E-Health and M-Health in Obesity Management: A Systematic Review and Meta-Analysis of RCTs †
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Search Strategy and Data Sources
2.3. Study Selection
- Title and Abstract Screening: Initial screening was performed based on the titles and abstracts of the articles.
- Full-Text Review: Full texts of potentially eligible studies were obtained and assessed for final inclusion.
- Evaluated digital health interventions (e-Health and m-Health), either as standalone interventions or as part of mixed approaches combining digital components with limited in-person elements, compared to no treatment or traditional management approaches providing educational materials.
- Focused on overweight or obese adults aged over 18 years with BMIs above 25 kg/m2.
- Utilized a randomized controlled trial (RCT) design that compared two or more groups, one of which received an e-Health or m-Health intervention.
- Reported health endpoints, including anthropometric endpoints (BMI, weight, waist circumference, and body-fat percentage) and lifestyle behavior outcomes (physical activity, energy intake and eating behaviors) for individuals enrolled in the study.
2.4. Data Extraction and Synthesis
2.5. Quality Assessment
2.6. Statistical Analysis
Strategy for Data Synthesis
3. Results
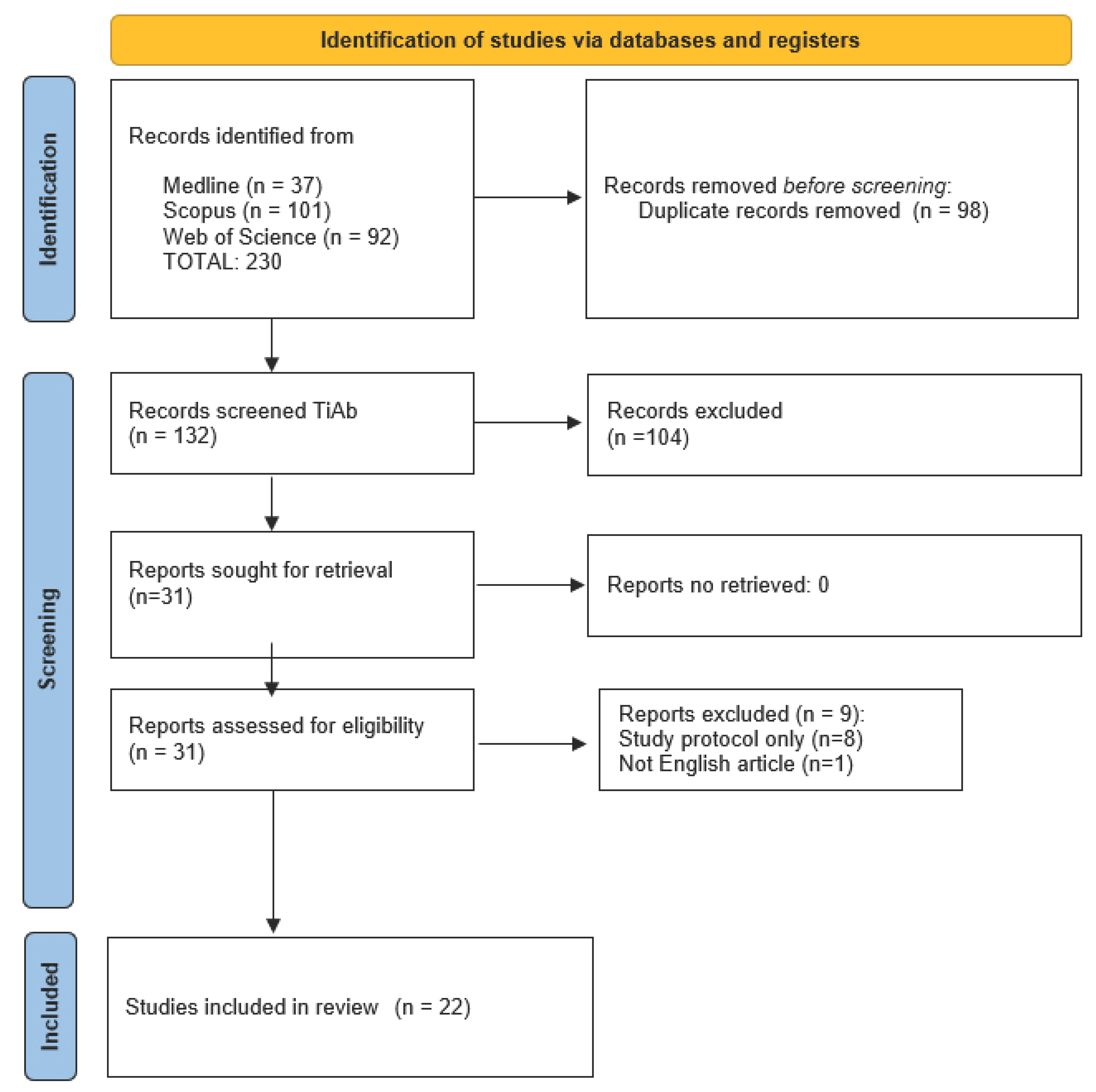
3.1. Study Selection
3.2. Results of Systematic Review
3.3. Results of GRADE
| Outcome | Certainty | Reasons for Downgrade |
|---|---|---|
| BMI | Low | High inconsistency, imprecision |
| Weight | Moderate | Imprecision |
| Waist circumference | Moderate | Inconsistency |
| Body Fat % | Low | Inconsistency, imprecision |
| Physical activity | Low | High inconsistency, imprecision |
| Energy intake | Very Low | Very high inconsistency, imprecision, few studies |
| Eating behavior | Low | High inconsistency, imprecision |
3.4. Results of Metanalysis
3.4.1. Anthropometric Indicators
3.4.2. Lifestyle Behaviors
3.4.3. Publication Bias
4. Discussion
