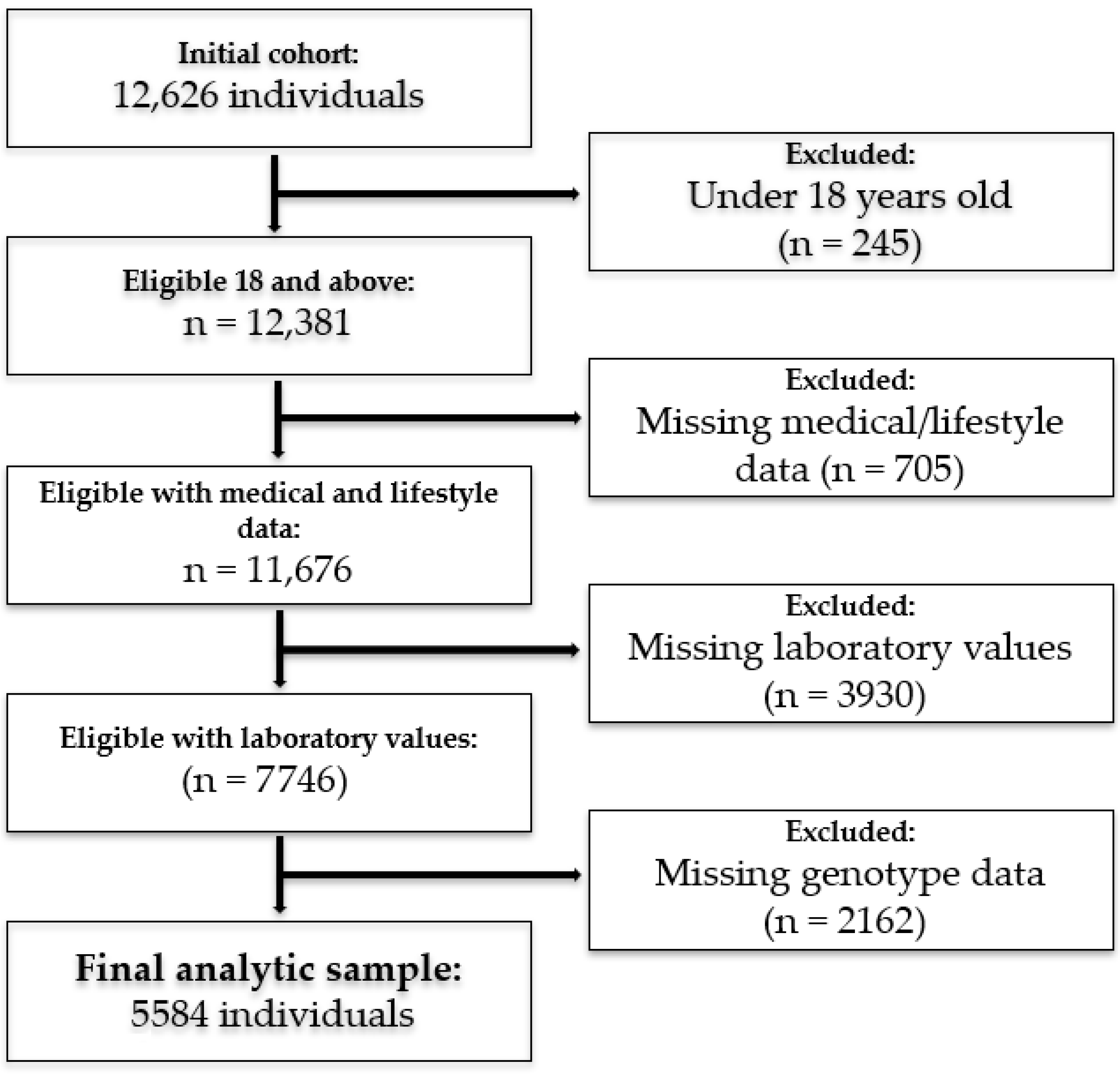
Polygenic Risk, Modifiable Lifestyle Behaviors, and Metabolic Factors: Associations with HDL-C, Triglyceride Levels, and Cardiovascular Risk
Highlights
- High wPRSs were significantly associated with unfavorable lipid profiles (higher TG and lower HDL-C levels) and increased TG/HDL-C ratios.
