Does Vitamin D Supplementation Slow Brain Volume Loss in Multiple Sclerosis? A 4-Year Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Aged over 18 years;
- EDSS ≤ 6.5;
- Stable DMT treatment for at least one year at the baseline.
- 5.
- Progressive subtypes of MS, including secondary progressive MS (SPMS) and primary progressive MS (PPMS);
- 6.
- Relapse in the last 4 weeks;
- 7.
- Steroid use in the last 6 weeks;
- 8.
- An EDSS > 6.5;
- 9.
- Pregnancy and breast-feeding;
- 10.
- Acute or chronic renal failure;
- 11.
- Hypercalcemia in medical history;
- 12.
- DMT switch from platform to high-efficacy treatment agents or DMT discontinuation/termination;
- 13.
- Other types of MS treatment (mitoxantrone and cyclophosphamide).
2.2. Atrophy Assessment
2.3. Statistical Analysis
2.4. Ethics Approval
3. Results
3.1. Study Group Characteristics
3.2. Vitamin D Serum Levels
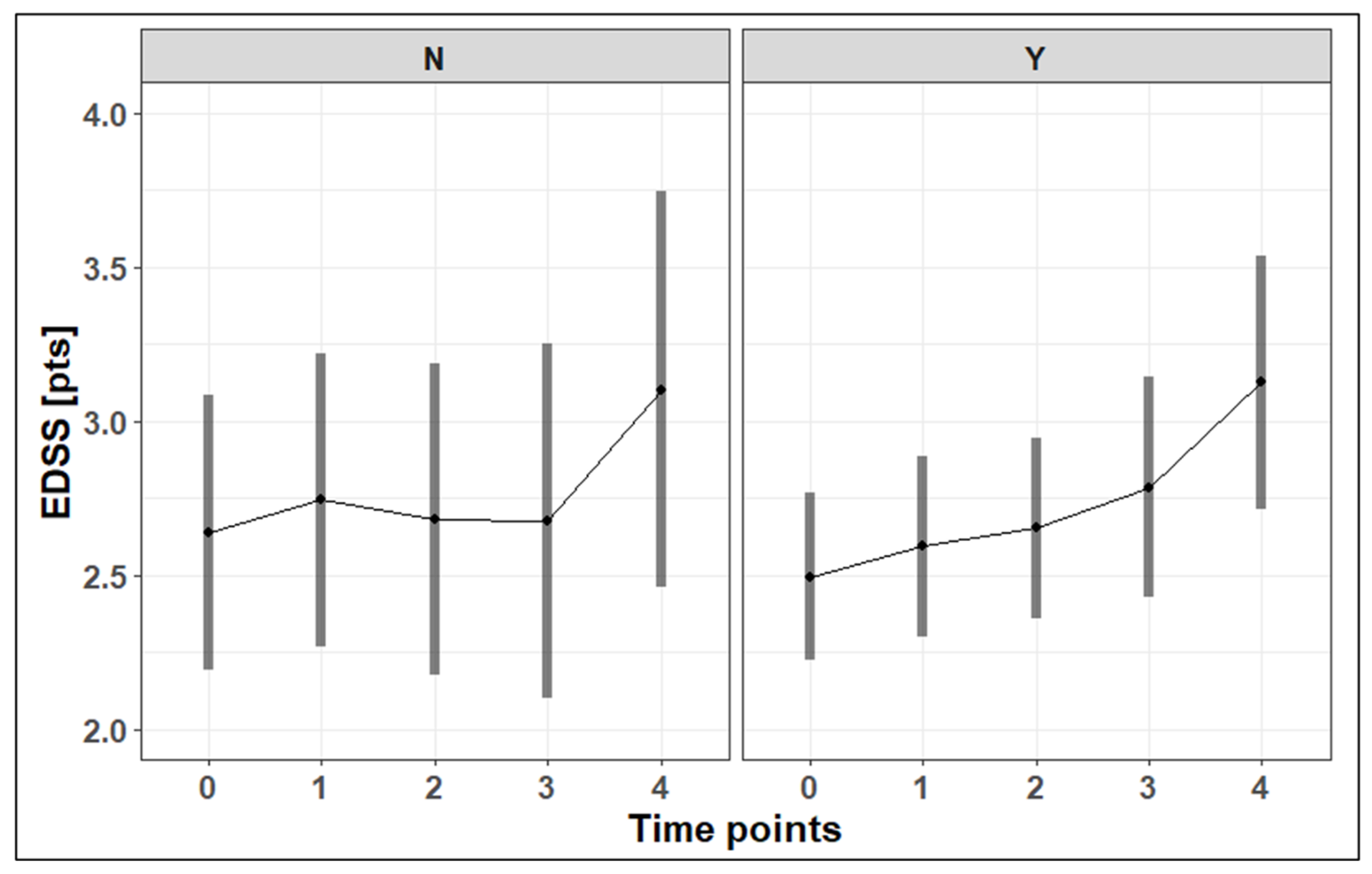
3.3. EDSS Progression
3.4. Vitamin D Supplementation and Clinical and Radiological Disease Activity
3.5. Vitamin D Supplementation and Brain Atrophy
3.6. Sun Exposure
4. Discussion
4.1. Vitamin D Supplementation and Clinical and Radiological Disease Activity
4.2. Vitamin D Supplementation and Brain Atrophy
4.3. Potential Mechanism Underlying the Association Between Vitamin D Supplementation and Reduction in Brain Atrophy
4.4. Consideration of Disability Progression
4.5. Sun Exposure Impact on Disability Progression
4.6. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Multiple sclerosis |
| RRMS | Relapsing–remitting multiple sclerosis |
| CNS | Central nervous system |
| EDSS | Expanded Disability Status Scale |
| DMT | Disease-modifying therapy |
| MRI | Magnetic resonance imaging |
| T2-w | T2-weighted |
| GEL | Gadolinium-enhancing lesion |
| 2D | Two-dimensional |
| FH | Frontal horn distance |
| CC | Intercaudate distance |
| TV | Third ventricle width |
| IT | Inner table of the skull |
| mIT | Maximum inner table of the skull |
| BCR | Bicaudate ratio |
| 25(OH)D | 25-hydroxyvitamin D |
| CI | Confidence interval |
| BMI | Body mass index |
| HETA | High-efficacy treatment agent |
References
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Leung, P.S.; Adamopoulos, I.E.; Gershwin, M.E. The implication of vitamin D and autoimmunity: A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.K.; Fujinami, R.S. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 2007, 184, 37–44. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Niedziela, N.; Kalinowska, A.; Kułakowska, A.; Mirowska-Guzel, D.; Rejdak, K.; Siger, M.; Stasiołek, M.; Adamczyk-Sowa, M. Clinical and therapeutic challenges of smouldering multiple sclerosis. Neurol. Neurochir. Pol. 2024, 58, 245–255. [Google Scholar] [CrossRef]
- Andravizou, A.; Dardiotis, E.; Artemiadis, A.; Sokratous, M.; Siokas, V.; Tsouris, Z.; Aloizou, A.M.; Nikolaidis, I.; Bakirtzis, C.; Tsivgoulis, G.; et al. Brain atrophy in multiple sclerosis: Mechanisms, clinical relevance and treatment options. Autoimmun. Highlights 2019, 10, 7. [Google Scholar] [CrossRef]
