Impact of Probiotic/Synbiotic Supplementation on Post-Bariatric Surgery Anthropometric and Cardiometabolic Outcomes: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Data Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias and Certainty of Evidence
2.7. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Characteristics of Included Studies
3.3. Risk of Bias Assessment
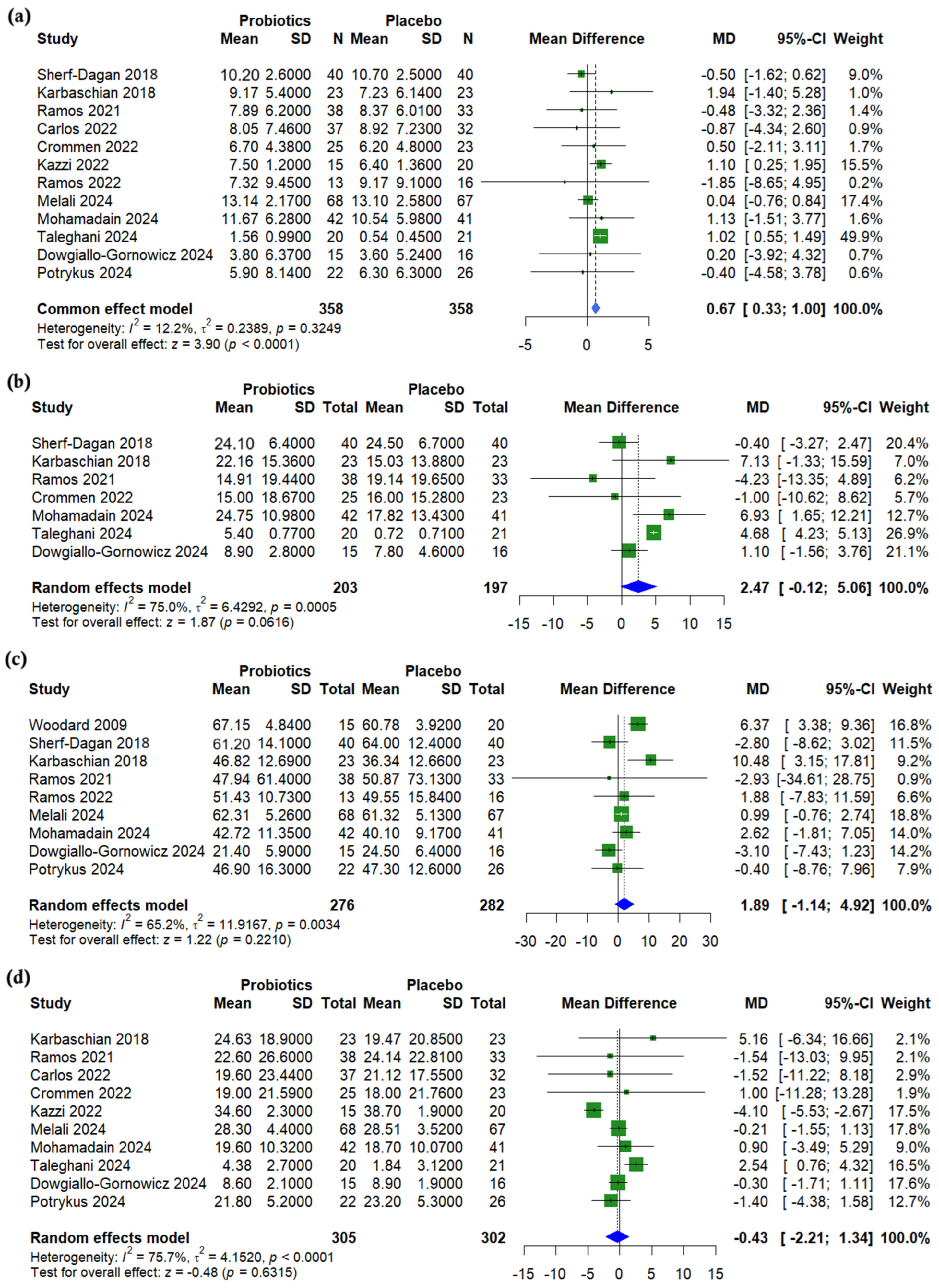
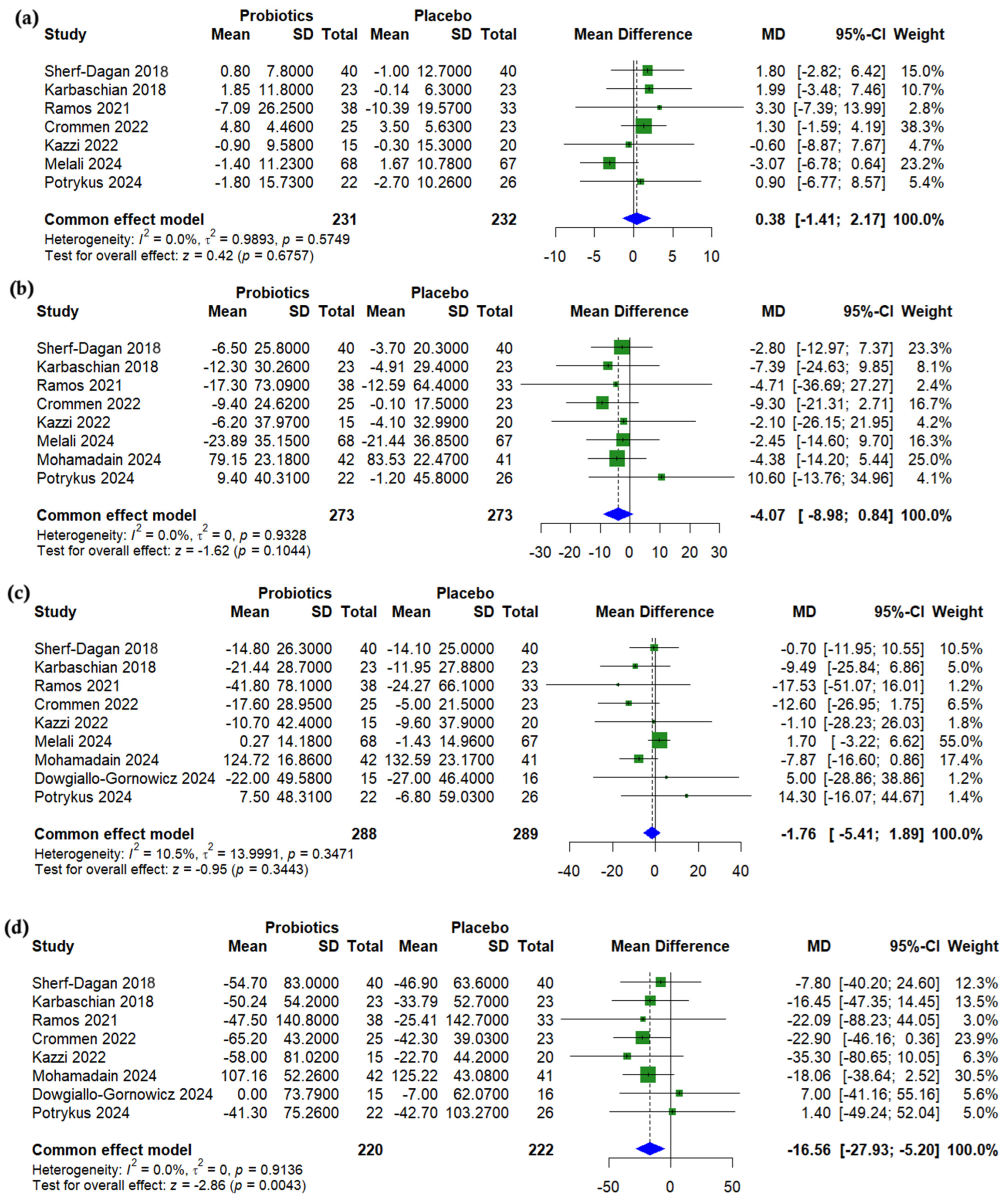
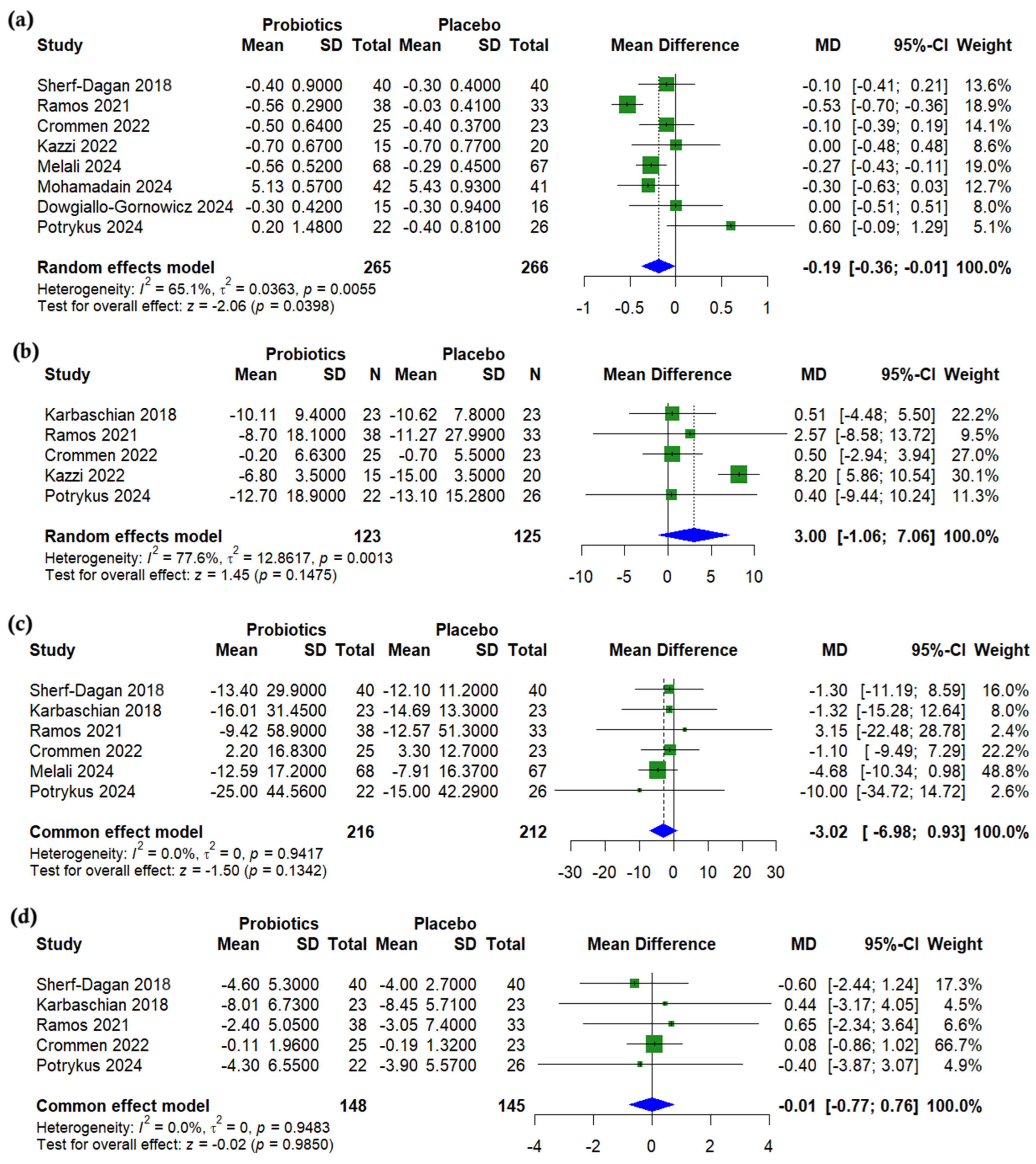
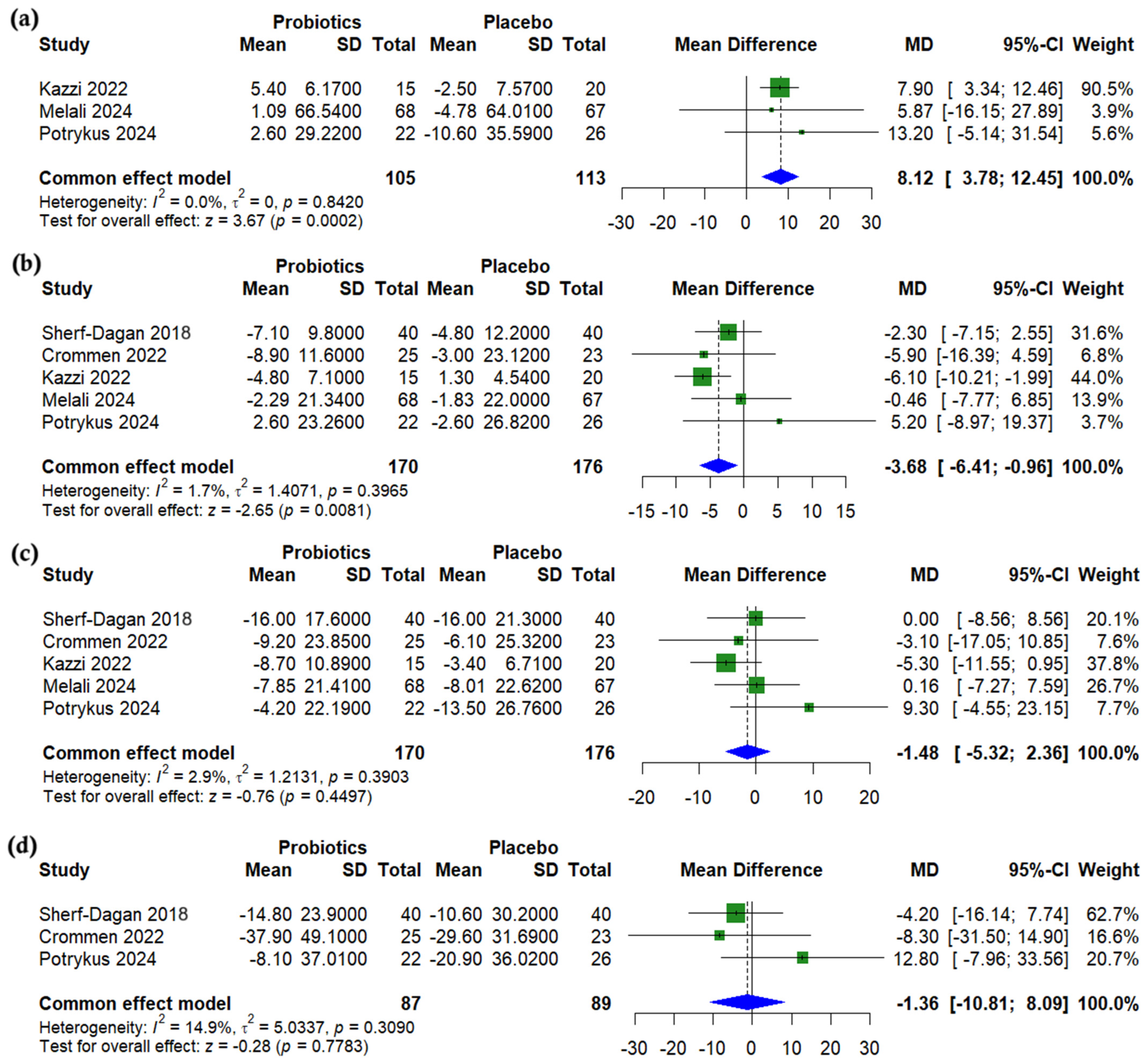
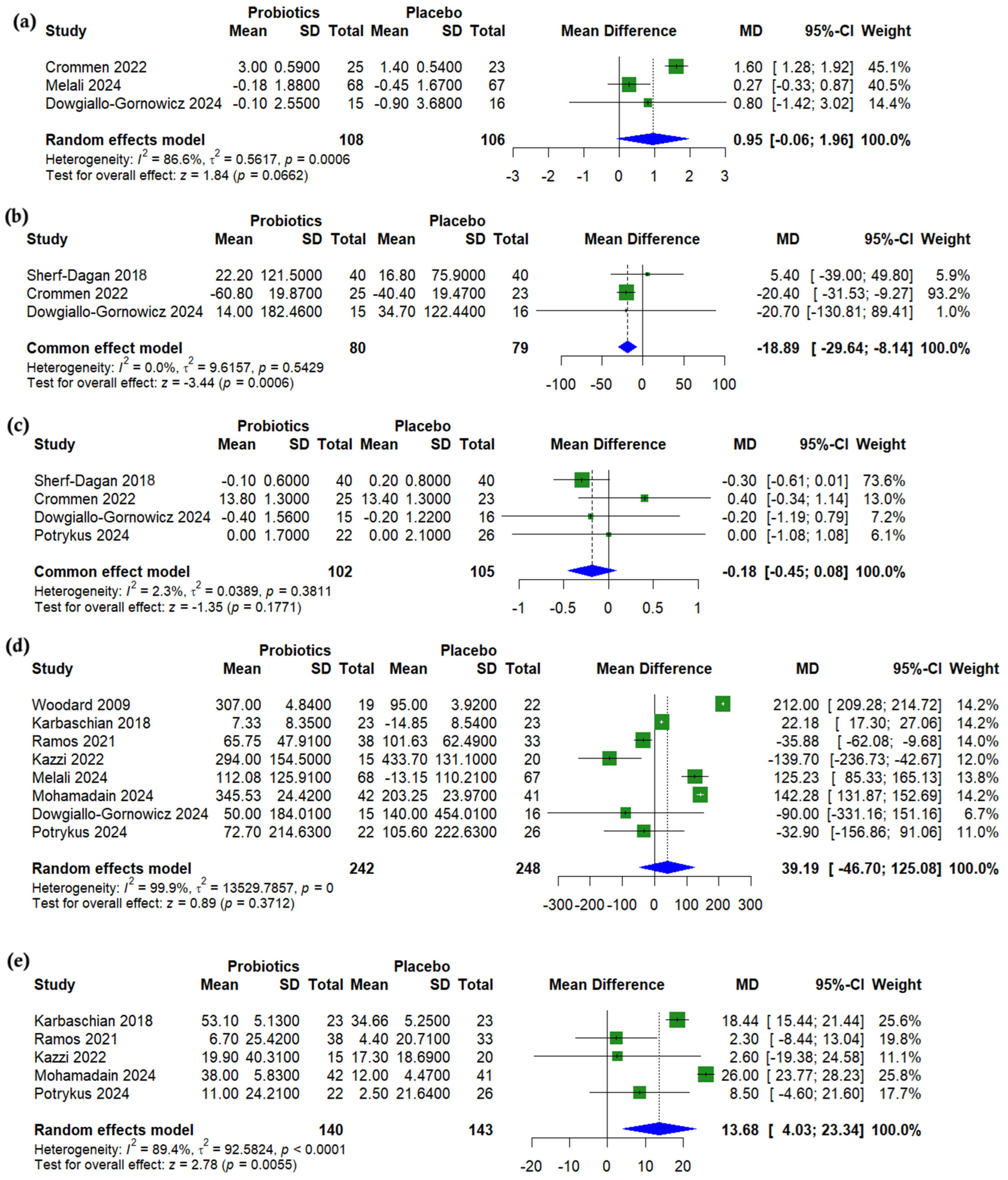
3.4. Anthropometric Outcomes
3.5. Lipid Profile
3.6. Glycemic Outcomes
3.7. Liver Enzymes
3.8. Nutritional Outcomes
3.9. Subgroup Analysis
3.10. Synbiotic-Specific Pooled Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation List
