Effects of Obesogenic Diet Consumption During Pregnancy and Lactation on the Oxidative Balance and Hepatic Metabolism of Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Evaluation of Food Consumption
2.3. Measurement of Murinometric Parameters, Body Mass, and Hepatic and Adipose Tissue Mass
2.4. Serum Biochemical Profile
2.5. Triglyceride and Total Cholesterol Levels in the Liver
2.6. Oral Glucose Tolerance Test (OGTT)
2.7. Preparation of Liver for mRNA Expression
2.8. Liver Preparation for Biochemical Analysis
2.9. Determination of β-Hydroxyacyl-CoA Dehydrogenase (β-HAD) Activity
2.10. Evaluation of Fatty Acid Synthase Activity
2.11. Evaluation of Lipid Peroxidation
2.12. Evaluation of Protein Oxidation
2.13. Evaluation of Total Superoxide Dismutase (t-SOD) Activity
2.14. Evaluation of Catalase (CAT) Activity
2.15. Evaluation of Glutathione-s-Transferase (GST) Activity
2.16. Evaluation of REDOX State (GSH/GSSG Ratio)
2.17. Evaluation of Total Thiol (SH) Groups
2.18. Histology of Hepatocytes
2.19. Statistical Analysis
3. Results
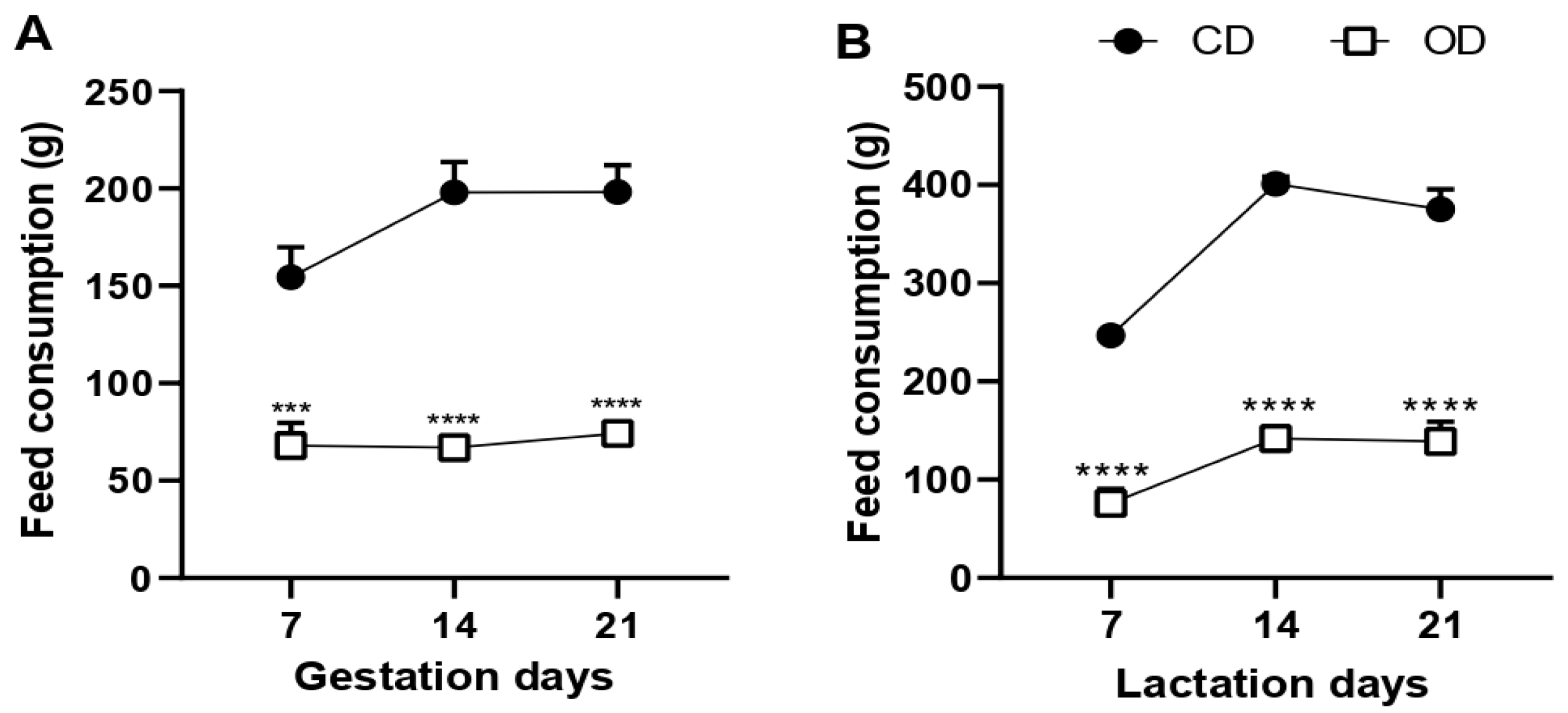
3.1. Food Consumption
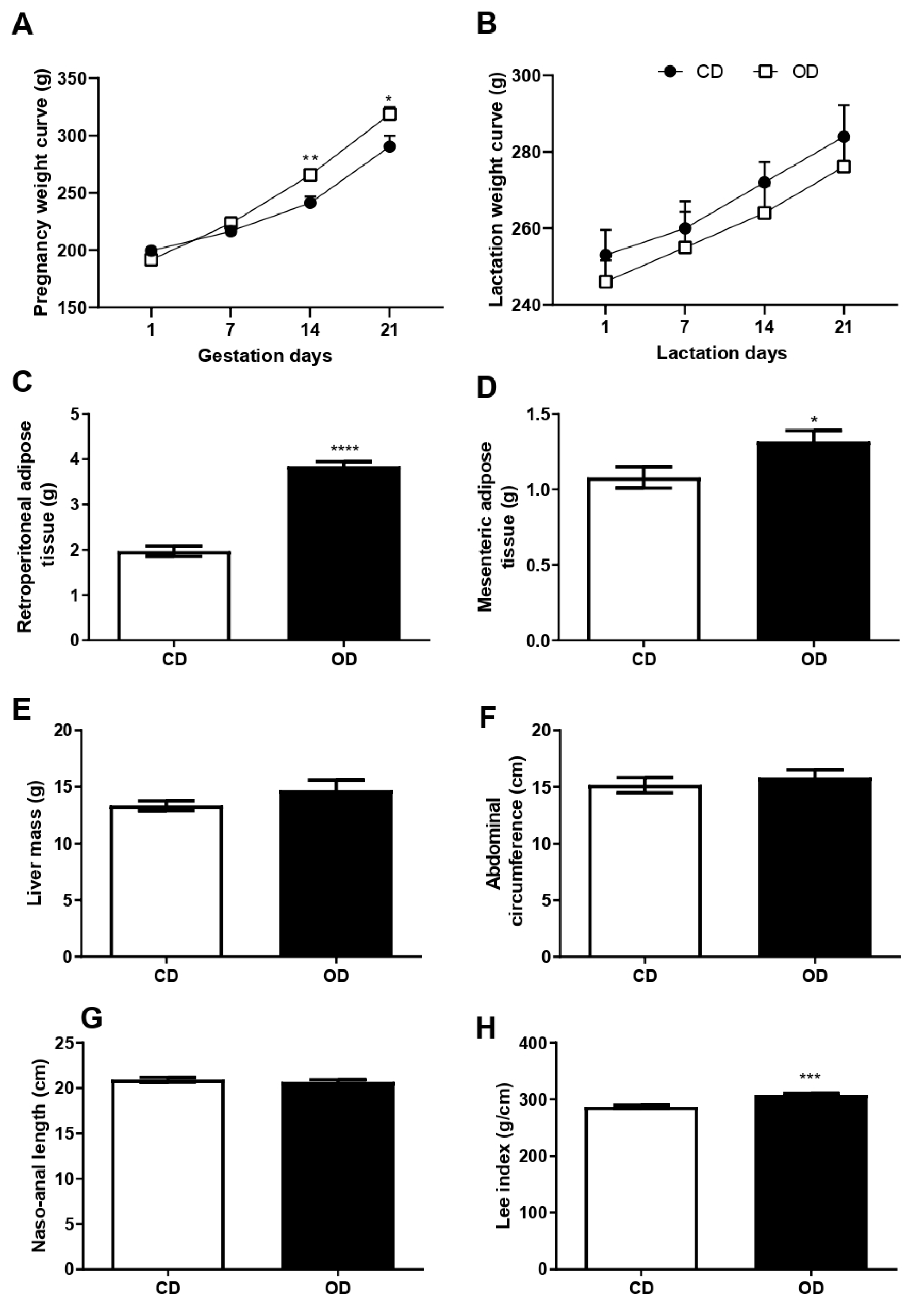
3.2. Body Mass Curve, Adipose and Hepatic Tissue Mass, and Murinometric Parameters
3.3. Serum and Liver Biochemical Profile
3.4. Oral Glucose Tolerance Test
3.5. Metabolic Enzymes
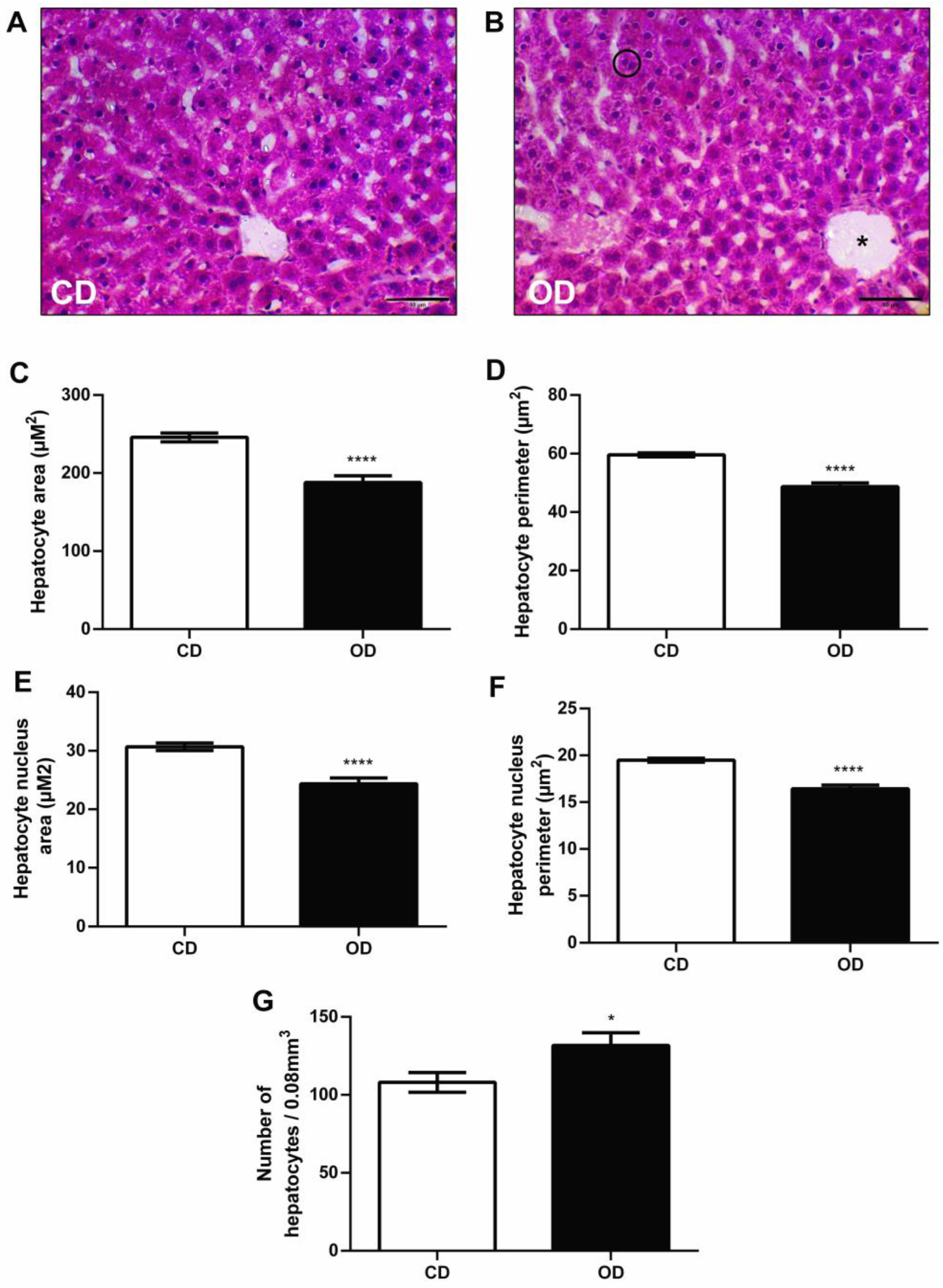
3.6. Histological and Morphometric Analysis of the Liver
3.7. mRNA Expression of Inflammatory Markers
3.8. Pro-Oxidant Markers and Enzymatic and Non-Enzymatic Antioxidant Defenses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Reichetzeder, C. Overweight and obesity in pregnancy: Their impact on epigenetics. Eur. J. Clin. Nutr. 2021, 75, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Snelgrove-Clarke, E.; Macdonald, D.; Helwig, M.; Alsius, A. Women’s experiences of living with obesity during pregnancy, birthing, and postpartum: A qualitative systematic review protocol. JBI Evid. Synth. 2021, 19, 3183–3189. [Google Scholar] [CrossRef]
- Almutairi, F.S.; Alsaykhan, A.M.; Almatrood, A.A. Obesity Prevalence and Its Impact on Maternal and Neonatal Outcomes in Pregnant Women: A Systematic Review. Cureus 2024, 16, e75262. [Google Scholar] [CrossRef] [PubMed]
- Foo, R.X.; Ma, J.J.; Du, R.; Goh, G.B.B.; Chong, Y.S.; Zhang, C.; Li, L.J. Gestational diabetes mellitus and development of intergenerational non-alcoholic fatty liver disease (NAFLD) after delivery: A systematic review and meta-analysis. Eclinicalmedicine 2024, 72, e102609. [Google Scholar] [CrossRef]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef]
- Hershman, M.; Mei, R.; Kushner, T. Implications of Nonalcoholic Fatty Liver Disease on Pregnancy and Maternal and Child Outcomes. Gastroenterol. Hepatol. 2019, 15, 221–228. [Google Scholar]
