The Biological Value of Proteins for Pediatric Growth and Development: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Update on the Biological Value of Proteins
3.1. Understanding Biological Value in Proteins
3.2. Protein Requirements for Human Growth and Development
3.3. Protein Sources
3.4. Growth and Development with Regard to Protein Consumption
3.5. Alternative Proteins
4. Discussion
5. Future Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiong, T.; Wu, Y.; Hu, J.; Xu, S.; Li, Y.; Kong, B.; Zhang, Z.; Chen, L.; Tang, Y.; Yao, P.; et al. Associations between High Protein Intake, Linear Growth, and Stunting in Children and Adolescents: A Cross-Sectional Study. Nutrients 2023, 15, 4821. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Ferdousi, A.; Biswas, S.; Begum, A.; Datta, M.; Shil, S.; Reza, F.H.; Talukdar, M. The Essential Role of Right Amount and Quality of Protein for Ensuring Child Growth and Maintenance of Bone and Muscle Mass. Am. J. Pediatr. 2025, 11, 14–25. [Google Scholar] [CrossRef]
- Tahergorabi, R.; Hosseini, S.V. Chapter 2—Proteins, Peptides, and Amino Acids. In Nutraceutical and Functional Food Components; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 15–38. [Google Scholar]
- La Pelusa, A.; Kaushik, R. Physiology, Proteins. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555990/ (accessed on 7 March 2025).
- O’Brien, E.C.; Tsoi, K.Y.; Ma, R.C.W.; Hanson, M.A.; Hod, M.; McAuliffe, F.M. Nutrition Through the Life Cycle: Pregnancy. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 49–74. [Google Scholar]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Schop, M.; de Boer, I.J.M.; Huppertz, T. Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef]
- Reeds, P.J. Dispensable and indispensable amino acids for humans. J. Nutr. 2000, 130, 1835S–1840S. [Google Scholar] [CrossRef]
- Richter, M.; Baerlocher, K.; Bauer, J.M.; Elmadfa, I.; Heseker, H.; Leschik-Bonnet, E.; Stangl, G.; Volkert, D.; Stehle, P.; on behalf of the German Nutrition Society (DGE). Revised Reference Values for the Intake of Protein. Ann. Nutr. Metab. 2019, 74, 242–250. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Escobedo Monge, M.F.; Barrado, E.; Alonso Vicente, C.; Redondo del Río, M.P.; Manuel Marugán de Miguelsanz, J. Zinc Nutritional Status in Patients with Cystic Fibrosis. Nutrients 2019, 11, 150. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Alonso Vicente, C.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M.; Redondo del Río, M.P. Copper and Copper/Zinc Ratio in a Series of Cystic Fibrosis Patients. Nutrients 2020, 12, 3344. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Marcos-Temprano, M.; Marugán-Miguelsanz, J.M. Magnesium Status and Calcium/Magnesium Ratios in a Series of Cystic Fibrosis Patients. Nutrients 2022, 14, 1793. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Marcos-Temprano, M.; Parodi-Román, J.; Escobedo-Monge, M.A.; Alonso-Vicente, C.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Calcium, Phosphorus, and Vitamin D Levels in a Series of Cystic Fibrosis Patients: A Cross-Sectional Study. Int. J. Mol. Sci. 2024, 25, 1900. [Google Scholar] [CrossRef] [PubMed]
- Tome, D.; Xipsiti, M.; Shertukde, S.P.; Calvez, J.; Vasilopoulou, D.; Wijesinha-Bettoni, R.; Owino, V.O. Context and Perspectives for Establishing a Novel Database for Protein Quality of Human Foods, as Proposed by a Joint Food and Agriculture Organization of the United Nations/International Atomic Energy Agency Expert Technical Meeting in October 2022. J. Nutr. 2024, 154, 294–299. [Google Scholar] [CrossRef]
- Xipsiti, M. Protein quality evaluation: FAO perspective. Front. Nutr. 2024, 11, 1446879. [Google Scholar] [CrossRef]
- Lamb, M.W.; Harden, M.L. 7—Protein as a Source of Amino Acids. In The Meaning of Human Nutrition; Lamb, M.W., Harden, M.L., Eds.; Pergamon Bio-Medical Sciences Series; Elsevier: Amsterdam, The Netherlands, 1973; pp. 153–191. [Google Scholar]
- Dupont, C.; Bocquet, A.; Tomé, D.; Bernard, M.; Campeotto, F.; Dumond, P.; Essex, A.; Frelut, M.L.; Guénard-Bilbault, L.; Lack, G.; et al. Hydrolyzed Rice Protein-Based Formulas, a Vegetal Alternative in Cow’s Milk Allergy. Nutrients 2020, 12, 2654. [Google Scholar] [CrossRef]
- Forester, S.M.; Jennings-Dobbs, E.M.; Sathar, S.A.; Layman, D.K. Perspective: Developing a Nutrient-Based Framework for Protein Quality. J. Nutr. 2023, 153, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; Food and Nutrition Paper 92; Report of an FAO Expert Consultation; Food and Agriculture Organization of the United Nations: Roma, Italy, 2013; 66p. [Google Scholar]
- Shivakumar, N.; Jackson, A.A.; Courtney-Martin, G.; Elango, R.; Ghosh, S.; Hodgkinson, S.; Xipsiti, M.; Lee, W.T.K.; Kurpad, A.V.; Tomé, D. Protein Quality Assessment of Follow-up Formula for Young Children and Ready-to-Use Therapeutic Foods: Recommendations by the FAO Expert Working Group in 2017. J. Nutr. 2020, 150, 195–201. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant-versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- McNamara, D.J. Eggs. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 132–138. [Google Scholar]
- Hoffman, J.R.; Falvo, M.J. Protein—Which is Best? J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Mariotti, F. 35—Plant Protein, Animal Protein, and Protein Quality. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Mariotti, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 621–642. [Google Scholar]
- Faber, T.A.; Bechtel, P.J.; Hernot, D.C.; Parsons, C.M.; Swanson, K.S.; Smiley, S.; Fahey, G.C., Jr. Protein digestibility evaluations of meat and fish substrates using laboratory, avian, and ileally cannulated dog assays. J. Anim. Sci. 2010, 88, 1421–1432. [Google Scholar] [CrossRef]
- WHO; FAO; UNU Protein and Amino Acid Requirements in Human Nutrition. World Health Organ Technical Report Series 935. 2007. Available online: https://iris.who.int/bitstream/handle/10665/43411/WHO_TRS_935_eng.pdf (accessed on 16 June 2025).