References
- Maciejewski, M.L.; Arterburn, D.E.; Van Scoyoc, L.; Smith, V.A.; Yancy, W.S., Jr.; Weidenbacher, H.J.; Livingston, E.H.; Olsen, M.K. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016, 151, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Doumouras, A.G.; Yu, J.; Aditya, I.; Gmora, S.; Anvari, M.; Hong, D. Laparoscopic Sleeve Gastrectomy Versus Laparoscopic Roux-en-Y Gastric Bypass: A Systematic Review and Meta-analysis of Weight Loss, Comorbidities, and Biochemical Outcomes From Randomized Controlled Trials. Ann. Surg. 2021, 273, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ionut, V.; Bergman, R.N. Mechanisms responsible for excess weight loss after bariatric surgery. J. Diabetes Sci. Technol. 2011, 5, 1263–1282. [Google Scholar] [CrossRef]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Vrieze, A.; Holleman, F.; Zoetendal, E.G.; de Vos, W.M.; Hoekstra, J.B.; Nieuwdorp, M. The environment within: How gut microbiota may influence metabolism and body composition. Diabetologia 2010, 53, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Dao, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.L.; Chevallier, J.M.; et al. Major microbiota dysbiosis in severe obesity: Fate after bariatric surgery. Gut 2019, 68, 70–82. [Google Scholar] [CrossRef]
- Yang, J.; Qin, S.; Zhang, H. Precise strategies for selecting probiotic bacteria in treatment of intestinal bacterial dysfunctional diseases. Front. Immunol. 2022, 13, 1034727. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y. Multidisciplinary combined treatment based on bariatric surgery for metabolic syndrome: A review article. Int. J. Surg. 2024, 110, 3666–3679. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Kuang, L.; Yang, K.; Xie, J.; Liu, X.; Shen, S.; Li, X.; Wu, S.; Yang, Y.; et al. Effects of probiotics in patients with morbid obesity undergoing bariatric surgery: A systematic review and meta-analysis. Int. J. Obes. 2023, 47, 1029–1042. [Google Scholar] [CrossRef]
- de Sousa, D.F.; Salaroli, L.B. Effects of the Use of Probiotics in Post-Bariatric Surgery Obesity: Meta-Umbrella of Systematic Reviews. Obesities 2024, 4, 491–508. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Rayyan. AI-Powered Systematic Review Management Platform. Available online: https://www.rayyan.ai/ (accessed on 20 April 2024).