- Sferruzzi-Perri, A.N.; Lopez-Tello, J.; Napso, T.; Yong, H.E.J. Exploring the causes and consequences of maternal metabolic maladaptations during pregnancy: Lessons from animal models. Placenta 2020, 98, 43–51. [Google Scholar] [CrossRef]
- Leth-Møller, M.; Kampmann, U.; Hede, S.; Ovesen, P.G.; Hulman, A.; Knorr, S. Breastfeeding and infant growth in relation to childhood overweight—A longitudinal cohort study. Am. J. Clin. Nutr. 2025, 12, 835–842. [Google Scholar] [CrossRef]
- Álvarez-Zaragoza, C.; Vásquez-Garibay, E.M.; Sánchez-Ramírez, C.A. Adiposity and feeding practices in the first two years of life among toddlers in Guadalajara, Mexico. BMC Pediatr. 2023, 23, e61. [Google Scholar] [CrossRef]
- Musial, B.; Vaughan, O.R.; Fernandez-Twinn, D.S.; Voshol, P.; Ozanne, S.E.; Fowden, A.L.; Sferruzzi-Perri, A.N. A Western-style obesogenic diet alters maternal metabolic physiology with consequences for fetal nutrient acquisition in mice. J. Physiol. 2017, 595, 4875–4892. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Basak, S.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Dietary Fats and the Gut Microbiota: Their impacts on lipid-induced metabolic syndrome. J. Funct. Foods 2022, 91, e105026. [Google Scholar] [CrossRef]
- Gomes, R.M.; Bueno, F.G.; Schamber, C.R.; de Mello, J.C.P.; de Oliveira, J.C.; Francisco, F.A.; Moreira, V.M.; Junior, M.D.F.; Pedrino, G.R.; Mathias, P.C.d.F.; et al. Maternal diet-induced obesity during suckling period programs offspring obese phenotype and hypothalamic leptin/insulin resistance. J. Nutr. Biochem. 2018, 61, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mennitti, L.V.; Carpenter, A.A.M.; Loche, E.; Pantaleão, L.C.; Fernandez-Twinn, D.S.; Schoonejans, J.M.; Blackmore, H.L.; Ashmore, T.J.; Pisani, L.P.; Tadross, J.A.; et al. Effects of maternal diet-induced obesity on metabolic disorders and age-associated miRNA expression in the liver of male mouse offspring. Int. J. Obes. 2022, 46, 269–278. [Google Scholar] [CrossRef]
- Lean, S.C.; Candia, A.A.; Gulacsi, E.; Lee, G.C.L.; Sferruzzi-Perri, A.N. Obesogenic diet in mice compromises maternal metabolic physiology and lactation ability leading to reductions in neonatal viability. Acta Physiol. 2022, 236, 13861. [Google Scholar] [CrossRef]
- Pomar, C.A.; Castillo, P.; Palou, M.; Palou, A.; Picó, C. Implementation of a healthy diet to lactating rats attenuates the early detrimental programming effects in the offspring born to obese dams. Putative relationship with milk hormone levels. J. Nutr. Biochem. 2022, 107, 109043. [Google Scholar] [CrossRef]
- Carli, F.; Della Pepa, G.; Sabatini, S.; Vidal Puig, A.; Gastaldelli, A. Lipid metabolism in MASLD and MASH: From mechanism to the clinic. JHEP Rep. 2024, 6, 101185. [Google Scholar] [CrossRef]
- Szudzik, M.; Hutsch, T.; Chabowski, D.; Zajdel, M.; Ufnal, M. Normal caloric intake with high-fat diet induces metabolic dysfunction-associated steatotic liver disease and dyslipidemia without obesity in rats. Sci. Rep. 2024, 14, 22796. [Google Scholar] [CrossRef]