- Individuals with high wPRSHDL had significantly greater odds of developing CVD.
- Individuals with both high wPRSHDL and wPRSTG exhibited a significantly increased risk of CVD.
- Obesity (BMI > 30) and HbA1c ≥ 5.7% were significantly linked to elevated TG and reduced HDL-C levels, particularly in those with high wPRSHDL and WPRSTG.
- Moderate wine (1–3 drinks/week) consumption and coffee intake (≥1 cup/day) mitigated these effects, particularly among individuals with high wPRS.
- Risk stratification predicated on genetics, lifestyle and metabolic profiles may inform personalized prevention strategies for dyslipidemia.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. SNPs Selection and PRS Construction
2.3. Demographic, Metabolic and Lifestyle Variables
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Association Between wPRS and Lipid Profiles
3.3. Association Effect Between Demographic, Metabolic, and Lifestyle Factors and Lipid Trait Predisposition on Lipid Levels (mg/dL)
3.4. wPRS Association with CVD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK343489/ (accessed on 1 December 2024).
- Ruiz-García, A.; Arranz-Martínez, E.; López-Uriarte, B.; Rivera-Teijido, M.; Palacios-Martínez, D.; Dávila-Blázquez, G.M.; Rosillo-González, A.; Delgado, J.A.G.-P.; Mariño-Suárez, J.E.; Revilla-Pascual, E.; et al. Prevalence of hypertriglyceridemia in adults and related cardiometabolic factors. SIMETAP-HTG study. Clin. Investig. Arterioscler. 2020, 32, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Marston, N.A.; Giugliano, R.P.; Im, K.; Silverman, M.G.; O’Donoghue, M.L.; Wiviott, S.D.; Ference, B.A.; Sabatine, M.S. Association Between Triglyceride Lowering and Reduction of Cardiovascular Risk Across Multiple Lipid-Lowering Therapeutic Classes. Circulation 2019, 140, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport: Basic Mechanisms and their Roles in Vascular Health and Disease. Circ. Res. 2019, 124, 1505. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2023, 44, 1394–1407. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio as a Risk Marker for Metabolic Syndrome and Cardiovascular Disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Optimal Cutoff of the TG/HDL-c Ratio for Cardiovascular Risk in Hypertensive and Diabetic Patients Monitored by Primary Health Care in a City in Minas Gerais. Available online: https://www.scielo.br/j/ijcs/a/3xx76DnVjjnDsJTNdpvzKXC/ (accessed on 9 January 2025).
- Nie, G.; Hou, S.; Zhang, M.; Peng, W. High TG/HDL ratio suggests a higher risk of metabolic syndrome among an elderly Chinese population: A cross-sectional study. BMJ Open 2021, 11, e041519. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Daimon, T. Comparison of discrimination for cardio-metabolic risk by different cut-off values of the ratio of triglycerides to HDL cholesterol. Lipids Health Dis. 2019, 18, 156. [Google Scholar] [CrossRef]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Weiss, L.A.; Pan, L.; Abney, M.; Ober, C. The sex-specific genetic architecture of quantitative traits in humans. Nat. Genet. 2006, 38, 218–222. [Google Scholar] [CrossRef]
- Ordovas, J.M. Genetic influences on blood lipids and cardiovascular disease risk: Tools for primary prevention. Am. J. Clin. Nutr. 2009, 89, 1509S–1517S. [Google Scholar] [CrossRef] [PubMed]
- Dowaidar, M. Gene-environment interactions that influence CVD, lipid traits, obesity, diabetes, and hypertension appear to be able to influence gene therapy. Mol. Asp. Med. 2023, 94, 101213. [Google Scholar] [CrossRef] [PubMed]
- Torkamani, A.; Wineinger, N.E.; Topol, E.J. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 2018, 19, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Vassos, E. Polygenic risk scores: From research tools to clinical instruments. Genome Med. 2020, 12, 44. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.; Lehman, T.C.; Ledsky, R.; Blanck, H.M. Occasions, locations, and reasons for consuming sugar-sweetened beverages among U.S. adults. Nutrients 2023, 15, 920. [Google Scholar] [CrossRef]
- Israel Ministry of Health. Mabat National Health and Nutrition Survey: Food Frequency Questionnaire (FFQ). Available online: https://www.gov.il/BlobFolder/guide/mabat-survey/he/units_malbam_ffq.pdf (accessed on 24 June 2025).