- Cappelle, S.; Pareto, D.; Tintoré, M.; Vidal-Jordana, A.; Alyafeai, R.; Alberich, M.; Sastre-Garriga, J.; Auger, C.; Montalban, X.; Rovira, À. A validation study of manual atrophy measures in patients with Multiple Sclerosis. Neuroradiology 2020, 62, 955–964. [Google Scholar] [CrossRef]
- Pérez-Álvarez, A.I.; Suárez-Santos, P.; González-Delgado, M.; Oliva-Nacarino, P. Quantification of brain atrophy in multiple sclerosis using two-dimensional measurements. Neurología 2020, 35, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Ajitomi, S.; Fujimori, J.; Nakashima, I. Usefulness of two-dimensional measurements for the evaluation of brain volume and disability in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2022, 8, 20552173211070749. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention—An Update. Semin. Neurol. 2016, 36, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Rhead, B.; Bäärnhielm, M.; Gianfrancesco, M.; Mok, A.; Shao, X.; Quach, H.; Shen, L.; Schaefer, C.; Link, J.; Gyllenberg, A.; et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol. Genet. 2016, 2, e97. [Google Scholar] [CrossRef]
- Jiang, X.; Ge, T.; Chen, C.Y. The causal role of circulating vitamin D concentrations in human complex traits and diseases: A large-scale Mendelian randomization study. Sci. Rep. 2021, 11, 184. [Google Scholar] [CrossRef]
- Galoppin, M.; Kari, S.; Soldati, S.; Pal, A.; Rival, M.; Engelhardt, B.; Astier, A.; Thouvenot, E. Full spectrum of vitamin D immunomodulation in multiple sclerosis: Mechanisms and therapeutic implications. Brain Commun. 2022, 4, fcac171. [Google Scholar] [CrossRef]
- Correale, J.; Ysrraelit, M.C.; Gaitán, M.I. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain 2009, 132, 1146–1160. [Google Scholar] [CrossRef]
- Sangha, A.; Quon, M.; Pfeffer, G.; Orton, S.M. The Role of Vitamin D in Neuroprotection in Multiple Sclerosis: An Update. Nutrients 2023, 15, 2978. [Google Scholar] [CrossRef]
- Sparaco, M.; Bonavita, S. Vitamin D Supplementation: Effect on Cytokine Profile in Multiple Sclerosis. J. Clin. Med. 2024, 13, 835. [Google Scholar] [CrossRef]
- Walawska-Hrycek, A.; Galus, W.; Hrycek, E.; Kaczmarczyk, A.; Krzystanek, E. The Impact of Vitamin D Low Doses on Its Serum Level and Cytokine Profile in Multiple Sclerosis Patients. J. Clin. Med. 2021, 10, 2781. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Mahatanan, R.; Lee, C.H.; Charoenpong, P.; Hong, J.P. Associations of serum 25(OH) vitamin D levels with clinical and radiological outcomes in multiple sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2020, 411, 116668. [Google Scholar] [CrossRef] [PubMed]