- Sucedaram, Y.; Johns, E.J.; Husain, R.; Abdul Sattar, M.; HAbdulla, M.; Nelli, G.; Rahim, N.S.; Khalilpourfarshbafi, M.; Abdullah, N.A. Exposure to High-Fat Style Diet Induced Renal and Liver Structural Changes, Lipid Accumulation and Inflammation in Intact and Ovariectomized Female Rats. J. Inflamm. Res. 2021, 14, 689–710. [Google Scholar] [CrossRef]
- Paulsen, G.; Hamarsland, H.; Cumming, K.T.; Johansen, R.E.; Hulmi, J.J.; Børsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014, 592, 5391–5408. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.A.; De Almeida, M.M.; Rocha, C.P.D.D.; de Brito Fassarella, L.; De Souza, L.L.; Souza, A.F.P.; Andrade, C.B.V.; Fortunato, R.S.; Pazos-Moura, C.C.; Trevenzoli, I.H. Maternal high-fat diet consumption induces sex-dependent alterations of the endocannabinoid system and redox homeostasis in liver of adult rat offspring. Sci. Rep. 2018, 8, 14751. [Google Scholar] [CrossRef]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Fisch, J.; de Moura, A.C.; Feistauer, V.; Reinhardt, L.S.; Molz, P.; Morás, A.M.; Moura, D.J.; de Souza, P.O.; Braganhol, E.; Almeida, S.; et al. Effects of different maternal diets on adipose tissue inflammation and liver tissue oxidative stress in dams and their female offspring. Mol. Cell Biochem. 2024, 479, 1257–1266. [Google Scholar] [CrossRef]
- George, G.; Draycott, S.A.V.; Muir, R.; Clifford, B.; Elmes, M.J.; Langley-Evans, S.C. The impact of exposure to cafeteria diet during pregnancy or lactation on offspring growth and adiposity before weaning. Sci. Rep. 2019, 9, 14173. [Google Scholar] [CrossRef]
- Dearden, L.; Buller, S.; Furigo, I.C.; Fernandez-Twinn, D.S.; Ozanne, S.E. Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways. Mol. Metab. 2020, 42, 101079. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef]
- Ferro Cavalcante, T.C.; Lima da Silva, J.M.; da Marcelino da Silva, A.A.; Muniz, G.S.; da Luz Neto, L.M.; Lopes de Souza, S.; Manhães de Castro, R.; Ferraz, K.M.; do Nascimento, E. Effects of a westernized diet on the reflexes and physical maturation of male rat offspring during the perinatal period. Lipids 2013, 48, 1157–1168. [Google Scholar] [CrossRef]
- Oliveira, W.A.; Silva, G.S.M.; Silva, R.N.; Santos, J.W.O.; Pachêco, L.S.; Correia, D.A.; Lemos, M.D.T.B.; Aguiar Júnior, F.C.A.; Oliveira, T.R.P.; Lagranha, C.J.; et al. Repercussions of the Maternal Obesogenic Diet on the Oxidative Balance and Pancreatic Metabolism in Male Juvenile Offspring. Nutrients 2025, 17, 578. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.; Selen Alpergin, E.S.; Zhao, L.; Hartung, T.; Scafidi, S.; Riddle, R.C.; Wolfgang, M.J. Loss of Hepatic Mitochondrial Long-Chain Fatty Acid Oxidation Confers Resistance to Diet-Induced Obesity and Glucose Intolerance. Cell Rep. 2017, 20, 655–667. [Google Scholar] [CrossRef]