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Hefnawy, M.T.; Negida, A. Meta-analysis accelerator: A comprehensive tool for statistical data conversion in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 2024, 24, 243. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; McKenzie, J.E.; Veroniki, A.A. (Eds.) Chapter 10: Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2024. [Google Scholar]
- DeVito, N.J.; Goldacre, B. Catalogue of bias: Publication bias. BMJ Evid. Based Med. 2019, 24, 53–54. [Google Scholar] [CrossRef]
- Mohamadain, A.H.; Eltaweel, M.M. The Adjuvant Therapeutic Effect of Probiotics on the Anthropometric Measurements and Metabolic Parameters in Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Clinical Trial. Al-Azhar Int. Med. J. 2024, 5, 169–176. [Google Scholar] [CrossRef]
- Ghafouri-Taleghani, F.; Tafreshi, A.S.; Doost, A.H.; Tabesh, M.; Abolhasani, M.; Amini, A.; Saidpour, A. Effects of Probiotic Supplementation Added to a Weight Loss Program on Anthropometric Measures, Body Composition, Eating Behavior, and Related Hormone Levels in Patients with Food Addiction and Weight Regain After Bariatric Surgery: A Randomized Clinical Trial. Obes. Surg. 2024, 34, 3181–3194. [Google Scholar] [CrossRef]
- Woodard, G.A.; Encarnacion, B.; Downey, J.R.; Peraza, J.; Chong, K.; Hernandez-Boussard, T.; Morton, J.M. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: A prospective randomized trial. J. Gastrointest. Surg. 2009, 13, 1198–1204. [Google Scholar] [CrossRef]
- Karbaschian, Z.; Mokhtari, Z.; Pazouki, A.; Kabir, A.; Hedayati, M.; Moghadam, S.S.; Mirmiran, P.; Hekmatdoost, A. Probiotic Supplementation in Morbid Obese Patients Undergoing One Anastomosis Gastric Bypass-Mini Gastric Bypass (OAGB-MGB) Surgery: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Obes. Surg. 2018, 28, 2874–2885. [Google Scholar] [CrossRef]
- Dowgiallo-Gornowicz, N.; Mysiorska, D.; Sosnowska-Turek, E.; Botulinska, A.; Lech, P. Initial Study on the Impact of Probiotics on Postoperative Gastrointestinal Symptoms and Gut Microbiota after Sleeve Gastrectomy: A Placebo-Controlled Study. Nutrients 2024, 16, 3498. [Google Scholar] [CrossRef]
- Melali, H.; Abdolahi, A.; Sheikhbahaei, E.; Vakili, K.; Mahmoudieh, M.; Keleidari, B.; Shahabi, S. Impact of Probiotics on Gastrointestinal Function and Metabolic Status After Roux-en-Y Gastric Bypass: A Double-Blind, Randomized Trial. Obes. Surg. 2024, 34, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Crommen, S.; Rheinwalt, K.P.; Plamper, A.; Simon, M.C.; Rosler, D.; Fimmers, R.; Egert, S.; Metzner, C. A Specifically Tailored Multistrain Probiotic and Micronutrient Mixture Affects Nonalcoholic Fatty Liver Disease-Related Markers in Patients with Obesity after Mini Gastric Bypass Surgery. J. Nutr. 2022, 152, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.R.Z.; de Oliveira Carlos, L.; Wagner, N.R.F.; Felicidade, I.; da Cruz, M.R.; Taconeli, C.A.; Fernandes, R.; Filho, A.J.B.; Campos, A.C.L. Effects of Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 Supplementation on Nutritional and Metabolic Parameters in the Early Postoperative Period after Roux-en-Y Gastric Bypass: A Randomized, Double-Blind, Placebo-Controlled Trial. Obes. Surg. 2021, 31, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Kazzi, F.; Daher, N.; Zimmerman, G.; Garcia, M.; Schmidt, N.; Scharf, K. Effect of Bacillius Coagulans and Galactomannans on Obese Patients Undergoing Sleeve Gastrectomy, A Randomized-Controlled Clinical Trial. Altern. Ther. Health Med. 2021, 27, 138–145. [Google Scholar]
- Ramos, M.R.Z.; Felicidade, I.; de Oliveira Carlos, L.; Wagner, N.R.F.; Mantovani, M.S.; de Lima, L.V.A.; Ribeiro, L.R.; Lopes, T.I.B.; Henrique-Bana, F.C.; Zimmerman, J.V.; et al. Effect of probiotic supplementation on plasma metabolite profile after Roux-Y gastric bypass: A prospective, randomized, double-blind, placebo-controlled clinical trial. Int. J. Obes. 2022, 46, 2006–2012. [Google Scholar] [CrossRef]
- Sherf-Dagan, S.; Zelber-Sagi, S.; Zilberman-Schapira, G.; Webb, M.; Buch, A.; Keidar, A.; Raziel, A.; Sakran, N.; Goitein, D.; Goldenberg, N.; et al. Probiotics administration following sleeve gastrectomy surgery: A randomized double-blind trial. Int. J. Obes. 2018, 42, 147–155. [Google Scholar] [CrossRef]
- Carlos, L.O.; Ramos, M.R.Z.; Wagner, N.R.F.; Freitas, L.A.C.; Felicidade, I.; Campos, A.C.L. Probiotic Supplementation Attenuates Binge Eating and Food Addiction 1 Year after Roux-En-Y Gastric Bypass: A Randomized, Double-Blind, Placebo-Controlled Trial. Arq. Bras. Cir. Dig. 2022, 35, e1659. [Google Scholar] [CrossRef]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Szymanski, M.; Loniewski, I.; Kaska, L.; Proczko-Stepaniak, M. Preoperative Multistrain Probiotic Supplementation Does Not Affect Body Weight Changes or Cardiometabolic Risk Factors in Bariatrics: Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 2055. [Google Scholar] [CrossRef]