- Moosazadeh, M.; Nabinezhad-Male, F.; Afshari, M.; Nasehi, M.M.; Shabani, M.; Kheradmand, M.; Aghaei, I. Vitamin D status and disability among patients with multiple sclerosis: A systematic review and meta-analysis. AIMS Neurosci. 2021, 8, 239–253. [Google Scholar] [CrossRef]
- Yuan, X.; Guo, L.; Jiang, C.; Yang, X.; Huang, J. The Effect of Different Administration Time and Dosage of Vitamin D Supplementation in Patients with Multiple Sclerosis: A Meta-Analysis of Randomized Controlled Trials. Neuroimmunomodulation 2021, 28, 118–128. [Google Scholar] [CrossRef]
- Hanaei, S.; Sahraian, M.A.; Mohammadifar, M.; Ramagopalan, S.V.; Ghajarzadeh, M. Effect of Vitamin D Supplements on Relapse Rate and Expanded Disability Status Scale (EDSS) in Multiple Sclerosis (MS): A Systematic Review and Meta-Analysis. Int. J. Prev. Med. 2021, 12, 42. [Google Scholar] [CrossRef]
- Zheng, C.; He, L.; Liu, L.; Zhu, J.; Jin, T. The efficacy of vitamin D in multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2018, 23, 56–61. [Google Scholar] [CrossRef]
- Doosti-Irani, A.; Tamtaji, O.R.; Mansournia, M.A.; Ghayour-Mobarhan, M.; Ferns, G.; Daneshvar Kakhaki, R.; Rezaei Shahmirzadi, A.; Asemi, Z. The effects of vitamin D supplementation on expanded disability status scale in people with multiple sclerosis: A critical, systematic review and meta-analysis of randomized controlled trials. Clin. Neurol. Neurosurg. 2019, 187, 105564. [Google Scholar] [CrossRef]
- Jagannath, V.A.; Filippini, G.; Di Pietrantonj, C.; Asokan, G.V.; Robak, E.W.; Whamond, L.; Robinson, S.A. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst. Rev. 2018, 9, CD008422. [Google Scholar] [CrossRef]
- McLaughlin, L.; Clarke, L.; Khalilidehkordi, E.; Butzkueven, H.; Taylor, B.; Broadley, S.A. Vitamin D for the treatment of multiple sclerosis: A meta-analysis. J. Neurol. 2018, 265, 2893–2905. [Google Scholar] [CrossRef]
- Mahler, J.V.; Solti, M.; Apóstolos-Pereira, S.L.; Adoni, T.; Silva, G.D.; Callegaro, D. Vitamin D3 as an add-on treatment for multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. Mult. Scler. Relat. Disord. 2024, 82, 105433. [Google Scholar] [CrossRef] [PubMed]
- Piędel, F.; Rocka, A.; Piwek, M.; Jasielski, P.P.; Petit, V.; Rejdak, K. Correlation between vitamin D and alterations in MRI among patients with multiple sclerosis. Ann. Agric. Environ. Med. 2021, 28, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Hajeer, S.; Nasr, F.; Nabha, S.; Saab, M.B.; Harati, H.; Desoutter, A.; Al Ahmar, E.; Estephan, E. Association between vitamin D deficiency and multiple sclerosis—MRI significance: A scoping review. Heliyon 2023, 9, e15754. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D supplementation guidelines for the general population and groups at risk of vitamin D deficiency in Poland—Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel. Front. Endocrinol. 2018, 9, 246. [Google Scholar] [CrossRef]
- Evans, W.A. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch. Neuropsychol. 1942, 47, 931–937. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef]
