Vandenbogaerde, T.J.; Hopkins, W.G. Career performance trajectories of Olympic swimmers: Benchmarks for talent development. Eur. J. Sport Sci. 2014, 14, 643–651. [Google Scholar] [CrossRef]

| Characteristic | Mean ± SD (n = 60) |
|---|---|
| Age (years) | 23.20 ± 3.64 |
| Height (cm) | 182.20 ± 5.21 |
| Weight (kg) | 81.6 ± 4.42 |
| Strains | Dosage (CFU) |
|---|---|
| Lactiplantibacillus plantarum BP06 | 0.43 × 1011 |
| Lacticaseibacillus casei BP07 | 0.65 × 1011 |
| Lactobacillus acidophilus BA05 | 0.94 × 1011 |
| Lactobacillus bulgaricus BD08 | 0.36 × 1011 |
| Bifidobacterium infantis BI04 | 0.57 × 1011 |
| Bifidobacterium longum BL03 | 0.73 × 1011 |
| Bifidobacterium breve BB02 | 0.62 × 1011 |
| Streptococcus thermophilus BT01 | 0.20 × 1011 |
| Total | 4.5 × 1011 |
| CON | USRPT | PLA + USRPT | OMEGA + USRPT | PRO + USRPT | PRO + OMEGA + USRPT | ||||
|---|---|---|---|---|---|---|---|---|---|
| In-water tests | 50 m fr (s) | Mean | Pre | 25.66 | 25.70 | 25.48 | 25.59 | 25.63 | 25.99 |
| SD | 1.91 | 1.59 | 1.97 | 1.83 | 1.70 | 1.66 | |||
| Mean | Post | 25.71 | 25.51 | 25.27 | 25.30 | 25.28 | 25.49 | ||
| SD | 1.83 | 1.59 | 1.98 | 1.73 | 1.70 | 1.45 | |||
| 100 m fr (s) | Mean | Pre | 58.05 | 57.64 | 56.93 | 57.70 | 57.51 | 57.82 | |
| SD | 3.02 | 2.58 | 3.17 | 3.16 | 3.12 | 3.15 | |||
| Mean | Post | 57.82 | 57.34 | 56.60 | 57.17 | 57.05 | 57.09 | ||
| SD | 2.86 | 2.38 | 2.93 | 2.93 | 3.12 | 2.80 | |||
| WVJH (cm) | Mean | Pre | 66.60 | 66.70 | 68.30 | 66.90 | 67.90 | 66.80 | |
| SD | 3.56 | 2.16 | 3.62 | 3.44 | 3.34 | 3.15 | |||
| Mean | Post | 67.80 | 68.00 | 69.60 | 69.50 | 69.30 | 69.60 | ||
| SD | 5.61 | 2.05 | 4.88 | 4.71 | 3.26 | 3.20 | |||
| RWVJ (times) | Mean | Pre | 29.50 | 29.80 | 30.40 | 30.70 | 30.40 | 30.20 | |
| SD | 1.43 | 1.87 | 2.54 | 2.49 | 1.95 | 2.29 | |||
| Mean | Post | 29.40 | 30.60 | 31.80 | 32.40 | 32.40 | 32.30 | ||
| SD | 1.64 | 2.63 | 2.57 | 2.45 | 2.79 | 3.05 | |||
| SD (m) | Mean | Pre | 12.40 | 12.52 | 12.81 | 12.93 | 12.76 | 12.47 | |
| SD | 0.82 | 0.53 | 0.85 | 0.78 | 0.92 | 1.14 | |||
| Mean | Post | 12.40 | 12.66 | 13.07 | 13.51 | 13.00 | 13.23 | ||
| SD | 0.94 | 0.77 | 0.97 | 0.90 | 0.99 | 1.06 | |||
| PD (m) | Mean | Pre | 8.12 | 8.34 | 8.70 | 8.80 | 8.64 | 8.39 | |
| SD | 0.75 | 0.54 | 0.82 | 0.86 | 1.01 | 1.17 | |||
| Mean | Post | 8.26 | 8.51 | 8.96 | 9.40 | 9.06 | 9.09 | ||
| SD | 0.72 | 0.71 | 0.98 | 1.22 | 1.15 | 1.35 | |||
| SI (kgm2/s3) | Mean | Pre | 49.71 | 49.13 | 49.84 | 50.27 | 49.03 | 48.24 | |