- Senarathna, S.; Mel, R.; Malalgoda, M. Utilization of cereal-based protein ingredients in food applications. J. Cereal Sci. 2024, 116, 103867. [Google Scholar] [CrossRef]
- Grant, G.; Duncan, M.; Alonso, R.; Marzo, F. Peas and Lentils. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 4433–4440. [Google Scholar]
- Podgórska-Kryszczuk, I. Spirulina—An Invaluable Source of Macro and Micronutrients with Broad Biological Activity and Application Potential. Molecules 2024, 29, 5387. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Shaghaghian, S.; McClements, D.J.; Khalesi, M.; Garcia-Vaquero, M.; Mirzapour-Kouhdasht, A. Digestibility and bioavailability of plant-based proteins intended for use in meat analogues: A review. Trends Food Sci. Technol. 2022, 129, 646–656. [Google Scholar] [CrossRef]
- Boyle, F.; Lynch, G.; Reynolds, C.M.; Green, A.; Parr, G.; Howard, C.; Knerr, I.; Rice, J. Determination of the Protein and Amino Acid Content of Fruit, Vegetables and Starchy Roots for Use in Inherited Metabolic Disorders. Nutrients 2024, 16, 2812. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- WHO The Optimal Duration of Exclusive Breastfeeding: Report of An Expert Consultation. In World Health Organization (Issue March). 2001. Available online: https://apps.who.int/iris/bitstream/10665/67219/1/WHO_NHD_01.09.pdf (accessed on 23 June 2025).
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Parikh, P.; Semba, R.; Manary, M.; Swaminathan, S.; Udomkesmalee, E.; Bos, R.; Poh, B.K.; Rojroongwasinkul, N.; Geurts, J.; Sekartini, R.; et al. Animal source foods, rich in essential amino acids, are important for linear growth and development of young children in low- and middle- income countries. Matern. Child. Nutr. 2022, 18, e13264. [Google Scholar] [CrossRef]
- Eaton-Evans, J. Nutritional Assessment: Anthropometry. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 227–232. [Google Scholar]
- World Health Organization. Child Growth Standards. 2025. Available online: https://www.who.int/news-room/questions-and-answers/item/child-growth-standards (accessed on 16 June 2025).
- Verduci, E.; Köglmeier, J. Immunomodulation in Children: The Role of the Diet. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 293–298. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clinical medicine insights. Pediatrics 2016, 10, 67–83. [Google Scholar]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M. The Triple Burden of Malnutrition in the Era of Globalization. Nestle Nutr. Inst. Workshop Ser. 2023, 97, 51–61. [Google Scholar]
- UNICEF for Every Child. New Insights: 21st Century Malnutrition. Unpacking the Triple Burden for Children Nutritional Wellbeing. Available online: https://www.unicef.org/innocenti/stories/new-insights-21st-century-malnutrition (accessed on 10 April 2025).