- Liao, W.-Z.; Zhou, Z.-Y.; Wu, X.-N.; Zhu, X.-F.; Li, S.-A.; Zheng, J.-H.; Tan, J.-N.; Chen, H.-K.; Gu, T.-Y.; Xu, Z.; et al. Coffee and caffeine consumption and risk of periodontitis: National Health and Nutrition Examination Survey 2009–2014. Med. Adv. 2024, 1, e5. [Google Scholar] [CrossRef]
- ACC/AHA Task Force on Clinical Practice Guidelines. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation 2019. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000678 (accessed on 26 August 2024).
- Bilen, O.; Virani, S.; Nambi, V. Chapter 42—Hyperlipidemia. In Cardiology Secrets, 5th ed.; Levine, G.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 377–386. [Google Scholar] [CrossRef]
- Lee, W.J.; Cheng, H.; Whitney, B.M.; Nance, R.M.; Britton, S.R.; Jordahl, K.; Lindstrom, S.; Ruderman, S.A.; Kitahata, M.M.; Saag, M.S.; et al. Polygenic risk scores point toward potential genetic mechanisms of type 2 myocardial infarction in people with HIV. Int. J. Cardiol. 2023, 384, 15–23. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef]
- Wu, H.; Forgetta, V.; Zhou, S.; Bhatnagar, S.R.; Paré, G.; Richards, J.B. Polygenic Risk Score for Low-Density Lipoprotein Cholesterol Is Associated with Risk of Ischemic Heart Disease and Enriches for Individuals with Familial Hypercholesterolemia. Circ. Genom. Precis. Med. 2021, 14, e003106. [Google Scholar] [CrossRef]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knock, D.R.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Products—Data Briefs—Number 290—October 2017. Available online: https://www.cdc.gov/nchs/products/databriefs/db290.htm (accessed on 4 September 2024).
- Chen, J.X.; Li, Y.; Zhang, Y.B.; Wang, Y.; Zhou, Y.F.; Geng, T.; Liu, G.; Pan, A.; Liao, Y.-F. Nonlinear relationship between high-density lipoprotein cholesterol and cardiovascular disease: An observational and Mendelian randomization analysis. Metabolism 2024, 154, 155817. [Google Scholar] [CrossRef] [PubMed]
- Kohli, A.; Siddhu, A.; Pandey, R.M.; Reddy, K.S. Relevance of the triglyceride-to-high-density lipoprotein cholesterol ratio as an important lipid fraction in apparently healthy, young, and middle-aged Indian men. Indian J. Endocrinol. Metab. 2017, 21, 113–118. [Google Scholar] [CrossRef] [PubMed]
- da Luz, P.L.; Favarato, D.; Junior, J.R.F.N.; Lemos, P.; Chagas, A.C.P. High Ratio of Triglycerides to HDL-Cholesterol Predicts Extensive Coronary Disease. Clinics 2008, 63, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.H.; Zhong, Y.Y.; Wen, Z.G.; Kuang, C.Q.; Liao, J.R.; Chen, L.H.; Wang, P.S.; Wu, Y.X.; Ouyang, C.; Chen, Z. Triglyceride to HDL-C ratio and increased arterial stiffness in apparently healthy individuals. Int. J. Clin. Exp. Med. 2015, 8, 4342–4348. [Google Scholar]