| SD | 10.05 | 8.77 | 11.20 | 11.98 | 11.01 | 10.16 | |||
| Mean | Post | 49.83 | 49.42 | 50.17 | 51.82 | 49.55 | 50.00 | ||
| SD | 10.33 | 8.83 | 11.07 | 13.07 | 11.86 | 10.76 | |||
| FI (s–1) | Mean | Pre | 0.44 | 0.39 | 0.37 | 0.37 | 0.38 | 0.38 | |
| SD | 0.06 | 0.08 | 0.11 | 0.12 | 0.12 | 0.11 | |||
| Mean | Post | 0.44 | 0.39 | 0.37 | 0.37 | 0.37 | 0.37 | ||
| SD | 0.06 | 0.08 | 0.11 | 0.13 | 0.11 | 0.10 | |||
| V (m/s) | Mean | Pre | 2.11 | 2.12 | 2.14 | 2.12 | 2.12 | 2.10 | |
| SD | 0.23 | 0.13 | 0.17 | 0.26 | 0.20 | 0.22 | |||
| Mean | Post | 2.11 | 2.13 | 2.15 | 2.12 | 2.13 | 2.12 | ||
| SD | 0.24 | 0.13 | 0.16 | 0.25 | 0.19 | 0.20 | |||
| Dry land tests | VJH (cm) | Mean | Pre | 43.20 | 43.00 | 43.30 | 43.60 | 43.60 | 44.30 |
| SD | 3.64 | 3.01 | 4.00 | 3.23 | 3.43 | 4.11 | |||
| Mean | Post | 44.40 | 44.00 | 44.80 | 46.20 | 45.90 | 47.70 | ||
| SD | 4.14 | 3.71 | 3.55 | 3.55 | 3.41 | 3.71 | |||
| RVJ (times) | Mean | Pre | 40.30 | 40.80 | 41.20 | 41.10 | 41.00 | 40.90 | |
| SD | 1.33 | 1.54 | 2.09 | 2.51 | 2.30 | 2.64 | |||
| Mean | Post | 41.00 | 41.40 | 42.10 | 42.30 | 42.50 | 42.90 | ||
| SD | 1.56 | 2.31 | 1.91 | 2.26 | 2.79 | 2.92 | |||
| RT (s) | Mean | Pre | 0.35 | 0.35 | 0.35 | 0.35 | 0.34 | 0.34 | |
| SD | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 | |||
| Mean | Post | 0.35 | 0.35 | 0.35 | 0.34 | 0.34 | 0.33 | ||
| SD | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | |||
| TM (m) | Mean | Pre | 8.19 | 8.38 | 8.42 | 8.51 | 8.56 | 8.39 | |
| SD | 0.41 | 0.45 | 0.40 | 0.30 | 0.31 | 0.52 | |||
| Mean | Post | 8.22 | 8.40 | 8.51 | 8.75 | 8.67 | 8.66 | ||
| SD | 0.57 | 0.41 | 0.32 | 0.43 | 0.46 | 0.50 | |||
| FP (%) | Mean | Pre | 18.60 | 19.40 | 19.30 | 19.70 | 18.80 | 19.70 | |
| SD | 2.17 | 2.06 | 2.05 | 1.94 | 2.14 | 1.88 | |||
| Mean | Post | 18.20 | 18.80 | 18.70 | 18.80 | 17.10 | 17.70 | ||
| SD | 1.68 | 2.14 | 1.88 | 1.68 | 0.73 | 1.82 | |||
| MP (%) | Mean | Pre | 68.00 | 66.80 | 68.30 | 66.40 | 68.40 | 67.90 | |
| SD | 6.32 | 6.98 | 7.30 | 7.12 | 6.93 | 7.21 | |||
| Mean | Post | 68.40 | 67.30 | 68.90 | 67.00 | 69.40 | 69.50 | ||
| SD | 6.13 | 6.53 | 6.38 | 6.01 | 5.73 | 5.56 | |||
| Agility (s) | Mean | Pre | 11.40 | 11.28 | 10.90 | 10.80 | 10.89 | 11.14 | |
| SD | 0.60 | 0.41 | 0.84 | 0.74 | 0.64 | 0.68 | |||
| Mean | Post | 11.30 | 11.00 | 10.70 | 10.41 | 10.54 | 10.60 | ||
| SD | 0.86 | 0.53 | 0.77 | 0.58 | 0.83 | 0.51 | |||
| Variables | CON | USRPT | PLA + USRPT | OMEGA + USRPT | PRO + USRPT | PRO + OMEGA + USRPT | |||