- Diversity in sample sizes, which varied dramatically from 14 participants (Fenton et al. [17]—enhanced intervention subgroup) to over 1000 participants (Bijlholt et al. [16], with 487 controls and 533 interventions), and target populations, which included obese adults, overweight individuals, postpartum women, patients with eating disorders, and populations with high cardiovascular risk. The variable dropout rate between studies (e.g., Bijlholt et al. [16] lost approximately 30% of participants during follow-up) represents an additional source of heterogeneity.
- Variety in measurement instruments. For example, for physical activity, some studies used METs via IPAQ (Ruiz-Cortes et al. [14], Múzquiz-Barberá et al. [15]), while others measured sedentary time in minutes/day. For energy intake, units included both kcal/day and kJ/day, and values were measured using different assessment methodologies. For eating behaviors, different questionnaires (Dutch Eating Behavior Questionnaire DEBQ, FFQ) with variable scoring scales were used.
- Heterogeneity of digital interventions: the range of technologies used includes biofeedback devices (Bernardo et al. [11], Choi et al. [12]), smartphone apps with coaching (Domal et al. [13], Fenton et al. [17], Duncan et al. [22], Lee et al. [6]), e-coaching systems (Yu et al. [19], Alencar et al. [20], Johnson et al. [23]), web-based interventions (Ruiz-Cortes et al. [14], Múzquiz-Barberá et al. [15]), and mixed approaches. Each technology presents significantly different mechanisms of action, levels of interactivity, and frequencies of use. Additionally, the inclusion of both purely digital interventions and mixed approaches (combining digital tools with limited in-person components) contributed to the observed heterogeneity, as the hybrid nature of some interventions may have enhanced their effectiveness through mechanisms that differ from those of standalone digital tools.
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2021 Adult BMI Collaborators. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagi, M.A.; Ahmed, H.; Rezq, M.A.A.; Sangroongruangsri, S.; Chaikledkaew, U.; Almalki, Z.; Thavorncharoensap, M. Economic costs of obesity: A systematic review. Int. J. Obes. 2024, 48, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Balasundaram, P. Public Health Considerations Regarding Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572122/ (accessed on 1 June 2025).