- Burton, J.; Kimball, S.; Vieth, R.; Bar-Or, A.; Dosch, H.-M.; Cheung, R.; Gagne, D.; D’SOuza, C.; Ursell, M.; O’COnnor, P. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology 2010, 74, 1852–1859. [Google Scholar] [CrossRef]
- Soilu-Hänninen, M.; Åivo, J.; Lindström, B.-M.; Elovaara, I.; Sumelahti, M.-L.; Färkkilä, M.; Tienari, P.; Atula, S.; Sarasoja, T.; Herrala, L.; et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add-on treatment to interferon β-1b in patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 565–571. [Google Scholar] [CrossRef]
- Camu, W.; Lehert, P.; Pierrot-Deseilligny, C.; Hautecoeur, P.; Besserve, A.; Deleglise, A.-S.J.; Payet, M.; Thouvenot, E.; Souberbielle, J.C. Cholecalciferol in relapsing-remitting MS: A randomized clinical trial (CHOLINE). Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e597. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Liu, Y.; Gray, O.; Baker, J.; Kolbe, S.; Ditchfield, M.; Egan, G.; Mitchell, P.; Harrison, L.; Butzkueven, H.; et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 2011, 77, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi, G.; Ghazavi, A.; Ghasami, K.; Jand, Y.; Kokhaei, P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol. Investig. 2011, 40, 627–639. [Google Scholar] [CrossRef]
- Galus, W.; Chmiela, T.; Walawska-Hrycek, A.; Krzystanek, E. Radiological Benefits of Vitamin D Status and Supplementation in Patients with MS—A Two-Year Prospective Observational Cohort Study. Nutrients 2023, 15, 1465. [Google Scholar] [CrossRef]
- Simpson, S.; Taylor, B.; Blizzard, L.; Ponsonby, A.; Pittas, F.; Tremlett, H.; Dwyer, T.; Gies, P.; van der Mei, I. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann. Neurol. 2010, 68, 193–203. [Google Scholar] [CrossRef]
- Dörr, J.; Bäcker-Koduah, P.; Wernecke, K.-D.; Becker, E.; Hoffmann, F.; Faiss, J.; Brockmeier, B.; Hoffmann, O.; Anvari, K.; Wuerfel, J.; et al. High-dose vitamin D supplementation in multiple sclerosis—Results from the randomized EVIDIMS trial. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320903474. [Google Scholar] [CrossRef]
- Giordano, A.; Clarelli, F.; Pignolet, B.; Mascia, E.; Sorosina, M.; Misra, K.; Ferrè, L.; Bucciarelli, F.; Manouchehrinia, A.; Moiola, L.; et al. Vitamin D affects the risk of disease activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2025, 96, 170–176. [Google Scholar] [CrossRef]
- Giovannoni, G.; Butzkueven, H.; Dhib-Jalbut, S.; Hobart, J.; Kobelt, G.; Pepper, G.; Sormani, M.P.; Thalheim, C.; Traboulsee, A.; Vollmer, T. Brain health: Time matters in multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 9, S5–S48. [Google Scholar] [CrossRef]
- De Stefano, N.; Giorgio, A.; Battaglini, M.; Rovaris, M.; Sormani, M.; Barkhof, F.; Korteweg, T.; Enzinger, C.; Fazekas, F.; Calabrese, M.; et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 2010, 74, 1868–1876. [Google Scholar] [CrossRef]