- Chen, J.C.; Lee, W.J.; Tsou, J.J.; Liu, T.P.; Tsai, P.L. Effect of probiotics on postoperative quality of gastric bypass surgeries: A prospective randomized trial. Surg. Obes. Relat. Dis. 2016, 12, 57–61. [Google Scholar] [CrossRef]
- Fernandes, R.; Beserra, B.T.; Mocellin, M.C.; Kuntz, M.G.; da Rosa, J.S.; de Miranda, R.C.; Schreiber, C.S.; Frode, T.S.; Nunes, E.A.; Trindade, E.B. Effects of Prebiotic and Synbiotic Supplementation on Inflammatory Markers and Anthropometric Indices After Roux-en-Y Gastric Bypass: A Randomized, Triple-blind, Placebo-controlled Pilot Study. J. Clin. Gastroenterol. 2016, 50, 208–217. [Google Scholar] [CrossRef]
- Ball, G.D.C.; Merdad, R.; Birken, C.S.; Cohen, T.R.; Goodman, B.; Hadjiyannakis, S.; Hamilton, J.; Henderson, M.; Lammey, J.; Morrison, K.M.; et al. Managing obesity in children: A clinical practice guideline. Can. Med. Assoc. J. 2025, 197, E372–E389. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.W.; Hung, K.C. Impact of Probiotics on Triglyceride Level After Bariatric Surgery: A Trial Sequential Analysis. Obes. Surg. 2025, 35, 651–654. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; D’Alessio, D.A. Going with the flow: Adaptation of beta-cell function to glucose fluxes after bariatric surgery. Diabetes 2013, 62, 3671–3673. [Google Scholar] [CrossRef]
- Dulai, A.S.; Min, M.; Sivamani, R.K. The Gut Microbiome’s Influence on Incretins and Impact on Blood Glucose Control. Biomedicines 2024, 12, 2719. [Google Scholar] [CrossRef]
- Tikka, T.; Mohd Slim, M.A.; Ton, T.; Sheldon, A.; Clark, L.J.; Kontorinis, G. Investigation of serum calcium and vitamin D levels in superior semicircular canal dehiscence syndrome: A case control study. J. Otol. 2023, 18, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Schramm, S.; Lahner, H.; Jockel, K.H.; Erbel, R.; Fuhrer, D.; Moebus, S.; Heinz Nixdorf Recall Study, G. Impact of season and different vitamin D thresholds on prevalence of vitamin D deficiency in epidemiological cohorts—A note of caution. Endocrine 2017, 56, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Need, A.G. Bone resorption markers in vitamin D insufficiency. Clin. Chim. Acta 2006, 368, 48–52. [Google Scholar] [CrossRef]
- Wei, J.H.; Lee, W.J.; Chong, K.; Lee, Y.C.; Chen, S.C.; Huang, P.H.; Lin, S.J. High Incidence of Secondary Hyperparathyroidism in Bariatric Patients: Comparing Different Procedures. Obes. Surg. 2018, 28, 798–804. [Google Scholar] [CrossRef]
- Mele, C.; Caputo, M.; Ferrero, A.; Daffara, T.; Cavigiolo, B.; Spadaccini, D.; Nardone, A.; Prodam, F.; Aimaretti, G.; Marzullo, P. Bone Response to Weight Loss Following Bariatric Surgery. Front. Endocrinol. 2022, 13, 921353. [Google Scholar] [CrossRef]
- Han, J.H.; Kwak, J.Y.; Lee, S.S.; Kim, H.G.; Jeon, H.; Cha, R.R. Markedly Elevated Aspartate Aminotransferase from Non-Hepatic Causes. J. Clin. Med. 2022, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Kular, K.S.; Manchanda, N.; Rutledge, R. Physiology of the MGB: How It Works for Long-Term Weight Loss. In Essentials of Mini—One Anastomosis Gastric Bypass; Deitel, M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 31–37. [Google Scholar]

| Study ID | Country | Total Participants | Procedure | Probiotic Type and Content | Probiotic Dose | Placebo Details | Reported Outcomes of Interest | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Dowgiallo-Gornowicz 2024 [23] | Poland | 31 | LSG | Lactobacillus plantarum AMT14 5 × 108 CFU g−1, Bifidobacterium animalis AMT30 1 × 1010 CFU g−1, Bifidobacterium breve AMT32 1 × 1010 CFU g−1 | Daily | Starch | The variables assessed include gastrointestinal symptoms (stool frequency, constipation occurrence, ease of defecation, and bowel movement completeness), anthropometric measurements (BMI, %TWL, %EWL, and waist circumference), biochemical parameters (hemoglobin, ferritin, albumin, protein, glycated hemoglobin, cholesterol, triglycerides, and vitamin B12), and stool microbiota composition (Enterococcus faecalis, Enterococcus faecium, and Clostridium perfringens). | 1 months |
| Melali 2024 [24] | Iran | 135 | RYGB | Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophilus (dose not reported) | N/A | N/A | The variables assessed include anthropometric measurements (BMI, %TWL, %EWL, and weight), glycemic parameters (FBS and HbA1c), lipid profile (cholesterol, HDL, and LDL), liver enzymes (AST, ALT, and ALK-P), coagulation markers (PT, PTT, and INR), vitamins and minerals (vitamin B12, calcium, sodium, potassium, phosphorus, magnesium, and zinc), renal function markers (BUN and creatinine), and GI symptoms evaluated via the GIQLI questionnaire. | 6 months |