|---|---|---|---|---|---|---|---|---|---|
| Post | |||||||||
| In-water tests | 50 m fr (s) | MD | Pre | 0.04 | −0.19 | −0.21 | −0.28 | −0.35 | −0.50 |
| Sig | 0.587 | 0.033 | 0.017 | 0.002 | 0.001 | 0.001 | |||
| 95% CI | −0.12–0.22 | −0.36–−0.01 | −0.39–−0.04 | −0.46–−0.11 | −0.52–−0.17 | −0.67–−0.32 | |||
| 100 m fr (s) | MD | Pre | −0.23 | −0.29 | −0.33 | −0.53 | −0.45 | −0.72 | |
| Sig | 0.104 | 0.043 | 0.024 | 0.001 | 0.002 | 0.001 | |||
| 95% CI | −0.52–0.05 | −0.58–−0.01 | −0.61–−0.04 | −0.81–−0.24 | −0.73–−0.16 | −1.01–−0.43 | |||
| WVJH (cm) | MD | Pre | 1.20 | 1.30 | 1.30 | 2.60 | 1.40 | 2.80 | |
| Sig | 0.141 | 0.111 | 0.111 | 0.002 | 0.087 | 0.001 | |||
| 95% CI | −0.41–2.81 | −0.31–2.91 | −0.31–2.91 | 0.99–4.21 | −0.21–3.01 | 1.19–4.41 | |||
| RWVJ (times) | MD | Pre | −0.10 | 0.80 | 1.40 | 1.70 | 2.00 | 2.10 | |
| Sig | 0.896 | 0.299 | 0.72 | 0.030 | 0.011 | 0.008 | |||
| 95% CI | −1.62–1.42 | −0.72–2.32 | −0.12–2.92 | 0.17–3.22 | 0.47–3.52 | 0.57–3.62 | |||
| SD (m) | MD | Pre | 0.00 | 0.14 | 0.26 | 0.58 | 0.24 | 0.76 | |
| Sig | 1.000 | 0.466 | 0.178 | 0.004 | 0.213 | 0.001 | |||
| 95% CI | −0.38–0.38 | −0.24–0.52 | −0.12–0.64 | 0.19–0.96 | −0.14–0.62 | 0.37–1.14 | |||
| PD (m) | MD | Pre | 0.14 | 0.17 | 0.26 | 0.60 | 0.42 | 0.70 | |
| Sig | 0.426 | 0.335 | 0.143 | 0.001 | 0.020 | 0.001 | |||
| 95% CI | −0.21–0.49 | −0.18–0.52 | −0.09–0.61 | 0.25–0.95 | 0.07–0.77 | 0.35–1.05 | |||
| SI (kgm2/s3) | MD | Pre | 0.11 | 0.29 | 0.33 | 1.55 | 0.51 | 1.76 | |
| Sig | 0.851 | 0.616 | 0.576 | 0.011 | 0.386 | 0.004 | |||
| 95% CI | −1.06–1.29 | −0.88–1.47 | −0.84–1.51 | 0.37–2.72 | −0.66–1.69 | 0.58–2.94 | |||
| FI (s−1) | MD | Pre | 0.000 | −0.001 | −0.001 | −0.003 | −0.005 | −0.005 | |
| Sig | 0.891 | 0.837 | 0.681 | 0.356 | 0.097 | 0.079 | |||
| 95% CI | −0.006–0.005 | −0.006–0.005 | −0.007–0.005 | −0.009–0.003 | −0.011–0.001 | −0.011–0.001 | |||
| V (m/s) | MD | Pre | 0.001 | 0.005 | 0.008 | 0.009 | 0.014 | 0.025 | |
| Sig | 0.901 | 0.533 | 0.320 | 0.264 | 0.085 | 0.003 | |||
| 95% CI | −0.015–0.017 | −0.011–0.021 | −0.008–0.024 | −0.007–0.025 | −0.002–0.030 | 0.009–0.041 | |||
| Dry land tests | VJH (cm) | MD | Pre | 1.20 | 1.00 | 1.50 | 2.60 | 2.30 | 3.40 |
| Sig | 0.085 | 0.150 | 0.033 | 0.001 | 0.001 | 0.001 | |||
| 95% CI | −0.17–2.57 | −0.37–2.37 | 0.12–2.87 | 1.22–3.97 | 0.92–3.67 | 2.02–4.77 | |||
| RVJ (times) | MD | Pre | 0.70 | 0.60 | 0.90 | 1.20 | 1.50 | 2.00 | |
| Sig | 0.247 | 0.321 | 0.139 | 0.061 | 0.015 | 0.002 | |||