- Wadden, T.A.; Tronieri, J.S.; Butryn, M.L. Lifestyle modification approaches for the treatment of obesity in adults. Am. Psychol. 2020, 75, 235–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, U.; Jung, G.; Ma, E.-Y.; Kim, J.S.; Kim, H.; Alikhanov, J.; Noh, Y.; Kim, H. Toward Data-Driven Digital Therapeutics Analytics: Literature Review and Research Directions. arXiv 2022, arXiv:2205.01851. [Google Scholar] [CrossRef]
- Bates, M.S.; Haskell, S.G. Mobile health and obesity: The role of mobile apps in obesity management. Obes. Rev. 2016, 17, 745–758. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Bernardo, D.; Bobadilla-Agouborde, C.; Festas, C.; Carvalho, C.; Abdalla, P.P.; Amezcua-Prieto, C.; Naia-Entonado, Z.; Mesquita, C.C.; Mota, J.; Santos, P.C. Feasibility, clinical efficacy, and maternal outcomes of a remote exercise program in pregnant women with obesity: The grob randomized control pilot study. Clin. Exp. Obstet. Gynecol. 2024, 51, 70. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.W.; Seo, J.; Sun, Y.; Jung, W.S.; Park, H.Y.; Kim, J.; Lim, K. Effects of a Mobile-Health Exercise Intervention on Body Composition, Vascular Function, and Autonomic Nervous System Function in Obese Women: A Randomized Controlled Trial. J. Multidiscip. Heal. 2023, 16, 1601–1615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Domal, S.V.; Chandrasekaran, B.; Palanisamy, H.P. Influence of smartphone-based physical activity intervention on executive functions and cardiometabolic disease risk in obese young adults: A pilot randomised controlled trial. J. Diabetes Metab. Disord. 2023, 22, 619–628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz-Cortés, M.; Múzquiz-Barberá, P.; Herrero, R.; Vara, M.D.; Escrivá-Martínez, T.; Carcelén, R.; Rodilla, E.; Baños, R.M.; Lisón, J.F. How the Presence of a Doctor Known to Patients Impacts a Web-Based Intervention to Promote Physical Activity and Healthy Eating Behaviour in Individuals with an Overweight/Obesity-Hypertension Phenotype: A Randomised Clinical Trial. Nutrients 2023, 15, 1624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Múzquiz-Barberá, P.; Ruiz-Cortés, M.; Herrero, R.; Vara, M.D.; Escrivá-Martínez, T.; Baños, R.M.; Rodilla, E.; Lisón, J.F. “Own doctor” presence in a web-based lifestyle intervention for adults with obesity and hypertension: A randomized controlled trial. Front. Public Health 2023, 11, 1115711. [Google Scholar] [CrossRef] [PubMed]
- Bijlholt, M.; Ameye, L.; Van Uytsel, H.; Devlieger, R.; Bogaerts, A. The INTER-ACT E-Health Supported Lifestyle Intervention Improves Postpartum Food Intake and Eating Behavior, but Not Physical Activity and Sedentary Behavior-A Randomized Controlled Trial. Nutrients 2021, 13, 1287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fenton, S.; Burrows, T.L.; Collins, C.E.; Rayward, A.T.; Murawski, B.; Duncan, M.J. Efficacy of a Multi-Component m-Health Diet, Physical Activity, and Sleep Intervention on Dietary Intake in Adults with Overweight and Obesity: A Randomised Controlled Trial. Nutrients 2021, 13, 2468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welzel, F.D.; Bär, J.; Stein, J.; Löbner, M.; Pabst, A.; Luppa, M.; Grochtdreis, T.; Kersting, A.; Blüher, M.; Luck-Sikorski, C.; et al. Using a brief web-based 5A intervention to improve weight management in primary care: Results of a cluster-randomized controlled trial. BMC Fam. Pract. 2021, 22, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Z.; Roberts, B.; Snyder, J.; Stuart, K.; Wilburn, J.; Pudwill, H.; Cortazzo, K. A Pilot Study of a Videoconferencing-Based Binge Eating Disorder Program in Overweight or Obese Females. Telemed. e-Health 2021, 27, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Alencar, M.; Johnson, K.; Gray, V.; Mullur, R.; Gutierrez, E.; Dionico, P. Telehealth-Based Health Coaching Increases m-Health Device Adherence and Rate of Weight Loss in Obese Participants. Telemed. e-Health 2020, 26, 365–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yousuf, H.; Reintjens, R.; Slipszenko, E.; Blok, S.; Somsen, G.A.; Tulevski, I.I.; Hofstra, L. Effectiveness