- Escobedo-Monge, M.F.; Ayala-Macedo, G.; Sakihara, G.; Peralta, S.; Almaraz-Gómez, A.; Barrado, E.; Marugán-Miguelsanz, J.M. Effects of Zinc Supplementation on Nutritional Status in Children with Chronic Kidney Disease: A Randomized Trial. Nutrients 2019, 11, 2671. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Torres-Hinojal, M.C.; Barrado, E.; Escobedo-Monge, M.A.; Marugán-Miguelsanz, J.M. Zinc Nutritional Status in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients 2021, 13, 1121. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients 2021, 13, 3578. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Magnesium Status and Ca/Mg Ratios in a Series of Children and Adolescents with Chronic Diseases. Nutrients 2022, 14, 2941. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Bahillo-Curieses, P.; Parodi-Román, J.; Escobedo-Monge, M.A.; Alonso-López, P.; Marugán-Miguelsanz, J.M. Calcium, Phosphate, and Vitamin D in Children and Adolescents with Chronic Diseases: A Cross-Sectional Study. Nutrients 2024, 16, 1349. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper/Zinc Ratio in Childhood and Adolescence: A Review. Metabolites 2023, 13, 82. [Google Scholar] [CrossRef]
- Escher, N.A.; Carrillo-Larco, R.M.; Parnham, J.C.; Curi-Quinto, K.; Ghosh-Jerath, S.; Millett, C.; Seferidi, P. Longitudinal transitions of the double burden of overweight and stunting from childhood to early adulthood in India, Peru, and Vietnam. Int. J. Epidemiol. 2024, 53, dyae151. [Google Scholar] [CrossRef]
- Mutasa, K.; Ntozini, R.; Mbuya, M.N.N.; Rukobo, S.; Govha, M.; Majo, F.D.; Tavengwa, N.; Smith, L.E.; Caulfield, L.; Swann, J.R.; et al. Biomarkers of environmental enteric dysfunction are not consistently associated with linear growth velocity in rural Zimbabwean infants. Am. J. Clin. Nutr. 2021, 113, 1185–1198. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023. Urbanization, Agrifood Systems Transformation and Healthy Diets Across the Rural–Urban Continuum; FAO: Rome, Italy, 2023. [CrossRef]
- Lenters, L.; Wazny, K.; Bhutta, Z.A. Management of Severe and Moderate Acute Malnutrition in Children. In Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, 3rd ed.; Black, R.E., Laxminarayan, R., Temmerman, M., Walker, N., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2016; Volume 2, Chapter 11. [Google Scholar] [CrossRef]
- Kurpad, A.V. The requirements of protein & amino acid during acute & chronic infections. Indian. J. Med. Res. 2006, 124, 129–148. [Google Scholar]
- Energy and protein requirements. Proceedings of an IDECG workshop, London, United Kingdom, 31 October–4 November 1994. Eur. J. Clin. Nutr. 1996, 50 (Suppl. S1), S1–S197.
- Mak, R.H.; Iyengar, A.; Lai, W.M.; McAlister, L.; Oliveira, E.A.; Xu, H.; Yap, H.K.; Shroff, R. Nutrition in Children With Chronic Kidney Disease: How to Thrive? J. Ren. Nutr. 2023, 33, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Kumar, V.; Sangwan, P.; Pant, N.C.; Saxena, A.; Joshi, S.; Yadav, A.N. Personalized Nutrition and -Omics. Compr. Foodomics 2021, 12, 495–507. [Google Scholar]
- Liu, K.; Sharma, P.; Bartle, J.; Gilbertson, H.; Cole, T.; McCarthy, M. Protein intake and requirements in children and adolescents undergoing Hematopoietic Stem Cell Transplant (HSCT): An international benchmarking survey and a scoping review. Clin. Nutr. ESPEN 2024, 63, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Rahman, F.; Bora, B.; Shameeh, M. Chapter 1—Importance and nutritive value of animal proteins in human diet. In Processing Technologies and Food Protein Digestion; Bhat, Z.F., Morton, J.D., Bekhit, A.A., Suleria, H.A.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–25. [Google Scholar]
- Font-i-Furnols, M. Meat Consumption, Sustainability and Alternatives: An Overview of Motives and Barriers. Foods 2023, 12, 2144. [Google Scholar] [CrossRef] [PubMed]
- Chouraqui, J.P. Vegetarian diets and diets which restrict animal-source foods during childhood in high-income countries. Paediatr. Int. Child. Health 2023, 43, 57–82. [Google Scholar] [CrossRef] [PubMed]
- Der Heijden, I.V.; Monteyne, A.J.; Stephens, F.B.; Wall, B.V.T. Alternative dietary protein sources to support healthy and active skeletal muscle aging. Nutr. Rev. 2023, 81, 206–230. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Children and Adoles-cents Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Re-view. Nutrients 2023, 15, 4341. [Google Scholar] [CrossRef]
- Reinehr, T.; Schnabel, D.; Wabitsch, M.; Bechtold-Dalla Pozza, S.; Bührer, C.; Heidtmann, B.; Jochum, F.; Kauth, T.; Körner, A.; Mihatsch, W.; et al. Vitamin D supplementation after the second year of life: Joint position of the Committee on Nutrition, German Society for Pediatric and Adolescent Medicine (DGKJ e.V.), and the German Society for Pediatric Endocrinology and Diabetology (DGKED e.V.). Mol. Cell Pediatr. 2019, 6, 3. [Google Scholar] [CrossRef]
- Alexy, U.; Fischer, M.; Weder, S.; Längler, A.; Michalsen, A.; Sputtek, A.; Keller, M. Nutrient Intake and Status of German Children and Adolescents Consuming Vegetarian, Vegan or Omnivore Diets: Results of the VeChi Youth Study. Nutrients 2021, 13, 1707. [Google Scholar] [CrossRef]
- Kiely, M.E. Risks and benefits of vegan and vegetarian diets in children. Proc. Nutr. Soc. 2021, 80, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Leroy, F. Children and adults should avoid consuming animal products to reduce the risk for chronic disease: Debate Consensus. Am. J. Clin. Nutr. 2020, 112, 937–940. [Google Scholar] [CrossRef]
- Soh, B.X.P.; Smith, N.W.; von Hurst, P.R.; McNabb, W.C. Evaluation of Protein Adequacy From Plant-Based Dietary Scenarios in Simulation Studies: A Narrative Review. J. Nutr. 2024, 154, 300–313. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture and U.S. Department of Health and Human Services: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf (accessed on 11 June 2024).