- Sultani, R.; Tong, D.C.; Peverelle, M.; Lee, Y.S.; Baradi, A.; Wilson, A.M. Elevated Triglycerides to High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio Predicts Long-Term Mortality in High-Risk Patients. Heart Lung Circ. 2020, 29, 414–421. [Google Scholar] [CrossRef]
- Lelis, D.F.; Calzavara, J.V.S.; Santos, R.D.; Sposito, A.C.; Griep, R.H.; Barreto, S.M.; Molina, M.; Schmidt, M.I.; Duncan, B.B.; Bensenor, I.; et al. Reference values for the triglyceride to high-density lipoprotein ratio and its association with cardiometabolic diseases in a mixed adult population: The ELSA-Brasil study. J. Clin. Lipidol. 2021, 15, 699–711. [Google Scholar] [CrossRef]
- Borrayo, G.; Basurto, L.; González-Escudero, E.; Diaz, A.; Vázquez, A.; Sánchez, L.; Hernández-González, G.O.; Barrera, S.; Degollado, J.A.; Córdova, N.; et al. TG/HDL-C Ratio as Cardio-Metabolic Biomarker Even in Normal Weight Women. Acta Endocrinol. 2018, 14, 261–267. [Google Scholar] [CrossRef]
- Nosrati, M.; Safari, M.; Alizadeh, A.; Ahmadi, M.; Mahrooz, A. The Atherogenic Index Log (Triglyceride/HDL-Cholesterol) as a Biomarker to Identify Type 2 Diabetes Patients with Poor Glycemic Control. Int. J. Prev. Med. 2021, 12, 160. [Google Scholar] [CrossRef]
- Link, J.C.; Reue, K. The Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu. Rev. Nutr. 2017, 37, 225–245. [Google Scholar] [CrossRef]
- Jia, Z.F.; Cao, X.Y.; Cao, D.H.; Kong, F.; Kharbuja, P.; Jiang, J. Polymorphisms of PTPN11 gene could influence serum lipid levels in a sex-specific pattern. Lipids Health Dis. 2013, 12, 72. [Google Scholar] [CrossRef]
- Gutierrez, D.A.; Puglisi, M.J.; Hasty, A.H. Impact of Increased Adipose Tissue Mass on Inflammation, Insulin Resistance, and Dyslipidemia. Curr. Diab. Rep. 2009, 9, 26–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimomura, I.; Bashmakov, Y.; Ikemoto, S.; Horton, J.D.; Brown, M.S.; Goldstein, J.L. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA 1999, 96, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- The Complex Metabolic Mechanisms Relating Obesity to Hypertriglyceridemia. Available online: https://www.ahajournals.org/doi/10.1161/ATVBAHA.111.233049 (accessed on 25 June 2024).
- Klop, B.; do Rego, A.T.; Cabezas, M.C. Alcohol and plasma triglycerides. Curr. Opin. Lipidol. 2013, 24, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Pankow, J.S.; Arnett, D.K.; Eckfeldt, J.H.; Myers, R.H.; Ellison, R.C. Apolipoprotein E polymorphism modifies the alcohol-HDL association observed in the National Heart, Lung, and Blood Institute Family Heart Study. Am. J. Clin. Nutr. 2004, 80, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Bochud, M.; Paccaud, F.; Waterworth, D.; Bergmann, S.; Preisig, M.; Waeber, M.; Vollenweider, P. No interaction between alcohol consumption and HDL-related genes on HDL cholesterol levels. Atherosclerosis 2010, 211, 551–557. [Google Scholar] [CrossRef]