- Negrin, K.A.; Roth Flach, R.J.; Distefano, M.T.; Matevossian, A.; Friedline, R.H.; Jung, D.; Kim, J.K.; Czech, M.P. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS ONE 2014, 9, 107265. [Google Scholar] [CrossRef]
- Kim, Y.J.; Bi, S. Knockdown of neuropeptide Y in the dorsomedial hypothalamus reverses high-fat diet-induced obesity and impaired glucose tolerance in rats. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.I.; Braz, G.R.F.; Silva, S.C.A.; Pedroza, A.A.D.S.; de Lima-Júnior, N.C.; Silva, T.L.A.; Lagranha, C.J. Body composition, biochemical, behavioral and molecular alterations in overfed rats after chronic exposure to SSRI. Behav. Brain Res. 2019, 356, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ito, M.; Jaswal, J.S.; Lam, V.H.; Oka, T.; Zhang, L.; Beker, D.L.; Lopaschuk, G.D.; Rebeyka, I.M. High levels of fatty acids increase contractile function of neonatal rabbit hearts during reperfusion following ischemia. Am. J. Physiol. Circ. Physiol. 2010, 298, 1426–1437. [Google Scholar] [CrossRef]
- Goodridge, A.G. Regulation of the activity of acetyl coenzyme A carboxylase by palmitoyl coenzyme A and citrate. J. Biol. Chem. 1972, 247, 6946–6952. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Simões-Alves, A.C.; Silva-Filho, R.C.; Braz, G.R.F.; Silva, S.C.A.; da Silva, A.I.; Lagranha, C.J.; Fernandes, M.P. Neonatal treatment with fluoxetine improves mitochondrial respiration and reduces oxidative stress in liver of adult rats. J. Cell. Biochem. 2018, 119, 6555–6565. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Alba-Loureiro, T.C.; Ribeiro, R.F.; Zorn, T.M.; Lagranha, C.J. Effects of glutamine supplementation on kidney of diabetic rat. Amino Acids 2010, 38, 1021–1030. [Google Scholar] [CrossRef]
- Ferreira, L.B.; Lobo, C.V.; Miranda, A.E.D.S.; Carvalho, B.D.C.; Santos, L.C.D. Dietary Patterns during Pregnancy and Gestational Weight Gain: A Systematic Review. Rev. Bras. Ginecol. Obs. 2022, 44, 540–547. [Google Scholar] [CrossRef]
- Akyol, A.; McMullen, S.; Langley-Evans, S.C. Glucose intolerance associated with early-life exposure to maternal cafeteria feeding is dependent upon post-weaning diet. Br. J. Nutr. 2012, 107, 964–978. [Google Scholar] [CrossRef]
- Desai, M.; Jellyman, J.K.; Han, G.; Beall, M.; Lane, R.H.; Ross, M.G. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 2014, 211, 237. [Google Scholar] [CrossRef]
- Lindqvist, C.; Holmer, M.; Hagström, H.; Petersson, S.; Tillander, V.; Brismar, T.B.; Stål, P. Macronutrient composition and its effect on body composition changes during weight loss therapy in patients with non-alcoholic fatty liver disease: Secondary analysis of a randomized controlled trial. Nutrition 2023, 110, 111982. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.M.; Estevam, W.M.; Coelho, P.M.; Haese, D.; Kobi, J.B.B.S.; Lima-Leopoldo, A.P.; Leopoldo, A.S. Differential Effects of High Sugar, High Lard or a Combination of Both on Nutritional, Hormonal and Cardiovascular Metabolic Profiles of Rodents. Nutrients 2018, 10, 1071. [Google Scholar] [CrossRef]