- Connolly, G.; Hudson, J.L.; Bergia, R.E.; Davis, E.M.; Hartman, A.S.; Zhu, W.; Carroll, C.C.; Campbell, W. Effects of Consuming Ounce-Equivalent Portions of Animal- vs. Plant-Based Protein Foods, as Defined by the Dietary Guidelines for Americans on Essential Amino Acids Bioavailability in Young and Older Adults: Two Cross-Over Randomized Controlled Trials. Nutrients 2023, 15, 2870. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yu, H.J.; He, Q.Q.; Heitmann, B.L.; Rangan, A.; McNaughton, S.A.; Campbell, K.J. Protein Intake During Infancy and Subsequent Body Mass Index in Early Childhood: Results from the Melbourne InFANT Program. J. Acad. Nutr. Diet. 2021, 121, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.; Campbell, K.J.; Yu, H.J.; Szymlek-Gay, E.A.; Abbott, G.; He, Q.Q.; Zheng, M. Protein Intake from Birth to 2 Years and Obesity Outcomes in Later Childhood and Adolescence: A Systematic Review of Prospective Cohort Studies. Adv. Nutr. 2021, 12, 1863–1876. [Google Scholar] [CrossRef]
- Arnesen, E.K.; Thorisdottir, B.; Lamberg-Allardt, C.; Bärebring, L.; Nwaru, B.; Dierkes, J.; Ramel, A.; Åkesson, A. Protein intake in children and growth and risk of overweight or obesity: A systematic review and meta-analysis. Food Nutr. Res. 2022, 66, 8284. [Google Scholar] [CrossRef]
- Ferré, N.; Luque, V.; Closa-Monasterolo, R.; Zaragoza-Jordana, M.; Gispert-Llauradó, M.; Grote, V.; Koletzko, B.; Escribano, J. Association of Protein Intake during the Second Year of Life with Weight Gain-Related Outcomes in Childhood: A Systematic Review. Nutrients 2021, 13, 583. [Google Scholar] [CrossRef]
- Alimujiang, A.; Colditz, G.A.; Gardner, J.D.; Park, Y.; Berkey, C.S.; Sutcliffe, S. Childhood diet and growth in boys in relation to timing of puberty and adult height: The Longitudinal Studies of Child Health and Development. Cancer Causes Control 2018, 29, 915–926. [Google Scholar] [CrossRef]
- Asare, H.; Rosi, A.; Faber, M.; Smuts, C.M.; Ricci, C. Animal-source foods as a suitable complementary food for improved physical growth in 6 to 24-month-old children in low- and middle-income countries: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2022, 128, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Desmond, M.A.; Fewtrell, M.S.; Wells, J.C.K. Plant-Based Diets in Children: Secular Trends, Health Outcomes, and a Roadmap for Urgent Practice Recommendations and Research-A Systematic Review. Nutrients 2024, 16, 723. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiong, J.; Wang, X.; He, F.; Cheng, G. Dietary protein sources, gut microbiome, and puberty timing in children: Findings from a cohort study. Sig Transduct. Target. Ther. 2024, 9, 167. [Google Scholar] [CrossRef]
- El Sharkawy, M.; Felix, J.F.; Grote, V.; Voortman, T.; Jaddoe, V.W.V.; Koletzko, B.; Küpers, L.K. Animal and plant protein intake during infancy and childhood DNA methylation: A meta-analysis in the NutriPROGRAM consortium. Epigenetics 2024, 19, 2299045. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gu, Y.; Liu, H.; Wang, L.; Li, W.; Li, W.; Leng, J.; Zhang, S.; Qi, L.; Yang, X.; et al. Daily branched-chain amino acid intake and risks of obesity and insulin resistance in children: A cross-sectional study. Obesity 2020, 28, 1310–1316. [Google Scholar] [CrossRef]
- Nuru, M.; Muradashvili, N.; Kalani, A.; Lominadze, D.; Tyagi, N. High methionine, low folate and low vitamin B6/B12 (HM-LF-LV) diet causes neurodegeneration and subsequent short-term memory loss. Metab. Brain Dis. 2018, 33, 1923–1934. [Google Scholar] [CrossRef]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef]
- Research and Innovation. Available online: https://research-and-innovation.ec.europa.eu/research-area/environment/bioeconomy_en (accessed on 23 March 2025).
- Food and Agriculture Organization of the United Nations. Sustainable Bioeconomy for Agrifood Systems Transformation. Alternative Proteins Top the Bill for the Latest FAO–International Sustainable Bioeconomy Working Group Webinar. Available online: https://www.fao.org/in-action/sustainable-and-circular-bioeconomy/resources/news/details/en/c/1507553/ (accessed on 7 March 2025).