- Andrade, A.C.; Cesena, F.H.; Consolim-Colombo, F.M.; Coimbra, S.R.; Benjó, A.M.; Krieger, E.M.; Lemos da Luz, P. Short-Term Red Wine Consumption Promotes Differential Effects on Plasma Levels of High-Density Lipoprotein Cholesterol, Sympathetic Activity, and Endothelial Function in Hypercholesterolemic, Hypertensive, and Healthy Subjects. Clinics 2009, 64, 435–442. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Zhou, A.; Hyppönen, E. Habitual coffee intake and plasma lipid profile: Evidence from UK Biobank. Clin. Nutr. 2021, 40, 4404–4413. [Google Scholar] [CrossRef]
- Chang, H.C.; Nfor, O.N.; Ho, C.C.; Chen, P.H.; Kung, Y.Y.; Hsu, S.Y.; Tantoh, D.M.; Liaw, Y.-C.; Hsieh, C.-F.; Liaw, Y.-P. Changes in High-Density Lipoprotein Cholesterol Levels in Relation to Coffee Consumption Among Taiwanese Adults. J. Multidiscip. Healthc. 2020, 13, 1427–1432. [Google Scholar] [CrossRef]
- Hsu, T.-W.; Tantoh, D.M.; Lee, K.-J.; Ndi, O.N.; Lin, L.-Y.; Chou, M.-C.; Liaw, Y.-P. Genetic and Non-Genetic Factor-Adjusted Association between Coffee Drinking and High-Density Lipoprotein Cholesterol in Taiwanese Adults: Stratification by Sex. Nutrients 2019, 11, 1102. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Zha, W.; Hong, X.; Luo, Q. Effect of coffee consumption on dyslipidemia: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Urgert, R.; Essed, N.; van der Weg, G.; Kosmeijer-Schuil, T.G.; Katan, M.B. Separate effects of the coffee diterpenes cafestol and kahweol on serum lipids and liver aminotransferases. Am. J. Clin. Nutr. 1997, 65, 519–524. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, S.; Peng, Z. Effects of cigarette smoking on HDL quantity and function: Implications for atherosclerosis. J. Cell Biochem. 2013, 114, 2431–2436. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.G.; Klarin, D.; Assimes, T.L.; Freiberg, M.S.; Ingelsson, E.; Lynch, J.; Natarajan, P.; O’Donnell, C.; Rader, D.J.; Tsao, P.S.; et al. Genetics of Smoking and Risk of Atherosclerotic Cardiovascular Diseases: A Mendelian Randomization Study. JAMA Netw. Open 2021, 4, e2034461. [Google Scholar] [CrossRef]
- Sonestedt, E.; Hellstrand, S.; Schulz, C.A.; Wallström, P.; Drake, I.; Ericson, U.; Gullberg, B.; Hedblad, B.; Orho-Melander, M. The Association between Carbohydrate-Rich Foods and Risk of Cardiovascular Disease Is Not Modified by Genetic Susceptibility to Dyslipidemia as Determined by 80 Validated Variants. PLoS ONE 2015, 10, e0126104. [Google Scholar] [CrossRef]