of web-based personalised e-Coaching lifestyle interventions. Neth. Heart. J. 2019, 27, 24–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duncan, M.J.; Fenton, S.; Brown, W.J.; Collins, C.E.; Glozier, N.; Kolt, G.S.; Holliday, E.G.; Morgan, P.J.; Murawski, B.; Plotnikoff, R.C.; et al. Efficacy of a Multi-component m-Health Weight-loss Intervention in Overweight and Obese Adults: A Randomised Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 6200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, K.E.; Alencar, M.K.; Coakley, K.E.; Swift, D.L.; Cole, N.H.; Mermier, C.M.; Kravitz, L.; Amorim, F.T.; Gibson, A.L. Telemedicine-Based Health Coaching Is Effective for Inducing Weight Loss and Improving Metabolic Markers. Telemed. e-Health 2019, 25, 85–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hutchesson, M.J.; Callister, R.; Morgan, P.J.; Pranata, I.; Clarke, E.D.; Skinner, G.; Ashton, L.M.; Whatnall, M.C.; Jones, M.; Oldmeadow, C.; et al. A Targeted and Tailored eHealth Weight Loss Program for Young Women: The Be Positive Be Healthe Randomized Controlled Trial. Healthcare 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.H.; Cheung, B.; Yi, G.H.; Oh, B.; Oh, Y.H. Mobile health, physical activity, and obesity: Subanalysis of a randomized controlled trial. Medicine 2018, 97, e12309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melchart, D.; Löw, P.; Wühr, E.; Kehl, V.; Weidenhammer, W. Effects of a tailored lifestyle self-management intervention (TALENT) study on weight reduction: A randomized controlled trial. Diabetes Metab. Syndr. Obes. 2017, 10, 235–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rader, S.; Dorner, T.E.; Schoberberger, R.; Wolf, H. Effects of a web-based follow-up intervention on self-efficacy in obesity treatment for women. Wien. Klin. Wochenschr. 2017, 129, 472–481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogers, R.J.; Lang, W.; Barone Gibbs, B.; Davis, K.K.; Burke, L.E.; Kovacs, S.J.; Portzer, L.A.; Jakicic, J.M. Applying a technology-based system for weight loss in adults with obesity. Obes. Sci. Pract. 2016, 2, 3–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wagner, B.; Nagl, M.; Dölemeyer, R.; Klinitzke, G.; Steinig, J.; Hilbert, A.; Kersting, A. Randomized Controlled Trial of an Internet-Based Cognitive-Behavioral Treatment Program for Binge-Eating Disorder. Behav Ther. 2016, 47, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Gregoski, M.J.; Newton, J.; Ling, C.G.; Blaylock, K.; Smith, S.A.; Paguntalan, J.; Treiber, F.A. Effective weight-loss using an e-health delivered physical activity and dietary intervention: A federal credit union pilot study. Work 2016, 54, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Tomkins-Lane, C.C.; Lafave, L.M.; Parnell, J.A.; Rempel, J.; Moriartey, S.; Andreas, Y.; Wilson, P.M.; Hepler, C.; Ray, H.A.; Hu, R. The spinal stenosis pedometer and nutrition lifestyle intervention (SSPANLI): Development and pilot. Spine J. 2015, 15, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Cadmus-Bertram, L.A.; Natarajan, L.; White, M.M.; Madanat, H.; Nichols, J.F.; Ayala, G.X.; Pierce, J.P. Wearable Sensor/Device (Fitbit One) and SMS Text-Messaging Prompts to Increase Physical Activity in Overweight and Obese Adults: A Randomized Controlled Trial. Telemed. e-Health 2015, 21, 782–792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papp-Zipernovszky, O.; Horváth, M.D.; Schulz, P.J.; Csabai, M. Generation Gaps in Digital Health Literacy and Their Impact on Health Information Seeking Behavior and Health Empowerment in Hungary. Front. Public Health 2021, 9, 635943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorgente, A.; Pietrabissa, G.; Manzoni, G.M.; Re, F.; Simpson, S.; Perona, S.; Rossi, A.; Cattivelli, R.; Innamorati, M.; Jackson, J.B.; et al. Web-Based Interventions for Weight Loss or Weight Loss Maintenance in Overweight and Obese People: A Systematic Review of Systematic Reviews. J. Med. Internet Res. 2017, 19, e229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flores Mateo, G.; Granado-Font, E.; Ferré-Grau, C.; Montaña-Carreras, X. Mobile Phone Apps to Promote Weight Loss and Increase Physical Activity: A Systematic Review and Meta-Analysis. J. Med. Internet Res. 2015, 17, e253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J.; et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev. Espanola De Cardiol. 2022, 75, 523. [Google Scholar] [CrossRef]