- Food and Agriculture Organization of the United Nations. Sustainable Bioeconomy for Agrifood Systems Transformation. The Need for Guidance on Alternative Proteins Highlighted to Codex Alimentarius Commission. Available online: https://www.fao.org/in-action/sustainable-and-circular-bioeconomy/resources/news/details/en/c/1459357/ (accessed on 7 March 2025).
- Aimutis, W.R.; Shirwaiker, R. A perspective on the environmental impact of plant-based protein concentrates and isolates. Proc. Natl. Acad. Sci. USA 2024, 121, e2319003121. [Google Scholar] [CrossRef]
- WHO. Plant-Based Diets and Their Impact on Health, Sustainability and the Environment: A Review of the Evidence; WHO European Office for the Prevention and Control of Noncommunicable Diseases: Copenhagen, Denmark, 2021.
- Desmond, M.A.; Sobiecki, J.G.; Jaworski, M.; Płudowski, P.; Antoniewicz, J.; Shirley, M.K.; Eaton, S.; Książyk, J.; Cortina-Borja, M.; De Stavola, B.; et al. Growth, body composition, and cardiovascular and nutritional risk of 5- to 10-y-old children consuming vegetarian, vegan, or omnivore diets. Am. J. Clin. Nutr. 2021, 113, 1565–1577. [Google Scholar] [CrossRef]
- Moughan, P.J. Population protein intakes and food sustainability indices: The metrics matter. Glob. Food Secur. 2021, 29, 100548. [Google Scholar] [CrossRef]
- World Register of Marine Species. Emerita Analog. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=467695 (accessed on 15 May 2025).
- FAO; WHO. Food Safety Aspects of Cell-Based Food; Food and Agriculture Organization of the United Nations and World Health Organization: Rome, Italy, 2023.
- Tso, R.; Forde, C.G. Unintended Consequences: Nutritional Impact and Potential Pitfalls of Switching from Animal- to Plant-Based Foods. Nutrients 2021, 13, 2527. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Hohoff, E.; Zahn, H.; Weder, S.; Fischer, M.; Längler, A.; Michalsen, A.; Keller, M.; Alexy, U. Food Costs of Children and Adolescents Consuming Vegetarian, Vegan or Omnivore Diets: Results of the Cross-Sectional VeChi Youth Study. Nutrients 2022, 14, 4010. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Kim, H.K.; Hirooka, R.; Tanaka, M.; Shimoda, T.; Chijiki, H.; Kojima, S.; Sasaki, K.; Takahashi, K.; Makino, S.; et al. Distribution of dietary protein intake in daily meals influences skeletal muscle hypertrophy via the muscle clock. Cell Rep. 2021, 36, 109336. [Google Scholar] [CrossRef] [PubMed]
- Pimpin, L.; Kranz, S.; Liu, E.; Shulkin, M.; Karageorgou, D.; Miller, V.; Fawzi, W.; Duggan, C.; Webb, P.; Mozaffarian, D. Effects of animal protein supplementation of mothers, preterm infants, and term infants on growth outcomes in childhood: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2019, 110, 410–429. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Hudson, J.L.; Baum, J.I.; Diaz, E.C.; Børsheim, E. Dietary Protein Requirements in Children: Methods for Consideration. Nutrients 2021, 13, 1554. [Google Scholar] [CrossRef]
- Xiao, X.; Zou, P.R.; Hu, F.; Zhu, W.; Wei, Z.J. Updates on Plant-Based Protein Products as an Alternative to Animal Protein: Technology, Properties, and Their Health Benefits. Molecules 2023, 28, 4016. [Google Scholar] [CrossRef]
- FAO; IAEA. Development of a Protein Database and the Way Forward for Reviewing Protein Requirements—Report of a Joint FAO-IAEA Technical Meeting; FAO: Rome, Italy; IAEA: Rome, Italy, 2024.
- FAO. Sustainable Bioeconomy for Agrifood Systems Transformation. Launch of Online Survey Towards the Development of a Multistakeholder Global Partnership on Bioeconomy for Sustainable Food and Agriculture. Available online: https://www.fao.org/in-action/sustainable-and-circular-bioeconomy/slides/detail/en/c/1734580/ (accessed on 23 June 2025).
- WHO; FAO. The joint FAO/WHO Expert Meetings on Nutrition (JEMNU): Nitrogen to Protein Conversion Factors for Soy-Based and Milk-Based Ingredients Used in Infant Formula and Follow-Up Formula; Report of the Meeting of the Expert Panel; WHO: Geneva, Switzerland, 2020.