| Mohamadain 2024 [19] | Egypt | 83 | LSG | 1 × 109 CFU capsule−1 lyophilised Lactobacillus acidophilus + Bifidobacterium animalis subsp. lactis; prebiotic inulin + oligofructose | BID | Inactive starch capsules | The primary outcome measured was anthropometric parameters including %EWL, BMI, weight, and WC. Secondary outcomes included lipid profile (cholesterol, triglycerides, and LDL), glycemic control (HbA1c), and serum vitamins (vitamin D [25-hydroxyvitamin D3] and vitamin B12). | 4 months (4 weeks Presurgery to 12 weeks postsurgery |
| Potrykus 2024 [31] | Poland | 48 | LSG or OAGB | Bifidobacterium bifidum W23, Bifidobacterium lactis W51 & W52, Lactobacillus acidophilus W37, Levilactobacillus brevis W63, Lacticaseibacillus casei W56, Ligilactobacillus salivarius W24, Lactococcus lactis W19 & W58; total 2 × 109 CFU day−1 | Four capsules daily with meals (two capsules in themorning and two capsules in the evening) | Maize starch and maltodextrin from maize | The variables assessed include anthropometric measurements (weight, BMI, %WL, %EWL, and %EBMIL), glycemic parameters (glucose, insulin, HbA1c, and HOMA-IR), lipid profile (TG, HDL, LDL, and total cholesterol), liver enzymes (LDH, ALT, AST, GGT, and ALP), vitamins and minerals (vitamin D, vitamin B12, folic acid, iron, and hemoglobin), and postoperative complications (classified according to the Clavien–Dindo scale). | 6 months |
| Ghafouri-Taleghani 2024 [20] | Iran | 41 | N/A (Bariatric surgery) | Per capsule: Lactobacillus acidophilus 1.8 × 109 CFU, Bifidobacterium bifidum 1.8 × 109 CFU, Bifidobacterium lactis 1.8 × 109 CFU, Bifidobacterium longum 1.8 × 109 CFU, Lactobacillus reuteri 1 × 109 CFU, Lactobacillus rhamnosus 1 × 109 CFU; excipients: magnesium stearate, maltodextrin | Two capsules daily | The placebo capsule contains 300 mg of starch. | The variables assessed include anthropometric measurements (weight, BMI, WC, WHR, PBF, MBF, MM, and %TWL), eating behavior scores (uncontrolled eating, cognitive restriction, emotional eating, and food addiction symptoms), serum biomarkers (leptin, oxytocin, and serotonin), dietary intake and physical activity (energy, carbohydrate, protein, fat, fiber intake, and MET), and biochemical parameters (ALT, AST, GGT, plasma glucose, insulin, HbA1c, HOMA-IR, TC, TG, LDL, HDL, IL-6, CRP, and ferritin). | 3 months |
| Carlos 2022 [30] | Brazil | 71 | RYGB | 5 × 109 CFU tablet−1 Lactobacillus acidophilus + Bifidobacterium lactis | 1 tablet/day | Inert manipulated tablet | The variables assessed include anthropometric measurements (weight and BMI), eating behavior scores (BES score and YFAS symptoms), and food addiction prevalence. | 3 months |
| Crommen 2022 [25] | Germany | 48 | MGB | Multistrain powder 15 × 109 CFU (4 g) containing Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus belveticus, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus casei, Lactococcus lactis subsp. lactis, Streptococcus thermophilus + 3.9 g micronutrient mix | Daily | Micronutrient mixture and a placebo powder | The variables assessed include anthropometric measurements (body mass, BMI, body fat mass, body fat-free mass, waist circumference, and visceral adiposity index), biochemical parameters (serum ALT, AST, GGT, insulin, HbA1c, HOMA-IR, TC, TG, LDL, HDL, IL-6, CRP, ferritin, hemoglobin, albumin, and total protein), liver function indices (fatty liver index, NAFLD fibrosis score, AST/ALT ratio, GGT/ALT ratio, and AST/platelet ratio), metabolic parameters (plasma glucose), and vital signs (blood pressure and heart rate). | 3 months |
| Sherf-Dagan 2018 [29] | Israel | 100 | LSG | 25 × 109 CFU capsule−1 mix: Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus paracasei, Lactobacillus plantarum, Lactococcus lactis, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Streptococcus thermophilus | 2 capsules/d | No supplement | Outcomes included fatty liver assessed by the hepatorenal index and abdominal ultrasound; liver stiffness measured by shear wave elastography; anthropometric measurements (weight, height, BMI, waist circumference, hip circumference, and excess weight loss), biochemical parameters (lipid profile, C-reactive protein, glucose, hemoglobin A1c, liver enzymes, insulin, ferritin, blood count, leptin, adiponectin, cytokeratin-18, TNF-α, IL-6, IL-10, and bile acids), and microbiota composition evaluated via 16S rRNA sequencing. | 3 months |