| Author; Year; Country | Population; Sample; Mean Age (SD); Male Population % | Targeted Risk Behaviors | Study Design; Follow Up; Frequency; Assessment | Intervention | Intervention Components | Comparison Group | Endpoints |
|---|---|---|---|---|---|---|---|
| Bernardo et al., 2024, Portugal [11] | Obese; n = 24; age ≥ 18 y; 0% males | Pregnant between the 6th and 20th gestational week | Two-arm trial Follow-up: 8 weeks | e-Health e-Health: Remote exercise program delivered by a Phoenix® biofeedback device | n = 12 Phoenix® Device available 24 h/7 days per week; pregnant women were encouraged to exercise at least three times a week. | n = 12 Standard care | Anthropometric measures Weight |
| Choi et al., 2023, Korea [12] | Obese; n = 30; age: 39 ± 11 y; 0% males | Body fat > 30% | Two-arm trial Follow-up: 12 weeks Muscle function almost two times/week, cardiorespiratory endurance and flexibility almost five times/week | m-Health m-Health: Fitbit Charge + Fitbit app linked to the AI Fit webpage | n = 15 Combined exercise (muscle function, cardiorespiratory endurance, flexibility); the exercise programs were followed while watching videos, and the participants were able to obtain feedback on exercise performance through a mobile chat program linked to AI fit. | n = 15 Normal daily routines | Anthropometric measures Weight BMI Body fat Waist circumference |
| Domal et al., 2023, India [13] | Obese; n = 20; age: 18–35 y; 50% males | Men, waist circumference >90 cm; women, waist circumference >80 cm | Two-arm trial Follow-up: 8 weeks | m-Health Smartphone-based application (EXi app.) | n = 10 Video conferencing | n = 10 Standard care | Anthropometric measures Weight BMI Body fat Waist circumference |
| Riuz-Cortes et al., 2023, Spain [14] | Overweight/obese; n = 132; age: 46–67 y; 55% males | Hypertension | Two-arm trial Follow-up: 12 weeks | e-Health eHealth: Living Better web-based program and web page (Wix) | n = 70 Psychological strategies that encourage a healthy lifestyle, healthy eating habits and increased PA, with the participants’ own doctor appearing in the audiovisual material | n = 62 Standard protocol with unknown doctor | Anthropometric measures BMI Behaviors Adherence to Mediterranean diet Eating behaviors Physical activity |
| Múzquiz-Barberá et al., 2023, Spain [15] | Overweight/obese; n = 132; age: 46–67 y; 55% males | Hypertension | Two-arm trial Follow-up: 12 weeks | e-Health eHealth: Living Better web-based program and web page (Wix) | n = 70 Psychological strategies that encourage a healthy lifestyle, healthy eating habits, and increased PA, with the participants’ own doctor appearing in the audiovisual material | n = 62 Standard protocol with unknown doctor | Anthropometric measures BMI Behaviors Physical activity |
| Bijlholt et al., 2021, Belgium [16] | Overweight/obese; n = 1450; age: ≥ 18y; 0% males | Women in the postpartum period | Two-arm trial Follow-up: 1 year Assessment at T1 6 months and T2 12 months after delivery | e-Health INTER-ACT e-Health | n = 193 OW; n = 89 OB | Standard care n = 181 OW and 74 OB | Behaviors Eating behaviors Energy intake Physical activity |
| Fenton et al., 2021, Australia [17] | Overweight/obese; n = 116; age: 19–65 y (44.5 ± 10.5); 30% males | BMI 31.7 kg/m2 | Three arms Follow-up: 1 year Assessment at T1 6 months and T2 12 months EG m-Health (pooled Enhanced + Traditional) two arms/CG one arm | m-Health Smartphone app providing educational materials, goal-setting, self-monitoring, and feedback and one face-to-face dietary consultation, a Fitbit, and scales. | m-Health: smartphone app n = 39 Enhanced-intervention group (provided with stress-management and relaxation techniques and received access to a sleep intervention via the app); n = 41 Traditional-intervention group (provided with stress-management and relaxation techniques but no sleep intervention) | n = 36 Standard care | Anthropometric measures Weight BMI Waist circumference (only at baseline!) Behaviors Energy intake Physical activity |