- FAO. Protein Quality Assessment in Follow-Up Formula for Young Children; Food and Agriculture Organizaton of the United Nations: Rome, Italy, 2024. [Google Scholar]
- Linseisen, J.; Renner, B.; Gedrich, K.; Wirsam, J.; Holzapfel, C.; Lorkowski, S.; Watzl, B.; Daniel, H.; Leitzmann, M. Working Group “Personalized Nutrition” of the German Nutrition Society. Data in Personalized Nutrition: Bridging Biomedical, Psycho-behavioral, and Food Environment Approaches for Population-wide Impact. Adv. Nutr. 2025, 100377. [Google Scholar] [CrossRef]
- Pandita, D.; Pandita, A. Omics Technology for the Promotion of Nutraceuticals and Functional Foods. Front. Physiol. 2022, 13, 817247. [Google Scholar] [CrossRef]
- Carrera, M. Proteomics and Food Analysis: Principles, Techniques, and Applications. Foods 2021, 10, 2538. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino acid-dependent control of mTORC1 signaling: A variety of regulatory modes. J. Biomed. Sci. 2020, 27, 87. [Google Scholar] [CrossRef] [PubMed]
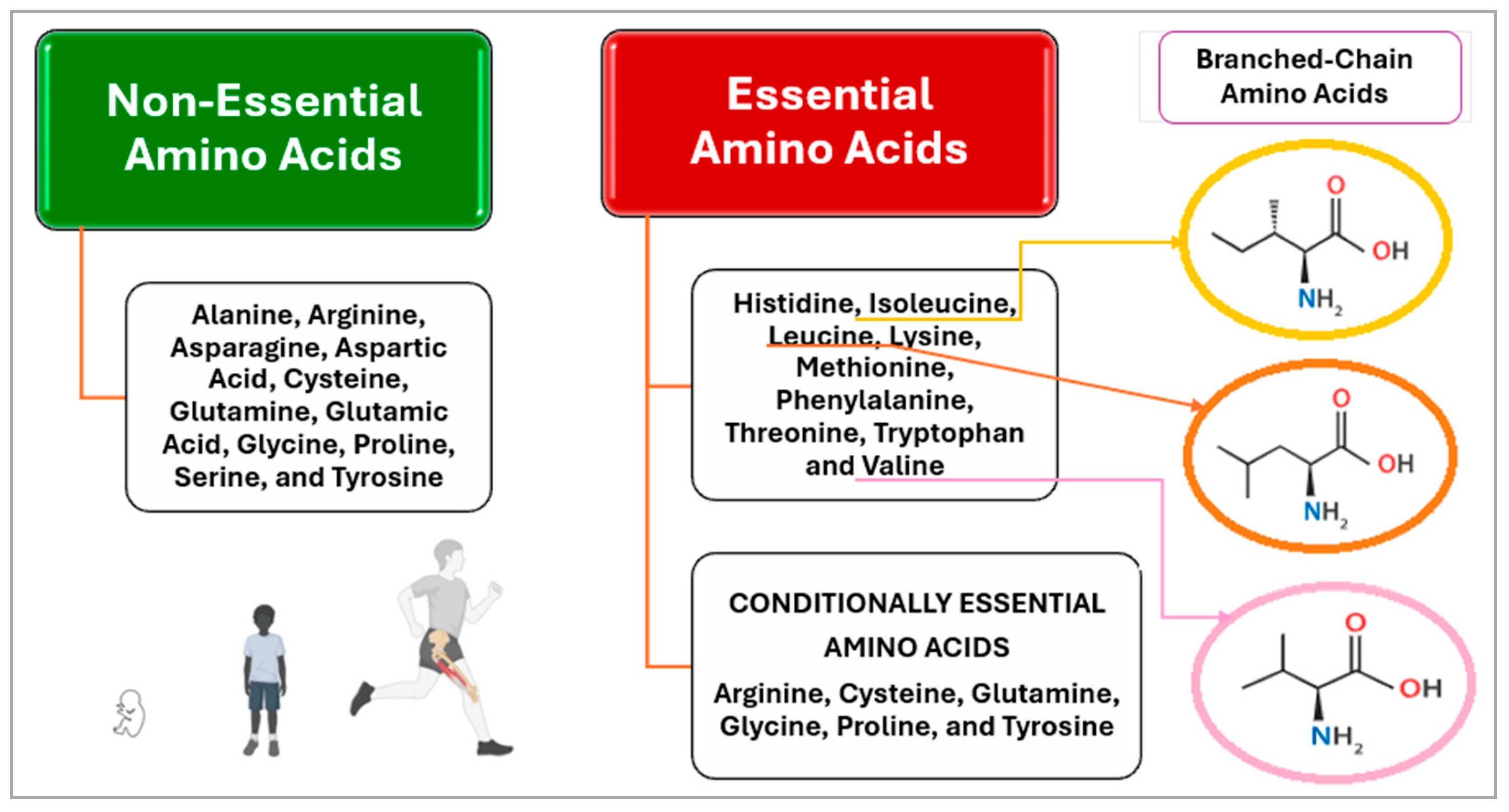
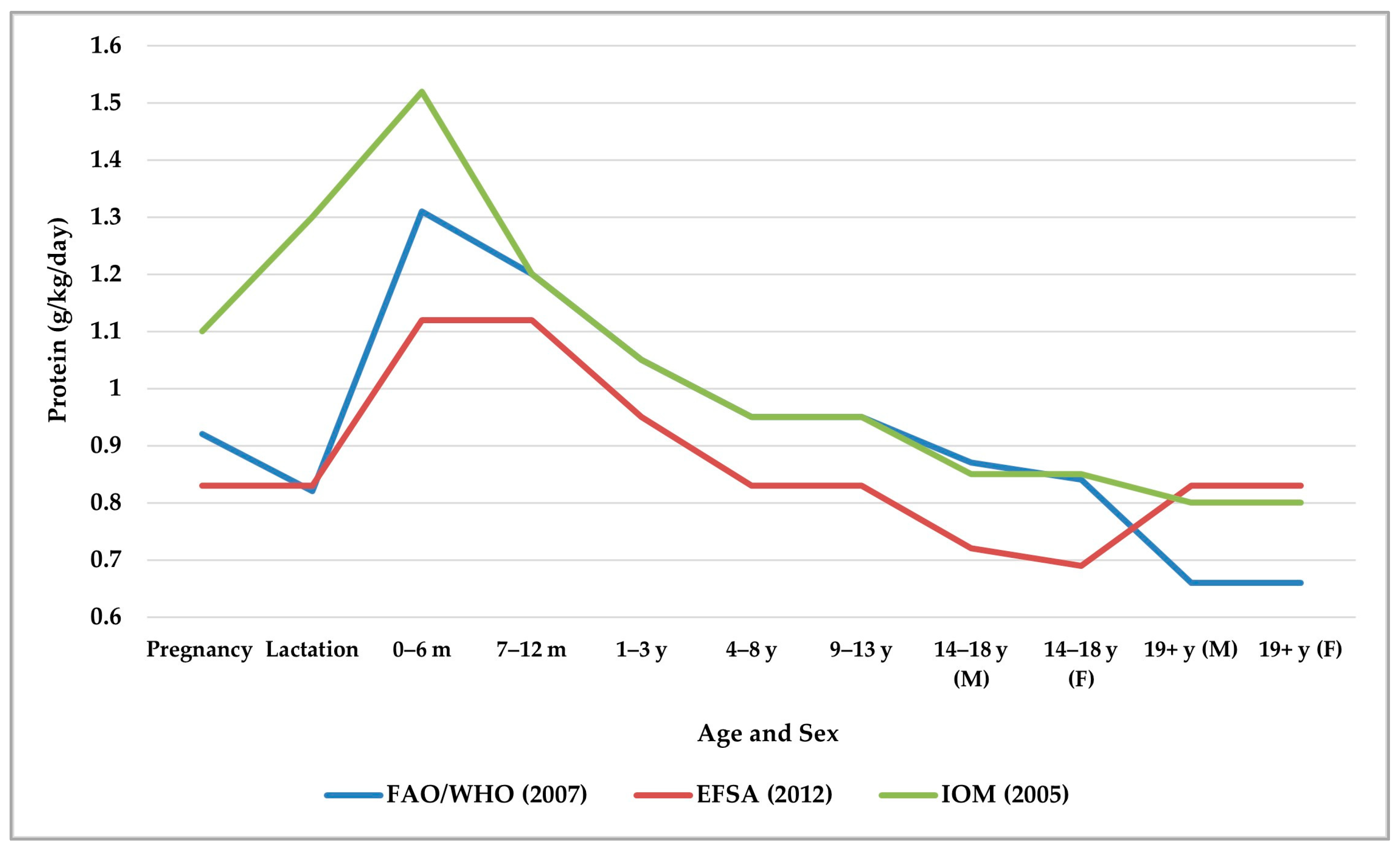
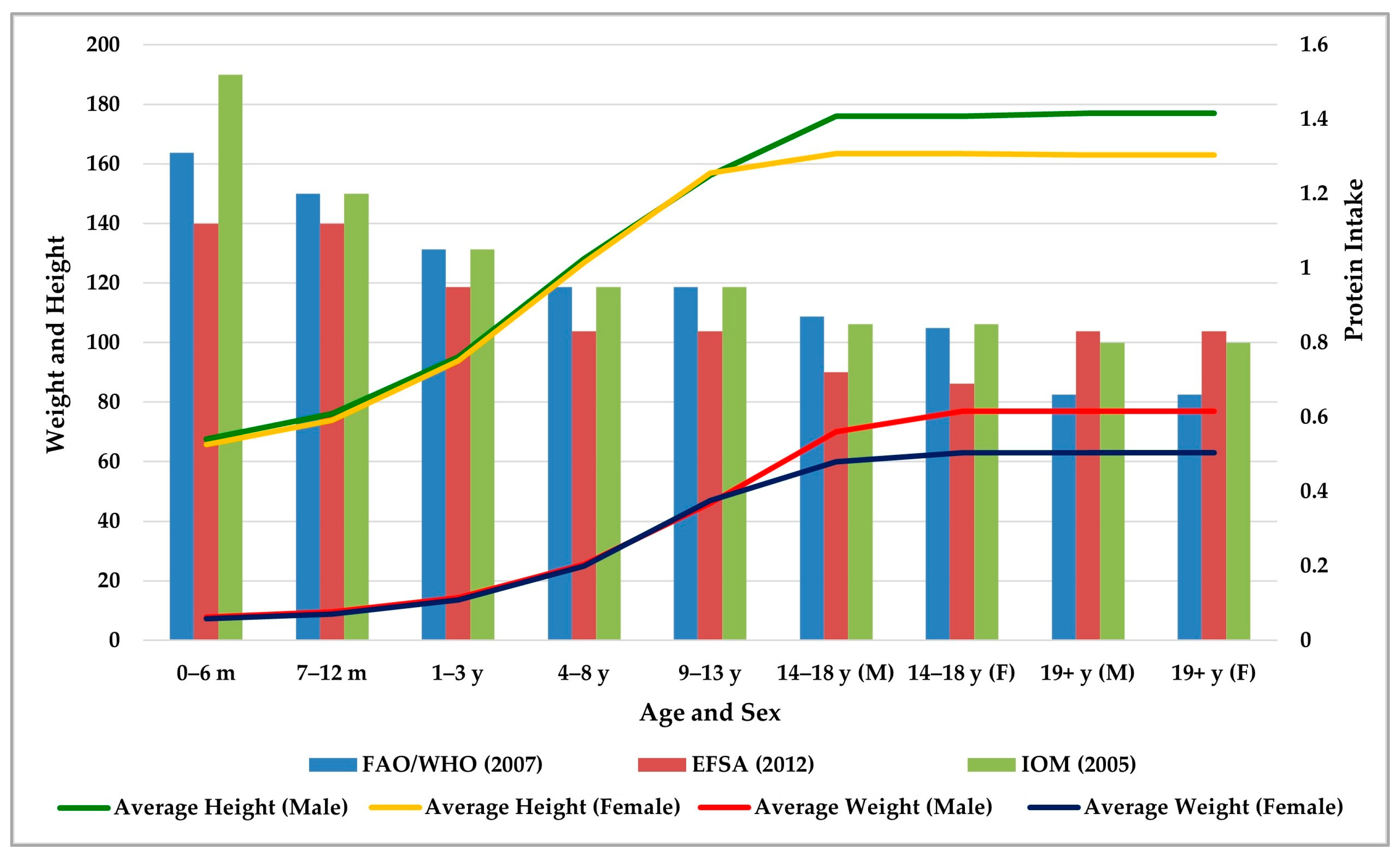
| Protein Source | Biological Value | Digestibility (%) | Net Protein Utilization | Amino Acid Content | Tips | Reference |
|---|---|---|---|---|---|---|