- Ladyman, S.R.; Khant Aung, Z.; Grattan, D.R. Impact of Pregnancy and Lactation on the Long-Term Regulation of Energy Balance in Female Mice. Endocrinology 2018, 159, 2324–2336. [Google Scholar] [CrossRef]
- Qiao, L.; Chu, K.; Wattez, J.S.; Lee, S.; Gao, H.; Feng, G.S.; Hay WWJr Shao, J. High-fat feeding reprograms maternal energy metabolism and induces long-term postpartum obesity in mice. Int. J. Obes. 2019, 43, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Yu, H.R.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Lin, I.C.; Lin, Y.J.; Chang, K.A.; Tsai, C.C.; Huang, L.T. A maternal high-fat diet during pregnancy and lactation, in addition to a postnatal high-fat diet, leads to metabolic syndrome with spatial learning and memory deficits: Beneficial effects of resveratrol. Oncotarget 2017, 8, 111998–112013. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Pham, M.; Hao, Y.; Wang, F.; Patel, D.; Jean-Baptiste, L.; Fan, L.; Wang, W.; Wang, Y.; Cheng, F. Sex, Age, and BMI Modulate the Association of Physical Examinations and Blood Biochemistry Parameters and NAFLD: A Retrospective Study on 1994 Cases Observed at Shuguang Hospital, China. Biomed. Res. Int. 2019, 2019, 1246518. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. Epidemiology and pathophysiology of the association between NAFLD and metabolically healthy or metabolically unhealthy obesity. Ann. Hepatol. 2020, 19, 359–366. [Google Scholar] [CrossRef]
- Chen, T.P.; Lin, W.Y.; Chiang, C.H.; Shen, T.H.; Huang, K.C.; Yang, K.C. Metabolically healthy obesity and risk of non-alcoholic fatty liver disease severity independent of visceral fat. J. Gastroenterol. Hepatol. 2021, 36, 2903–2910. [Google Scholar] [CrossRef]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Hodson, L.; Karpe, F. Hyperinsulinaemia: Does it tip the balance toward intrahepatic fat accumulation? Endocr. Connect. 2019, 8, 157–168. [Google Scholar] [CrossRef]
- Lewis, G.F.; Carpentier, A.C.; Pereira, S.; Hahn, M.; Giacca, A. Direct and indirect control of hepatic glucose production by insulin. Cell. Metab. 2021, 33, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.R.; Cross, E.; Sanna, F.; Hodson, L. Dysregulation of hepatic metabolism with obesity: Factors influencing glucose and lipid metabolism. Proc. Nutr. Soc. 2022, 81, 1–11. [Google Scholar] [CrossRef]
- Moyce, B.L.; Dolinsky, V.W. Maternal β-Cell Adaptations in Pregnancy and Placental Signalling: Implications for Gestational Diabetes. Int. J. Mol. Sci. 2018, 19, 3467. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Twinn, D.S.; Gascoin, G.; Musial, B.; Carr, S.; Duque-Guimaraes, D.; Blackmore, H.L.; Alfaradhi, M.Z.; Loche, E.; Sferruzzi-Perri, A.N.; Fowden, A.L.; et al. Exercise rescues obese mothers’ insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Sci. Rep. 2017, 7, 44650. [Google Scholar] [CrossRef]