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | All Population | wPRSTG < 90 | wPRSTG ≥ 90 | p-Value | ||||||
| Sex (female) n, (%) | 3861 (69.1) | 3461 (68.9) | 400 (71.7) | 0.18 | ||||||
| Age (years) (mean ± SD) | 58.0 ± 14.2 | 58.0 ± 14.2 | 58.1 ± 14.0 | 0.83 | ||||||
| BMI (kg/m2) (mean ± SD) | 31.3 ± 5.8 | 31.3 ± 5.8 | 30.9 ± 5.1 | 0.21 | ||||||
| HbA1C (%) (mean ± SD) | 5.1 ± 2.7 | 5.1 ± 2.5 | 5.2 ± 3.6 | 0.76 | ||||||
| Total Cholesterol (mg/dL) | 183.4 ± 48.6 | 182.5 ± 48.1 | 191.9 ± 51.9 | <0.0001 | ||||||
| HDL-C (mg/dL) | 52.9 ± 12.5 | 52.9 ± 12.5 | 52.5 ± 11.9 | 0.47 | ||||||
| LDL-C (mg/dL) | 109.2 ± 37.2 | 109.0 ± 36.5 | 110.9 ± 43.1 | 0.05 | ||||||
| TG (mg/dL) | 130.5 ± 65.8 | 127.1 ± 63.2 | 161.3 ± 79.4 | <0.0001 | ||||||
| T2DM n, (%) | 471 (8.4) | 420 (8.4) | 51 (9.1) | 0.58 | ||||||
| CVD n, (%) | 486 (8.7) | 437 (8.7) | 49 (8.8) | 0.94 | ||||||
| Smoking n, (%) | 507 (9.1) | 458 (9.1%) | 49 (8.8) | 0.85 | ||||||
| SSB (≥1 cup/day) n, (%) | 930 (16.7%) | 817 (16.3%) | 113 (20.3) | 0.01 | ||||||
| Coffee (≥1 cup/day) n, (%) | 4234 (75.8) | 3806 (75.7) | 428 (76.7) | 0.64 | ||||||
| Wine (1–3 drinks/week) n, (%) | 1721 (30.8) | 1555 (30.9) | 166 (29.7) | 0.47 | ||||||
| (b) | ||||||||||
| Variable | wPRSHDL < 90 | wPRSHDL ≥ 90 | p-Value | wPRSHDL < 90 | wPRSHDL ≥ 90 | p-Value | ||||
| Females | Males | |||||||||
| Age (years) (mean ± SD) | 57.2 ± 13.7 | 61.1 ± 14.1 | <0.0001 | 57.1 ± 15.0 | 64.3 ± 12.7 | <0.0001 | ||||
| BMI (kg/m2) (mean ± SD) | 30.9 ± 5.8 | 31.3 ± 6.0 | 0.09 | 31.8 ± 5.5 | 32.3 ± 5.6 | 0.12 | ||||
| HbA1C (%) (mean ± SD) | 5.1 ± 2.7 | 5.3 ± 3.3 | <0.001 | 5.1 ± 2.5 | 5.3 ± 2.1 | <0.001 | ||||
| Total Cholesterol (mg/dL) | 190.8 ± 46.9 | 179.0 ± 49.9 | <0.0001 | 174.6 ± 48.1 | 156.7 ± 47.0 | <0.0001 | ||||
| HDL-C (mg/dL) | 56.7 ± 12.0 | 53.8 ± 11.8 | <0.0001 | 45.9 ± 9.8 | 42.7 ± 9.3 | <0.0001 | ||||
| LDL-C (mg/dL) | 113.6 ± 35.7 | 104.3 ± 36.7 | <0.0001 | 106.3 ± 38.0 | 88.6 ± 39.4 | <0.0001 | ||||
| TG (mg/dL) | 124.5 ± 60.7 | 127.9 ± 60.5 | 0.08 | 142.0 ± 74.2 | 145.9 ± 75.9 | 0.39 | ||||
| T2DM n, (%) | 205 (6.2) | 59 (10.4) | <0.001 | 147 (10.8) | 60 (16.5) | <0.01 | ||||
| CVD n, (%) | 55 (1.7) | 206 (36.2) | <0.0001 | 18 (1.3) | 207 (56.9) | <0.0001 | ||||
| Smoking n, (%) | 299 (9.1) | 45 (7.9) | 0.40 | 137 (10.1) | 26 (7.1) | 0.10 | ||||
| SSB (≥1 cup/day) n, (%) | 481 (14.6) | 89 (15.6) | 0.56 | 290 (21.3) | 70 (19.2) | 0.42 | ||||
| Coffee (≥1 cup/day) n, (%) | 2494 (75.8) | 420 (73.8) | 0.32 | 1028 (75.6) | 292 (80.2) | 0.07 | ||||
| Wine (1–3 drinks/week) n, (%) | 909 (27.6) | 141 (24.8) | 0.31 | 522 (38.4) | 149 (40.9) | 0.68 | ||||
| wPRS Category * | β | OR | 95%CI | p-Value |
|---|---|---|---|---|