| Egg (whole) | 100 | ~98% | 94 | Complete; rich in leucine, lysine, methionine | Gold standard for protein quality | [22,23] |
| Whey protein | 104–110 | ~99% | 92 | High in BCAAs (leucine esp.); fast digesting | Rapid absorption, ideal for muscle synthesis | [24,25] |
| Casein (milk) | ~77 | ~97% | 76 | Complete; slow digestion profile | Good for sustained amino acid release | [24,25] |
| Milk (whole) | 91–93 | 100 | 82 | Complete, rich in lysine | High total protein content | [20,24] |
| Beef | 80 | ~94% | 78 | Complete; high in histidine and lysine | Also contains creatine and heme iron | [24,26] |
| Chicken | ~79 | ~95% | Complete; rich in lysine and leucine | Lean meat, low fat | [27] | |
| Fish (e.g., salmon) | ~83 | ~94% | High in lysine, methionine, taurine | Also provides omega-3 fatty acids | [3,27] | |
| Soy protein | ~74 | ~91% | 61 | Complete, but lower in methionine | Best plant-based complete protein | [24,28] |
| Pea protein | ~65 | ~89% | High in lysine, low in methionine | Often used in vegan supplements | [22] | |
| Rice protein | ~59 | ~88% | High in methionine, low in lysine | Often combined with pea for a complete profile | [29] | |
| Wheat protein (gluten) | ~54 | ~86% | 67 | Low in lysine and threonine | Incomplete on its own | [24,28] |