| Welzel et al., 2021, Germany [18] | Obese; n = 135; age: 43.3 ± 10.7 y; 38% males | BMI = 39 ± 6.0 kg/m2 | Two-arm trial Follow-up: 1 year Assessment at T1 6 months T2 12 months | e-Health Online tutorial | n = 65 Access to 5As of Obesity Management tutorial | n = 70 Standard care | Anthropometric measures Weight BMI |
| Yu et al., 2021, USA [19] | Overweight/obese; n = 13; age: 20–73 y; 0% males | Binge-eating disorder | Two arms Follow-up: 3 months | m-Health | n = 4 Videoconferencing-based treatment program | n = 9 Standard care | Anthropometric measures Weight BMI Weight in NO Disorder |
| Alencar et al., 2020, USA [20] | Obese; n = 25 (M and F); age: 41.5 ± 13.6 y; NR% males | BMI = 34.6 ± 4.33 kg/m2 | Two arms Follow-up: 12 weeks | m-Health Two wireless devices that connected them directly with the research team | n = 13 Health video-coaching; telehealth video conferencing for health coaching with a dietitian and a physician | n = 12 Standard care | Behaviors Adherence to wireless devices; |
| Anthropometric measures Weight (Weight Week Loss) | |||||||
| Yousuful et al., 2019; HAPPY NL, HAPPY AZM Netherlands [21] | Prospective cohort study NO RCT | ||||||
| Yousuful et al., 2019; HAPPY LONDON, England [21] | Overweight/obese; n = 402; age: 65.5 (40–74 y); 67% males | BMI E-coaching: 27.4 kg/m2 Standard: 27.1 kg/m2 a 10-year CVD risk score of ≥10%; excluded people with an established cardiovascular disease | Single RCT Follow-up: 6 months | e-Health e-Coaching MyCLIC: e-Coaching lifestyle intervention tool | n = ? e-Coaching received 6 months of tailored advice and were able to enter their MyCLIC pages with personal logins. | n = ? Standard care | Anthropometric measures Weight BMI |
| Duncan et al., 2020, Australia [22] | Overweight/obese; n = 116; age: 19–65 y (44.5 ± 10.5); 30% males | BMI 31.7 kg/m2 | Three arms Follow-up: 1 year Assessment at T1 6 months, T2 12 months EG m-Health (pooled Enhanced + Traditional) 2 arms/CG 1 arm | m-Health Smartphone app providing educational materials, goal-setting, self-monitoring and feedback, and also included one face-to-face dietary consultation, a Fitbit and scales. | n = 39 Enhanced-intervention group (received access to a sleep intervention via the app and participant handbook targeted a reduction in sleep timing variability, promoted sleep hygiene behaviors and provided stress management and relaxation techniques. n = 41 Traditional-intervention group (no sleep intervention) | n = 36 Standard care | Anthropometric measures Body weight BMI Waist circumference Behaviors Energy intake Physical activity |
| Johnson et al., 2019, USA [23] | Obese; n = 30 (M and F); age: 32–46 y; NR% males | Three arms Follow-up: 12 weeks | e-Health Telemedicine-based health coaching | n = 10 Video conference telemedicine-based health coaching | n = 10 In-person group n = 10 Standard care | Anthropometric measures Weight BMI Behaviors Physical activity | |
| Hutchesson et al., 2018, Australia [24] | Overweight/obese; n = 57; age: 18 ÷ 35 y (27.1 ± 4.7); 0% males | (56.1% overweight and 43.9% obese) | Two arms Follow-up: 6 months | e-Health e-Health: Multicomponent intervention consisting of five delivery modes: website, social media, smartphone application, email, text messages. | n = 29 | n = 28 Normal daily routines | Anthropometric measures Weight BMI Waist circumference Body fat Behaviors Energy intake Physical activity |
| Lee et al., 2018, South Korea [25] | Overweight/obese; n = 324; age: > 20 y; 47% males | BMI ≥ 25 kg/m2; metabolic syndrome | Two-arm trial Follow-up: 24 weeks | m-Health Smartphones equipped with the SmartCare application and a Bioimpedance Analyzer via Bluetooth to facilitate telemonitoring. | n = 177 | n = 147 Standard care | Anthropometric measures Weight BMI Waist circumference Body fat |