- Matthews, P.M.; Gupta, D.; Mittal, D.; Bai, W.; Scalfari, A.; Pollock, K.G.; Sharma, V.; Hill, N. The association between brain volume loss and disability in multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2023, 74, 104714. [Google Scholar] [CrossRef]
- Guenter, W.; Betscher, E.; Bonek, R. Predictive value of the third ventricle width for neurological status in multiple sclerosis. J. Clin. Med. 2022, 11, 2841. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Weinstock-Guttman, B.; Fishman, I.; Sharma, J.; Tjoa, C.W.; Bakshi, R. Prediction of neuropsychological impairment in multiple sclerosis: Comparison of conventional MRI measures of atrophy and lesion burden. Arch. Neurol. 2004, 61, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L.; White, R.; Köchert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.-P.; Miller, D.H.; Montalbán, X.; et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Zivadinov, R.; Qu, J.; Cookfair, D.; Duan, X.; Bang, E.; Bergsland, N.; Hussein, S.; Cherneva, M.; Willis, L.; et al. Vitamin D metabolites are associated with clinical and MRI outcomes in multiple sclerosis patients. J. Neurol. Neurosurg. Psychiatry 2011, 82, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Munger, K.L.; Köchert, K.; Arnason, B.G.W.; Comi, G.; Cook, S.; Goodin, D.S.; Filippi, M.; Hartung, H.-P.; Jeffery, D.R.; et al. Association of Vitamin D Levels with Multiple Sclerosis Activity and Progression in Patients Receiving Interferon Beta-1b. JAMA Neurol. 2015, 72, 1458–1465. [Google Scholar] [CrossRef]
- Nystad, A.E.; Wergeland, S.; Aksnes, L.; Myhr, K.-M.; Bø, L.; Torkildsen, Ø. Effect of high-dose 1.25 dihydroxyvitamin D3 on remyelination in the cuprizone model. Acta Pathol. Microbiol. Immunol. Scand. 2014, 122, 1178–1186. [Google Scholar] [CrossRef]
- Wergeland, S.; Torkildsen, Ø.; Myhr, K.-M.; Aksnes, L.; Mørk, S.J.; Bø, L.; Reindl, M. Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS ONE 2011, 6, e26262. [Google Scholar] [CrossRef]
- De La Fuente, A.G.; Lange, S.; Silva, M.E.; Gonzalez, G.A.; Tempfer, H.; van Wijngaarden, P.; Zhao, C.; Di Canio, L.; Trost, A.; Bieler, L.; et al. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep. 2017, 20, 1755–1764. [Google Scholar] [CrossRef]
- Baas, D.; Prüfer, K.; Ittel, M.E.; Kuchler-Bopp, S.; Labourdette, G.; Sarliève, L.L.; Brachet, P. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia 2000, 31, 59–68. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Rostami, A.; Zhang, G.-X. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp. Mol. Pathol. 2015, 98, 240–245. [Google Scholar] [CrossRef]
- Lee, Y.A.; Yoon, S.; Kim, S.; Youn, Y.C. Association of 25-hydroxyvitamin D status with brain volume changes. Food Sci. Nutr. 2021, 9, 4169–4175. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, E.; Zhang, Q.; Liu, J.; Luo, W. Vitamin D and Focal Brain Atrophy in PD with Non-Dementia: A VBM Study. Front. Hum. Neurosci. 2024, 18, 1474148. [Google Scholar] [CrossRef]