- Yang, M.; Ma, F.; Guan, M. Role of Steroid Hormones in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 320. [Google Scholar] [CrossRef]
- Yang, M.; Geng, C.A.; Liu, X.; Guan, M. Lipid Disorders in NAFLD and Chronic Kidney Disease. Biomedicines 2021, 9, 1405. [Google Scholar] [CrossRef] [PubMed]
- Petroianu, A.; Esquerdo, C.R.M.; Barbosa, A.J.A.; Alberti, L.R. Regeneração hepática induzida por ressecção segmentar do fígado, em rato. Rev. Col. Bras. Cir. 2004, 31, 10–14. [Google Scholar] [CrossRef]
- Pek, N.M.Q.; Liu, K.J.; Nichane, M.; Ang, L.T. Controversies Surrounding the Origin of Hepatocytes in Adult Livers and the in Vitro Generation or Propagation of Hepatocytes. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 273–290. [Google Scholar] [CrossRef]
- Altunkaynak, B.Z.; Ozbek, E. Overweight and structural alterations of the liver in female rats fed a high-fat diet: A stereological and histological study. Turk. J. Gastroenterol. 2009, 20, 93–103. [Google Scholar]
- Rodimova, S.; Mozherov, A.; Elagin, V.; Karabut, M.; Shchechkin, I.; Kozlov, D.; Krylov, D.; Gavrina, A.; Bobrov, N.; Zagainov, V.; et al. Effect of Hepatic Pathology on Liver Regeneration: The Main Metabolic Mechanisms Causing Impaired Hepatic Regeneration. Int. J. Mol. Sci. 2023, 24, 9112. [Google Scholar] [CrossRef]
- Michalopoulos, G.K. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 2017, 65, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Moteki, H.; Ogihara, M. Role of Hepatocyte Growth Regulators in Liver Regeneration. Cells 2023, 12, 208. [Google Scholar] [CrossRef]
- Brahma, M.K.; Gilglioni, E.H.; Zhou, L.; Trépo, E.; Chen, P.; Gurzov, E.N. Oxidative stress in obesity-associated hepatocellular carcinoma: Sources, signaling and therapeutic challenges. Oncogene 2021, 40, 5155–5167. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.G.; Ayoub, S.E.; Elkashefand, W.F.; Ibrahim, T.M. Caffeine affects HFD-induced hepatic steatosis by multifactorial intervention. Hum. Exp. Toxicol. 2018, 37, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Smirne, C.; Croce, E.; Di Benedetto, D.; Cantaluppi, V.; Comi, C.; Sainaghi, P.P.; Minisini, R.; Grossini, E.; Pirisi, M. Oxidative stress in nonalcoholic fatty liver disease. Livers 2022, 2, 30–76. [Google Scholar] [CrossRef]
- Frankenfeld, S.P.; Oliveira, L.P.; Ortenzi, V.H.; Rego-Monteiro, I.C.; Chaves, E.A.; Ferreira, A.C.; Leitão, A.C.; Carvalho, D.P.; Fortunato, R.S. The anabolic androgenic steroid nandrolone decanoate disrupts redox homeostasis in liver, heart and kidney of male Wistar rats. PLoS ONE 2014, 9, 102699. [Google Scholar] [CrossRef]