| Melchart et al., 2017, Germany [26] | Overweight/obese; n = 166; age: IHM: 49.9 (9.7) y, UC: 52.2 (19) y; IHM 25,2% males, UC: 27.3% males | Excluded pregnant people with diabetes, hypertension grade 2, disease | Two arms Follow-up: 1 year | e-Health Individual Health Management (IHM): lifestyle-modification program with a 3 month reduction phase and 9 month maintenance phase. The program encompasses access to a web-based health portal providing advice regarding food, exercise, and relaxation and allows personalized feedback for control of the progress. | n = 109 | n = 57 Standard care | Anthropometric measures Weight BMI Waist circumference |
| Rader et al., 2017, Austria [27] | Obese; n = 84; age: 18–80 y; 0% males | Two-arm trial Follow-up: 1 year Assessment at T1 6 months T2 12 months | e-Health Web-based intervention (WBI); e-Health web-based intervention (WBI) conducted subsequent to an initial face-to-face lifestyle treatment, with an introductory phase (4 months) and a training phase (2 months) in which each group was trained in using the appropriate instrument according to their study arm. In the following 6 months, participants used either the WBI or the PMI for follow-up support. | n = 21 | n = 22 Standard care | Anthropometric measures BMI | |
| Rogers et al.; 2016; USA [28] | Obese; n = 39; age: 39.9 ± 11.5 y; 79.5% males | Sedentary adults, BMI 39.5 ± 2.8 kg m2, excluded pregnant women, people taking medications or with chronical disease | Three arms Follow-up: 6 months Assessments at T1 3 months T2 6 months | e-Health Technology-based intervention combined with a monthly intervention telephone call (TECH); participants were provided with the BodyMedia® FIT System, a wearable device. Enhanced technology-based system combined with a monthly intervention telephone call (EN-TECH): BodyMedia® FIT System with the LINK activity monitor | n = 12 TECH; n = 13 EN-TECH | n = 14 Standard in-person group-based behavioral weight-loss intervention | Anthropometric measures Weight BMI Waist circumference Body fat Behaviors Eating behaviors Energy intake Physical activity |
| Wagner et al., 2016; Germany [29] | Overweight/obese; n 139; age: 35.1 (9.9) y; 3.6% males | Binge-eating disorder Excluded if BN or anorexia nervosa, severe major depression, acute suicidal symptoms, abuse, medical condition influencing weigh | Two arms Follow-up: 1 year Assessments at T1 3 months T2 6 months T3 12 months | e-Health 16-week intervention Internet-based cognitive–behavioral intervention or a wait-list condition. Internet-based cognitive–behavioral intervention Guided therapy with intensive therapist contact, 11 personalized structured writing assignments and individualized feedback from trained therapists, complimentary daily eating and activity diaries, week plans, and psychoeducation as applied in cognitive–behavioral treatments. | n = 69 | n = 70 | Anthropometric measures Weight BMI Behaviors Eating behaviors |
| Gregosky et al., 2015, USA [30] | Overweight/obese; n = 54; age: 37.7 ± 9.9 y; 7% males | BMI: 30.8 ± 8.6 | Two-arm trial Follow-up: 10 weeks; | e-Health e-Health: take home DVD. A prize of $15 was awarded to those who lost ≥ 15 lbs. | n = 45 Physical-activity program on DVD | n = 9 Standard care | Anthropometric measures Weight BMI |
| Tomkins Lane et al., 2015, Canada [31] | Overweight/obese; n= 10 age: 67.5 ± 6.7 y; 40% males | Lumbar spinal stenosis Excluded people with comorbidities that limit walking. | Intervention development and pilot Follow-up: 13 weeks | e-Health Lifestyle-modification approach of physical activity and nutrition education, delivered through an e-health platform. Participants received a pedometer and a personalized consultation with a dietitian and an exercise physiologist. For 12 weeks, participants logged on to the e-health Web site to access personal step goals, nutrition-education videos, and a discussion board. | n = ? | n = ? | Anthropometric measures Weight Waist circumference |