| 95% CI | −0.50–1.90 | −0.60–1.80 | −0.30–2.10 | −0.10–2.40 | 0.30–2.70 | 0.80–3.20 | |||
| RT (s) | MD | Pre | −0.001 | −0.002 | −0.001 | −0.004 | −0.002 | −0.013 | |
| Sig | 0.582 | 0.367 | 0.637 | 0.095 | 0.433 | 0.001 | |||
| 95% CI | −0.006–0.004 | −0.007–0.003 | −0.006–0.004 | −0.009–0.001 | −0.007–0.003 | −0.018–0.008 | |||
| TM (m) | MD | Pre | 0.02 | 0.01 | 0.08 | 0.24 | 0.11 | 0.27 | |
| Sig | 0.850 | 0.919 | 0.541 | 0.083 | 0.424 | 0.061 | |||
| 95% CI | −0.24–0.30 | −0.26–0.28 | −0.19–0.35 | −0.03–0.51 | −0.16–0.38 | −0.01–0.54 | |||
| FP (%) | MD | Pre | −0.40 | −0.60 | −0.60 | −0.90 | −1.70 | −2.00 | |
| Sig | 0.408 | 0.216 | 0.216 | 0.066 | 0.001 | 0.001 | |||
| 95% CI | −1.36–0.56 | −1.56–0.36 | −1.56–0.36 | −1.86–0.06 | −2.66–−0.73 | −2.96–−1.03 | |||
| MP (%) | MD | Pre | 0.40 | 0.50 | 0.60 | 0.60 | 1.00 | 1.60 | |
| Sig | 0.492 | 0.390 | 0.303 | 0.303 | 0.089 | 0.008 | |||
| 95% CI | −0.75–1.55 | −0.65–1.65 | −0.55–1.75 | −0.55–1.75 | −0.15–2.15 | 0.44–2.75 | |||
| Agility (s) | MD | Pre | −0.10 | −0.28 | −0.20 | −0.39 | −0.35 | −0.52 | |
| Sig | 0.488 | 0.066 | 0.172 | 0.010 | 0.019 | 0.001 | |||
| 95% CI | −0.39–0.19 | −0.57–0.01 | −0.49–0.09 | −0.68–−0.09 | −0.64–−0.05 | −0.81–−0.22 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maymandinejad, I.; Hemmatinafar, M.; Jäger, R.; Imanian, B.; Koushkie Jahromi, M.; Suzuki, K. Synergistic Effects of Probiotic and Omega-3 Supplementation with Ultra-Short Race Pace Training on Sprint Swimming Performance. Nutrients 2025, 17, 2296. https://doi.org/10.3390/nu17142296
Maymandinejad I, Hemmatinafar M, Jäger R, Imanian B, Koushkie Jahromi M, Suzuki K. Synergistic Effects of Probiotic and Omega-3 Supplementation with Ultra-Short Race Pace Training on Sprint Swimming Performance. Nutrients. 2025; 17(14):2296. https://doi.org/10.3390/nu17142296
Chicago/Turabian StyleMaymandinejad, Ideh, Mohammad Hemmatinafar, Ralf Jäger, Babak Imanian, Maryam Koushkie Jahromi, and Katsuhiko Suzuki. 2025. "Synergistic Effects of Probiotic and Omega-3 Supplementation with Ultra-Short Race Pace Training on Sprint Swimming Performance" Nutrients 17, no. 14: 2296. https://doi.org/10.3390/nu17142296
APA StyleMaymandinejad, I., Hemmatinafar, M., Jäger, R., Imanian, B., Koushkie Jahromi, M., & Suzuki, K. (2025). Synergistic Effects of Probiotic and Omega-3 Supplementation with Ultra-Short Race Pace Training on Sprint Swimming Performance. Nutrients, 17(14), 2296. https://doi.org/10.3390/nu17142296