- Boccardi, V.; Comanducci, C.; Baroni, M.; Mecocci, P. Of Energy and Entropy: The Ineluctable Impact of Aging in Old Age Dementia. Int. J. Mol. Sci. 2017, 18, 2672. [Google Scholar] [CrossRef]
- Zurawski, J.; Glanz, B.I.; Chua, A.; Lokhande, H.; Rotstein, D.; Weiner, H.; Engler, D.; Chitnis, T.; Healy, B.C. Time between expanded disability status scale (EDSS) scores. Mult. Scler. Relat. Disord. 2019, 30, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.L.; Nair, K.V.; Williams, I.M.; Alvarez, E. Multiple sclerosis phenotypes as a continuum: The role of neurologic reserve. Neurol. Clin. Pract. 2021, 11, 342–351. [Google Scholar] [CrossRef]
- Chen, T.C.; Chimeh, F.; Lu, Z.; Mathieu, J.; Person, K.S.; Zhang, A.; Kohn, N.; Martinello, S.; Berkowitz, R.; Holick, M.F. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch. Biochem. Biophys. 2007, 460, 213–217. [Google Scholar] [CrossRef]
- Wu, J.; Olsson, T.; Alfredsson, L.; Hedström, A.K. Association between sun exposure habits and disease progression in multiple sclerosis. Eur. J. Neurol. 2024, 31, e16269. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for preventing and treating vitamin D deficiency: A 2023 update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]

| Characteristics | Baseline (N = 132) |
|---|---|
| Men/women (n, %) | 34 (25.8%)/98 (74.2%) |
| Age (mean ± SD) | 45.8 ± 10.9 |
| BMI (mean ± SD) | 24.1 (21.9; 27.9) |
| Any comorbidities (n, %) | 20 (15.2%) |
| Smoking (n, %) | 25 (18.9%) |
| EDSS (median, min–max) | 2 (1.5–6.5) |
| Disease duration (mean, min–max) | 10.0 (6.0; 14.0) years |
| DMT (n, %) | 132 (100%) |
| Interferons | 21 (15.8%) |
| Glatiramer acetate | 9 (6.8%) |
| Dimethyl fumarate | 64 (48.5%) |
| Teriflunomide | 17 (12.9%) |
| Fingolimod | 11 (8.3%) |
| Natalizumab | 8 (6.1%) |
| Ocrelizumab | 1 (0.8%) |
| Alemtuzumab | 1 (0.8%) |
| Platform/HETA | 111/21 (84.1%/15.9%) |
| Patients Without Vitamin D Supplementation, N = 35 | Patients with Vitamin D Supplementation, N = 97 | p-Value | |
|---|---|---|---|
| Male (n, %) | 15 (42.9) | 19 (19.6) | <0.01 |
| Age (mean, ±SD) [years] | 44.7 ± 11.2 | 46.2 ± 10.9 | 0.48 |
| BMI (mean, ±SD) [kg/m2] | 25.0 ± 4.7 | 25.2 ± 4.5 | 0.88 |
| Sun exposure (n, %) | 16 (45.7) | 33 (43.0) | 0.22 |
| Comorbidities (n, %) | 2 (5.7) | 18 (18.6) | 0.10 |
| Smoking (n, %) | 5 (14.3) | 20 (20.6) | 0.41 |
| Duration of disease (median—lower quartile and upper quartile) [years] | 9 (5; 13) | 10 (6; 14) | 0.34 |
| HETA (n, %) | 5 (14.3) | 16 (16.5) | 0.76 |
| Patients Without Vitamin D Supplementation | Patients with Vitamin D Supplementation | ||||||
|---|---|---|---|---|---|---|---|
| Follow-Up | Mean Value | Lower CI | Upper CI | Mean Value | Lower CI | Upper CI | p-Value |
| Baseline | 21.7 | 13.9 | 29.4 | 41.2 | 36.5 | 45.9 | <0.001 |