| Wang et al., 2015, USA [32] | Overweight/obese; n = 67; age: 48.2 (11.7) y; 9% males | Not meeting recommended levels of physical activity (PA < 150 min/week of MVPA, 48 ability to safely increase PA) | Two-arm trial Follow-up: 6 weeks Assessment weekly Population wore a Fitbit One. The Fitbit One included a wearable tracker for instant feedback on performance and a website/mobile application (app) for detailed summaries. | e-Health Test the effects of daily text messaging as simple prompts to increase PA. Three daily SMS-based PAprompts. | n = 33 | n = 34; Self-monitoring with Fitbit One only | Behaviors Physical activity |
| WMD | Test of Heterogeneity | Publication Bias | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Effect-Sizes | Value (95% CI) | p | Q | I2% | p | p (Egger) | p (Begg) | |
| Anthropometric Indicators | |||||||||
| Overall | 13 | 46 | −0.12 (−0.24; 0.01) | 0.065 | 112.05 | 59.84 | <0.0001 | 0.596 | 0.698 |
| Excluding high-risk-of-bias studies (D1-D2 Robs tool 2) | 10 | 43 | −0.12 (−0.26; 0.02) | 0.088 | 111.6 | 62.36 | <0.0001 | 0.515 | 0.623 |
| Target group: | |||||||||
| Only women | 5 | 9 | −0.11 (−0.34; 0.11) | 0.335 | 6.75 | 0.00 | 0.574 | 0.754 | 0.835 |
| Healthy | 8 | 29 | −0.12 (−0.35; 0.11) | 0.301 | 106.89 | 73.81 | <0.0001 | 0.366 | 0.881 |
| Generation X (born after 1965) | 8 | 26 | −0.07 (−0.19; 0.05) | 0.268 | 17.54 | 0.00 | 0.861 | 0.743 | 0.440 |
| Intervention type: | |||||||||
| E-Health | 8 | 22 | −0.16 (−0.41; 0.1) | 0.230 | 92.59 | 77.32 | <0.0001 | 0.434 | 0.756 |
| M-Health | 5 | 24 | −0.06 (−0.16; 0.03) | 0.202 | 15.11 | 0.00 | 0.891 | 0.861 | 0.960 |
| Specific Outcomes | |||||||||
| BMI | 11 | 15 | −0.43 (−1.15; 0.30) | 0.247 | 104.71 | 86.63 | <0.0001 | 0.272 | 0.400 |
| WEIGHT | 10 | 15 | 0.42 (−0.45; 1.29) | 0.341 | 11.20 | 0.00 | 0.670 | 0.633 | 0.805 |
| WAIST | 5 | 8 | −1.77 (−3.10; −0.44) | 0.009 | 18.1 | 61.13 | 0.012 | 0.932 | 1.000 |
| BODY FAT % | 5 | 8 | −0.79 (−1.63; 0.06) | 0.068 | 16.94 | 58.67 | 0.018 | 0.128 | 0.805 |
| Cohen’s d | Test of heterogeneity | Publication bias | |||||||
| Lifestyle Behaviors | |||||||||
| Overall | 9 | 24 | −0.20 (−0.40; 0.00) | 0.052 | 211.45 | 89.12 | <0.0001 | 0.252 | 0.620 |
| Excluding high-risk-of-bias studies (D1-D2 Robs tool 2) | 6 | 21 | −0.01 (−0.16; 0.15) | 0.926 | 93.34 | 78.57 | <0.0001 | 0.973 | 0.856 |
| Target group: | |||||||||
| Only women | 2 | 7 | −0.04 (−0.21; 0.14) | 0.694 | 38.03 | 84.22 | <0.0001 | 0.711 | 0.652 |
| Healthy | 5 | 13 | −0.37 (−0.96; 0.23) | 0.225 | 126.26 | 90.50 | <0.0001 | 0.716 | 0.714 |
| Generation X (born after 1965) | 6 | 17 | −0.28 (−0.52; −0.04) | 0.020 | 168.07 | 90.40 | <0.0001 | 0.187 | 0.805 |
| Intervention type: | |||||||||
| E-Health | 8 | 22 | −0.07 (−0.25; 0.10) | 0.427 | 133.04 | 84.22 | <0.0001 | 0.750 | 0.978 |
| M-Health | 1 | 2 | / | / | / | / | / | / | / |
| Specific Outcomes | |||||||||
| Physical activity | 8 | 10 | −0.01 (−0.39; 0.36) | 0.939 | 67.26 | 86.62 | <0.0001 | 0.754 | 0.929 |
| Energy intake | 4 | 5 | −0.93 (−1.88; 0.01) | 0.052 | 62.81 | 93.63 | <0.0001 | 0.375 | 0.142 |
| Eating behavior | 4 | 9 | −0.13 (−0.40; 0.14) | 0.341 | 65.75 | 87.83 | <0.0001 | 0.508 | 0.404 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavarini, M.; Giacchetta, I.; Rosignoli, P.; Fabiani, R. E-Health and M-Health in Obesity Management: A Systematic Review and Meta-Analysis of RCTs. Nutrients 2025, 17, 2200. https://doi.org/10.3390/nu17132200
Chiavarini M, Giacchetta I, Rosignoli P, Fabiani R. E-Health and M-Health in Obesity Management: A Systematic Review and Meta-Analysis of RCTs. Nutrients. 2025; 17(13):2200. https://doi.org/10.3390/nu17132200
Chicago/Turabian StyleChiavarini, Manuela, Irene Giacchetta, Patrizia Rosignoli, and Roberto Fabiani. 2025. "E-Health and M-Health in Obesity Management: A Systematic Review and Meta-Analysis of RCTs" Nutrients 17, no. 13: 2200. https://doi.org/10.3390/nu17132200
APA StyleChiavarini, M., Giacchetta, I., Rosignoli, P., & Fabiani, R. (2025). E-Health and M-Health in Obesity Management: A Systematic Review and Meta-Analysis of RCTs. Nutrients, 17(13), 2200. https://doi.org/10.3390/nu17132200