| wPRSHDL males | 0.572 | 1.77 | 1.39–2.26 | <0.0001 |
| wPRSHDL females | 0.419 | 1.52 | 1.26–1.84 | <0.0001 |
| wPRSTG | 0.946 | 2.58 | 2.15–3.09 | <0.0001 |
| Combined wPRSHDL and wPRSTG | 0.692 | 2.00 | 1.26–3.17 | 0.003 |
| Variable | TG Levels (Mean ± SD) in High * wPRSTG | TG Levels (Mean ± SD) in Low * wPRSTG | p-Value (a) |
|---|---|---|---|
| Sex | |||
| Male | 172.46 ± 80.00 | 139.82 ± 73.32 | p < 0.0001 |
| Female | 156.77 ± 78.87 | 121.28 ± 57.10 | p < 0.0001 |
| p-value (b) within wPRSTG | 0.008 | p < 0.0001 | |
| Wine | |||
| Non-drinkers | 166.43 ± 81.23 | 129.70 ± 64.35 | p < 0.0001 |
| 1–3 drinks/week | 148.86 ± 74.37 | 121.56 ± 60.13 | p < 0.0001 |
| p-value (b) within wPRSTG | 0.02 | p < 0.0001 | |
| Coffee | |||
| Non-consumers | 166.62 ± 78.42 | 127.56 ± 63.75 | p < 0.0001 |
| 1–3 cups/day | 161.75 ± 77.70 | 127.92 ± 63.96 | p < 0.0001 |
| p-value (b) within wPRSTG | 0.41 | 0.94 | |
| Obesity | |||
| BMI < 30 | 140.85 ± 68.94 | 114.82 ± 58.64 | p < 0.0001 |
| BMI ≥ 30 | 178.98 ± 83.72 | 136.96 ± 64.97 | p < 0.0001 |
| p-value (b) within wPRSTG | p < 0.0001 | p < 0.0001 | |
| Smoking | |||
| Non-smoker | 159.23 ± 75.03 | 126.14 ± 62.41 | p < 0.0001 |
| Smoker | 181.76 ± 114.73 | 136.19 ± 69.82 | p < 0.01 |
| p-value (b) within wPRSTG | 0.55 | 0.002 | |
| SSB | |||
| None | 160.83 ± 82.55 | 125.29 ± 61.50 | p < 0.0001 |
| ≥1 cup/day | 162.72 ± 66.05 | 136.14 ± 70.56 | p < 0.0001 |
| p-value (b) within wPRSTG | 0.33 | 0.01 | |
| HbA1c | |||
| <5.7% | 149.98 ± 74.05 | 118.12 ± 57.41 | p < 0.0001 |
| ≥5.7% | 182.80 ± 84.99 | 143.42 ± 69.67 | p < 0.0001 |
| p-value (b) within wPRSTG | p < 0.0001 | p < 0.0001 |
| Variable | HDL-C Levels (Mean ± SD) in High * wPRSHDL | HDL-C Levels (Mean ± SD) in Low * wPRSHDL | p-Value (a) |
|---|---|---|---|
| Wine | |||
| Non-drinkers | 52.86 ± 11.98 | 56.18 ± 11.73 | p < 0.0001 |
| 1–3 drinks/week | 56.40 ± 10.46 | 57.59 ± 12.32 | 0.61 |
| p-value (b) within wPRSHDL | <0.001 | 0.01 | |
| Coffee | |||
| Non-consumers | 51.52 ± 12.29 | 55.98 ± 11.66 | p < 0.0001 |
| 1–3 cups/day | 54.38 ± 11.56 | 56.92 ± 12.18 | 0.001 |
| p-value (b) within wPRSHDL | 0.003 | 0.23 | |
| Obesity | |||
| BMI < 30 | 57.28 ± 11.90 | 59.14 ± 12.36 | 0.06 |
| BMI ≥ 30 | 51.12 ± 11.07 | 54.56 ± 11.26 | p < 0.0001 |
| p-value (b) within wPRSHDL | p < 0.0001 | p < 0.0001 | |
| Smoking | |||
| Non-smoker | 53.93 ± 11.82 | 57.03 ± 11.96 | p < 0.0001 |
| Smoker | 52.24 ± 11.97 | 53.82 ± 12.15 | 0.44 |
| p-value (b) within wPRSHDL | 0.33 | p < 0.0001 | |
| SSB | |||
| None | 53.87 ± 11.83 | 57.18 ± 12.17 | p < 0.0001 |
| ≥1 cup/day | 53.39 ± 11.87 | 54.12 ± 10.71 | 0.54 |
| p-value (b) within wPRSHDL | 0.77 | p < 0.0001 | |
| HbA1c | |||