| 12 months | 27.8 | 19.9 | 35.7 | 42.7 | 37.7 | 47.6 | <0.01 |
| 24 months | 28.9 | 20.9 | 37.0 | 42.5 | 38.0 | 47.1 | <0.01 |
| 36 months | 36.1 | 29.0 | 43.2 | 41.9 | 37.8 | 46.1 | 0.16 |
| 48 months | 39.9 | 32.9 | 46.9 | 41.1 | 36.7 | 45.4 | 0.78 |
| ∆48months vs. baseline | 18.3 | 9.8 | 26.7 | −0.2 | −5.4 | 5.1 | <0.001 |
| Time Point | Patients Without Vitamin D Supplementation | Patients with Vitamin D Supplementation | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Mean Value | Lower CI | Upper CI | Mean Value | Lower CI | Upper CI | ||
| Frontal horn width (FH) [mm] | |||||||
| Baseline | 35.7 | 34.1 | 37.2 | 33.8 | 32.8 | 34.7 | <0.05 |
| At 36 months | 36.3 | 34.4 | 38.1 | 34.2 | 33.1 | 35.2 | <0.05 |
| Intercaudate distance (CC) [mm] | |||||||
| Baseline | 12.0 | 11.0 | 13.0 | 11.1 | 10.5 | 11.7 | 0.13 |
| At 36 months | 12.7 | 11.6 | 13.8 | 11.4 | 10.8 | 12.0 | <0.05 |
| Inner table of the skull measured along the CC line (IT) [mm] | |||||||
| Baseline | 118 | 116 | 120 | 117 | 116 | 118 | NS |
| At 36 months | 118 | 116 | 120 | 117 | 116 | 119 | NS |
| Inner table of the skull measured at its maximum width (mIT) [mm] | |||||||
| Baseline | 135.0 | 133.0 | 136.9 | 132.7 | 131.5 | 133.8 | <0.05 |
| At 36 months | 134.5 | 132.5 | 136.5 | 133.3 | 132.1 | 134.4 | 0.29 |
| Third ventricle (TV) [mm] | |||||||
| Baseline | 8.2 | 7.4 | 9.1 | 7.1 | 6.6 | 7.6 | <0.05 |
| At 36 months | 8.7 | 7.8 | 9.6 | 7.4 | 6.8 | 7.9 | <0.05 |
| Bicaudate ratio (BCR) | |||||||
| Baseline | 0.100 | 0.092 | 0.108 | 0.095 | 0.090 | 0.100 | NS |
| At 36 months | 0.106 | 0.098 | 0.114 | 0.097 | 0.092 | 0.102 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galus, W.; Winder, M.; Owczarek, A.J.; Walawska-Hrycek, A.; Rzepka, M.; Kaczmarczyk, A.; Siuda, J.; Krzystanek, E. Does Vitamin D Supplementation Slow Brain Volume Loss in Multiple Sclerosis? A 4-Year Observational Study. Nutrients 2025, 17, 2271. https://doi.org/10.3390/nu17142271
Galus W, Winder M, Owczarek AJ, Walawska-Hrycek A, Rzepka M, Kaczmarczyk A, Siuda J, Krzystanek E. Does Vitamin D Supplementation Slow Brain Volume Loss in Multiple Sclerosis? A 4-Year Observational Study. Nutrients. 2025; 17(14):2271. https://doi.org/10.3390/nu17142271
Chicago/Turabian StyleGalus, Weronika, Mateusz Winder, Aleksander J. Owczarek, Anna Walawska-Hrycek, Michalina Rzepka, Aleksandra Kaczmarczyk, Joanna Siuda, and Ewa Krzystanek. 2025. "Does Vitamin D Supplementation Slow Brain Volume Loss in Multiple Sclerosis? A 4-Year Observational Study" Nutrients 17, no. 14: 2271. https://doi.org/10.3390/nu17142271
APA StyleGalus, W., Winder, M., Owczarek, A. J., Walawska-Hrycek, A., Rzepka, M., Kaczmarczyk, A., Siuda, J., & Krzystanek, E. (2025). Does Vitamin D Supplementation Slow Brain Volume Loss in Multiple Sclerosis? A 4-Year Observational Study. Nutrients, 17(14), 2271. https://doi.org/10.3390/nu17142271