| Lentils | ~49 | ~85% | 27–33 | High in lysine, low in sulfur-containing AAs | High in fiber, best when combined with grains | [30] |
| Spirulina | ~50–60 | ~84% | Rich in glutamic acid, contains all essential AAs | Algae source, nutrient-dense | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo-Monge, M.F.; Parodi-Román, J.; Escobedo-Monge, M.A.; Marugán-Miguelsanz, J.M. The Biological Value of Proteins for Pediatric Growth and Development: A Narrative Review. Nutrients 2025, 17, 2221. https://doi.org/10.3390/nu17132221
Escobedo-Monge MF, Parodi-Román J, Escobedo-Monge MA, Marugán-Miguelsanz JM. The Biological Value of Proteins for Pediatric Growth and Development: A Narrative Review. Nutrients. 2025; 17(13):2221. https://doi.org/10.3390/nu17132221
Chicago/Turabian StyleEscobedo-Monge, Marlene Fabiola, Joaquín Parodi-Román, María Antonieta Escobedo-Monge, and José Manuel Marugán-Miguelsanz. 2025. "The Biological Value of Proteins for Pediatric Growth and Development: A Narrative Review" Nutrients 17, no. 13: 2221. https://doi.org/10.3390/nu17132221
APA StyleEscobedo-Monge, M. F., Parodi-Román, J., Escobedo-Monge, M. A., & Marugán-Miguelsanz, J. M. (2025). The Biological Value of Proteins for Pediatric Growth and Development: A Narrative Review. Nutrients, 17(13), 2221. https://doi.org/10.3390/nu17132221