| <5.7% | 53.86 ± 11.73 | 57.62 ± 12.11 | p < 0.0001 |
| ≥5.7% | 53.68 ± 12.00 | 54.92 ± 11.60 | 0.20 |
| p-value (b) within wPRSHDL | 0.92 | p < 0.0001 |
| Variable | HDL-C Levels (Mean ± SD) in High * wPRSHDL | HDL-C Levels (Mean ± SD) in Low * wPRSHDL | p-Value (a) |
|---|---|---|---|
| Wine | |||
| Non-drinkers | 43.60 ± 8.82 | 44.50 ± 9.42 | p < 0.0001 |
| 1–3 drinks/week | 44.96 ± 9.67 | 46.14 ± 9.91 | 0.006 |
| p-value (b) within wPRSHDL | 0.03 | 0.007 | |
| Coffee | |||
| Non-consumers | 45.62 ± 11.75 | 45.61 ± 9.93 | 0.02 |
| 1–3 cups/day | 43.67 ± 7.98 | 45.29 ± 10.02 | 0.0001 |
| p-value (b) within wPRSHDL | 0.98 | 0.58 | |
| Obesity | |||
| BMI < 30 | 45.83 ± 10.55 | 47.82 ± 10.82 | <0.001 |
| BMI ≥ 30 | 42.89 ± 7.55 | 43.65 ± 8.69 | p < 0.0001 |
| p-value (b) within wPRSHDL | 0.002 | p < 0.0001 | |
| Smoking | |||
| Non-smoker | 44.36 ± 8.89 | 45.57 ± 9.85 | p < 0.0001 |
| Smoker | 42.61 ± 10.72 | 42.97 ± 9.21 | 0.004 |
| p-value (b) within wPRSHDL | 0.01 | <0.01 | |
| SSB | |||
| None | 44.63 ± 9.04 | 45.69 ± 10.06 | p < 0.0001 |
| ≥1 cup/day | 42.98 ± 9.15 | 43.86 ± 8.65 | 0.03 |
| p-value (b) within wPRSHDL | 0.43 | 0.002 | |
| HbA1c | |||
| <5.7% | 44.79 ± 9.85 | 45.89 ± 9.58 | p < 0.0001 |
| ≥5.7% | 43.18 ± 7.64 | 44.43 ± 10.13 | 0.00076 |
| p-value (b) within wPRSHDL | 0.30 | 0.008 |
| wPRS Category * | CVD n, (%) | β | OR | 95%CI | p-Value |
|---|---|---|---|---|---|
| wPRSHDL males | 207 (56.9) | 4.581 | 96.64 | 54.13–172.61 | p < 0.0001 |
| wPRSHDL females | 206 (36.2) | 3.453 | 31.58 | 22.83–43.70 | p < 0.0001 |
| High wPRSTG | 49 (8.8) | 0.023 | 1.023 | 0.74–1.4 | 0.89 |
| Combined high wPRSHDL and wPRSTG | 40 (42.6) | 2.343 | 10.41 | 6.51–16.66 | p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chermon, D.; Birk, R. Polygenic Risk, Modifiable Lifestyle Behaviors, and Metabolic Factors: Associations with HDL-C, Triglyceride Levels, and Cardiovascular Risk. Nutrients 2025, 17, 2244. https://doi.org/10.3390/nu17132244
Chermon D, Birk R. Polygenic Risk, Modifiable Lifestyle Behaviors, and Metabolic Factors: Associations with HDL-C, Triglyceride Levels, and Cardiovascular Risk. Nutrients. 2025; 17(13):2244. https://doi.org/10.3390/nu17132244
Chicago/Turabian StyleChermon, Danyel, and Ruth Birk. 2025. "Polygenic Risk, Modifiable Lifestyle Behaviors, and Metabolic Factors: Associations with HDL-C, Triglyceride Levels, and Cardiovascular Risk" Nutrients 17, no. 13: 2244. https://doi.org/10.3390/nu17132244
APA StyleChermon, D., & Birk, R. (2025). Polygenic Risk, Modifiable Lifestyle Behaviors, and Metabolic Factors: Associations with HDL-C, Triglyceride Levels, and Cardiovascular Risk. Nutrients, 17(13), 2244. https://doi.org/10.3390/nu17132244