- Rodríguez-González, G.L.; Vargas-Hernández, L.; Reyes-Castro, L.A.; Ibáñez, C.A.; Bautista, C.J.; Lomas-Soria, C.; Itani, N.; Estrada-Gutierrez, G.; Espejel-Nuñez, A.; Flores-Pliego, A.; et al. Resveratrol Supplementation in Obese Pregnant Rats Improves Maternal Metabolism and Prevents Increased Placental Oxidative Stress. Antioxidants 2022, 11, 1871. [Google Scholar] [CrossRef]
- Candia, A.A.; Lean, S.C.; Zhang, C.X.W.; McKeating, D.R.; Cochrane, A.; Gulacsi, E.; Herrera, E.A.; Krause, B.J.; Sferruzzi-Perri, A.N. Obesogenic Diet in Mice Leads to Inflammation and Oxidative Stress in the Mother in Association with Sex-Specific Changes in Fetal Development, Inflammatory Markers and Placental Transcriptome. Antioxidants 2024, 13, 411. [Google Scholar] [CrossRef]





| Ingredient/Amount per 100 g of Diet | Vivarium Diet | Hyperlipidic Diet (g) | Sweetened Condensed Milk |
|---|---|---|---|
| Corn starch | - | 11.8 | - |
| Flour | - | 12.0 | - |
| Cornstarch biscuit | - | 7.2 | - |
| Soy flour | - | 8.5 | - |
| Lard | - | 5.5 | - |
| Margarine (65% lipids) | - | 3.5 | - |
| Milk cream (20% lipids) | - | 3.0 | - |
| Casein (>85%) | - | 20.0 | - |
| Sucrose | - | 20.0 | - |
| Soy oil | - | 4.0 | - |
| Cellulose | - | 0.3 | - |
| Mineral mix * | - | 2.5 | - |
| Vitamin mix ** | - | 0.7 | - |
| DL-Methionine | - | 0.3 | - |
| Choline Bitartrate | - | 0.25 | - |
| BHT | - | 0.0014 | - |
| Sodium Chloride | - | 0.36 | 0.026 |
| Calcium | - | - | 0.058 |
| Total (g) | - | 100 | - |
| Kcal/100 g | 3.44 | 4.52 | 304 |
| % Total fat | 10.9 | 31.99 | 17.7 |
| % Proteins | 28.3 | 20.18 | 9.8 |
| % Carbohydrates | 60.8 | 47.82 | 72.3 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β2M | TGACCGTGATCTTTCTGGTG | ACTTGAATTTGGGGAGTTTTCTG |
| IL1β | GCCACCTTTTGACAGTGATG | CCCAGGTCAAAGGTTTGGA |
| TNFα | AGGCACTCCCCCAAAAGATG | TGAGGGTCTGGGCCATAGAA |
| 1° Week (kcal) | 2° Week (kcal) | 3° Week (kcal) | Total Period of Pregnancy (kcal) | ||
|---|---|---|---|---|---|
| Ptna | 154.4 ± 15.50 | 198.0 ± 15.65 | 198.2 ± 13.72 | 550.6 ± 43.39 | |
| Lip | 59.75 ± 5.99 | 76.63 ± 6.05 | 76.70 ± 5.31 | 213.1 ± 16.79 | |
| CHO | 331.7 ± 33.29 | 425.3 ± 33.61 | 407.1 ± 38.99 | 1164 ± 102.0 | |
| CD | Total | 545.8 ± 54.78 | 699.9 ± 55.31 | 682.0 ± 56.57 | 1928 ± 161.7 |
| Ptna | 96.87 ± 4.93 ** | 86.47 ± 4.51 *** | 84.71 ± 3.44 **** | 268.0 ± 11.71 *** | |
| Lip | 159.0 ±8.18 **** | 137.3 ± 6.08 *** | 138.5 ± 5.53 **** | 434.9 ± 18.36 **** | |
| CHO | 392.3 ± 36.78 | 307.7 ± 23.06 * | 285.4 ± 13.30 * | 985.4 ± 69.83 | |
| OD | Total | 648.2 ± 41.18 | 531.5 ± 31.11 * | 508.6± 20.52 * | 1688 ± 87.56 |
| 1° Week (kcal) | 2° Week (kcal) | 3° Week (kcal) | Total Period of Lactation (kcal) | ||
|---|---|---|---|---|---|
| Ptna | 247.6 ± 5.73 | 401.8 ± 7.41 | 375.4 ± 20.51 | 1025 ± 19.53 | |
| Lip | 95.82 ± 2.21 | 155.5 ±2.87 | 145.3 ± 7.93 | 396.6 ± 7.55 | |
| CHO | 531.8 ± 12.31 | 863.1 ± 15.93 | 806.4 ± 44.05 | 2201 ± 41.95 | |
| CD | Total | 875.3 ± 20.26 | 1420 ± 26.23 | 1327 ± 72.49 | 3623 ± 69.03 |
| Ptna | 84.02 ± 12.86 **** | 153.9 ± 11.87 **** | 154.1 ± 19.11 **** | 392.0 ± 42.71 **** | |
| Lip | 167.2 ± 21.54 * | 252.2 ± 19.36 ** | 255.3 ± 30.22 ** | 674.7 ± 50.52 *** | |
| CHO | 308.0 ± 37.82 *** | 555.0 ± 40.90 *** | 546.8 ± 39.15 ** | 1410 ± 108.4 *** | |
| OD | Total | 559.3 ± 52.29 *** | 961.1 ± 72.05 *** | 956.3 ± 84.91 * | 2477 ± 194.0 *** |
| Serum Biochemical Analysis (mg/dL) | CD Group | OD Group | p Value |
|---|---|---|---|
| Glucose | 106.3 ± 7.45 | 140.2 ± 10.25 | p = 0.0200 * |
| Triglycerides | 157.3 ± 19.67 | 283.3 ± 24.06 | p = 0.0037 ** |
| Total cholesterol | 71.41 ± 7.26 | 92.40 ± 5.50 | p = 0.0469 * |
| HDL cholesterol | 0.0367 ± 0.0004 | 0.0342 ± 0.0006 | p = 0.0080 ** |
| Biochemical analysis of liver tissue (mg/dL) | CD group | OD group | p value |
| Triglycerides | 179.7 ± 8.96 | 237.6 ± 18.53 | p = 0.0226 * |
| Total cholesterol | 45.63 ± 3.57 | 61.41 ± 1.74 | p = 0.0042 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.S.d.M.; Correia, D.d.A.; Oliveira, W.d.A.; Lima, T.A.; Pachêco, L.d.S.; Santos Junior, O.H.d.; Silva Filho, R.C.d.; Aguiar Júnior, F.C.A.d.; Vasconcelos, D.A.A.d.; Lagranha, C.J.; et al. Effects of Obesogenic Diet Consumption During Pregnancy and Lactation on the Oxidative Balance and Hepatic Metabolism of Female Rats. Nutrients 2025, 17, 2219. https://doi.org/10.3390/nu17132219
Silva GSdM, Correia DdA, Oliveira WdA, Lima TA, Pachêco LdS, Santos Junior OHd, Silva Filho RCd, Aguiar Júnior FCAd, Vasconcelos DAAd, Lagranha CJ, et al. Effects of Obesogenic Diet Consumption During Pregnancy and Lactation on the Oxidative Balance and Hepatic Metabolism of Female Rats. Nutrients. 2025; 17(13):2219. https://doi.org/10.3390/nu17132219
Chicago/Turabian StyleSilva, Gizele Santiago de Moura, Deisiane de Araújo Correia, Wellington de Almeida Oliveira, Talitta Arruda Lima, Letícia da Silva Pachêco, Osmar Henrique dos Santos Junior, Reginaldo Correia da Silva Filho, Francisco Carlos Amanajás de Aguiar Júnior, Diogo Antônio Alves de Vasconcelos, Claudia Jacques Lagranha, and et al. 2025. "Effects of Obesogenic Diet Consumption During Pregnancy and Lactation on the Oxidative Balance and Hepatic Metabolism of Female Rats" Nutrients 17, no. 13: 2219. https://doi.org/10.3390/nu17132219
APA StyleSilva, G. S. d. M., Correia, D. d. A., Oliveira, W. d. A., Lima, T. A., Pachêco, L. d. S., Santos Junior, O. H. d., Silva Filho, R. C. d., Aguiar Júnior, F. C. A. d., Vasconcelos, D. A. A. d., Lagranha, C. J., Souza, S. L. d., & Fernandes, M. P. (2025). Effects of Obesogenic Diet Consumption During Pregnancy and Lactation on the Oxidative Balance and Hepatic Metabolism of Female Rats. Nutrients, 17(13), 2219. https://doi.org/10.3390/nu17132219