| Karbaschian 2018 [22] | Iran | 46 | OAGB | Powder (w/38.5 mg FOS) per g: Lactobacillus casei 3.5 × 109 CFU, Lactobacillus rhamnosus 7.5 × 108 CFU, Streptococcus thermophilus 1 × 108 CFU, Bifidobacterium breve 1 × 1010 CFU, Lactobacillus acidophilus 1 × 109 CFU, Bifidobacterium longum 3.5 × 109 CFU, Lactobacillus bulgaricus 1 × 108 CFU | Daily | Maltodextrin daily | The primary outcome of the study was a significant reduction in inflammatory factor concentrations in serum. Secondary outcomes included anthropometric measurements (weight, height, waist circumference, hip circumference, and BMI), glycemic indices (plasma glucose, insulin, HOMA-IR, and QUICKI), lipid profile (total cholesterol, triglycerides, HDL, and LDL), nutrient and vitamin levels (vitamin B12, folate, and 25-hydroxyvitamin D3), and other biochemical markers, such as homocysteine. | 4 months 4 weeks pre-surgery to 12 weeks post-surgery |
| Kazzi 2021 [27] | USA | 35 | LSG | 4.5 × 109 CFU capsule−1 Bacillus coagulans with galactomannans (300 mg) | 1 capsule/d | 600 mg of calcium citrate yields 126 mg of elemental calcium. 1 capsule/day | The variables assessed include biochemical parameters, such as HbA1c, TC, LDL, HDL, TG, ALT, AST, ALP, TSH, insulin, B12, vitamin D, CRP, and fasting glucose. Additionally, anthropometric and clinical measures included SBP, DBP, % wt. loss, BMI, and % EWL. | 3 months |
| Woodard 2009 [21] | USA | 41 | RYGB | Lactobacillus spp. (strain and dose not specified) | 1 capsule/day. Each capsule contains 2.4 billion live cells | No supplementation | H2 levels, which were indicative of bacterial overgrowth, GI-related quality of life (GIQoL), serologies, weight loss, %EWL, and vitamin B12. | 6 months |
| Ramos 2021 [26] | Brazil | 101 | RYGB | 5 × 109 CFU tablet−1 Lactobacillus acidophilus + Bifidobacterium lactis | 1 tablet/d | Inert manipulated tablet consisting of starch and 190 mg lactose. | The variables assessed include anthropometric measurements (weight, BMI, WC, body fat, % EWL, and lean body mass), glycemic indices (FBS, HbA1c, insulin, HOMA-IR, and QUICKI), lipid profile (TC, TG, HDL, and LDL), and vitamin and biochemical markers (vitamin B12, 25-OH vitamin D3, and serum folate). | 3 months |
| Ramos 2022 [28] | Brazil | 29 | RYGB | 5 × 109 CFU tablet−1 Lactobacillus acidophilus and 5 × 109 CFU tablet−1 Bifidobacterium lactis | 2 tablets/d | Inert manipulated tablet consisting of starch and 190 mg lactose. | The variables assessed include anthropometric measurements (%EWL, BMI, and weight), glycemic indices (glucose, β-hydroxybutyrate [BHB]), lipid profile, plasma metabolites (trimethylamine-N-oxide [TMAO], alanine, acetate, lactate, lipids, and acetoacetate), and branched-chain amino acids (BCAAs) (valine, leucine, and isoleucine). | 3 months |
| Study | Patients, n. (%) | Age (Mean ± SD) | Male Gender, n. (%) | Weight, kg. (Mean ± SD) | BMI (Mean ± SD) | HbA1c, % (Mean ± SD) | Smokers, n. (%) | Diabetes, n. (%) | Hypertension, n. (%) | Dyslipidemia, n. (%) | Hypothyroidism, n. (%) | Fatty Liver n. (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | Probiotics | Placebo | |
| Dowgiallo-Gornowicz 2024 [23] | 15 (48.4%) | 16 (51.6%) | 40.9 ± 10.6 | 40.9 ± 11.9 | N/A | N/A | NA | NA | 43.2 ± 4.7 | 40.1 ± 3.8 | 5.7 ± 0.3 | 6.0 ± 0.8 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Melali 2024 [24] | 68 (50.4%) | 67 (49.6%) | 33.28 ± 9.18 | 31.83 ± 8.62 | 19 (27.9%) | 20 (29.8%) | 125.33 ± 5.02 | 125.95 ± 4.38 | 45.98 ± 3.92 | 46.50 ± 4.57 | 5.98 ± 0.42 | 6.05 ± 0.18 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Mohamadain 2024 [19] | 42 (50.6%) | 41 (49.4%) | 42.72 ± 11.35 | 40.10 ± 9.17 | 7 (16.77%) | 5 (12.19%) | 123.14 ± 13.10 | 119.61 ± 12.83 | 43.85 ± 5.42 | 44.61 ± 4.35 | 6.15 ± 1.03 | 6.34 ± 1.15 | NA | NA | 7 (16.76%) | 9 (21.95%) | 11 (26.19%) | 13 (31.71%) | 24 (57.14%) | 29 (70.73%) | 3 (7.14%) | 4 (9.76%) | NA | NA |
| Potrykus 2024 [31] | 22 (45.8%) | 26 (54.2%) | 41.0 ± 11.2 | 42.2 ± 11.6 | 9 (40.9%) | 6 (23.1%) | 138.3 ± 27.1 | 135.9 ± 21.8 | 46.2 ± 6.2 | 45.9 ± 5.0 | 6.1 ± 1.3% | 5.7 ± 0.7% | 1 (4.5%) | 7 (26.9%) | 6 (27.3%) | 6 (23.1%) | 12 (54.5%) | 14 (53.8%) | 15 (68.2%) | 17 (65.4%) | 7 (31.8%) | 9 (34.6%) | 19 (86.4%) | 21 (80.8%) |
| Ghafouri-Taleghani 2024 [20] | 20 (48.8%) | 21 (51.2%) | 39.10 ± 6.96 | 39.09 ± 8.36 | 4 (20%) | 3 (14.3%) | 93.87 ± 15.24 | 94.93 ± 18.63 | 33.87 ± 4.14 | 34.21 ± 4.82 | NA | NA | 4 (20%) | 4 (19%) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Carlos 2022 [30] | 38 (53.5%) | 33 (46.5%) | 40.21 ± 11.25 | 12.7% | 113.61 ± 23.21 | 111.21 ± 17.57 | 42.84 ± 5.40 | 43.51 ± 5.51 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Crommen 2022 [25] | 25 (52.1%) | 23 (47.9%) | 40 ± 11 | 41 ± 9 | 6 (24%) | 4 (17%) | 127 ± 16.2 | 124 ± 16.9 | 44.3 ± 3.0 | 43.2 ± 3.4 | 5.8 ± 1.0 | 5.6 ± 0.7 | NA | NA | 7 (28%) | 4 (17%) | 12 (48%) | 5 (22%) | NA | NA | NA | NA | 25 (100%) | 23 (100%) |
| Sherf-Dagan 2018 [29] | 50 (50%) | 50 (50%) | 41.9 ± 9.0 | 41.8 ± 10.6 | NA | NA | NA | NA | 42.1 ± 5.0 | 42.5 ± 4.4 | NA | NA | NA | NA | 7 (14%) | 6 (12%) | NA | NA | NA | NA | NA | NA | 50 (100%) | 50 (100%) |
| Karbaschian 2018 [22] | 23 (50%) | 23 (50%) | 32.35 ± 6.88 | 36.95 ± 11.00 | 0% | 0% | 120.04 ± 15.10 | 119.34 ± 15.83 | 44.59 ± 4.30 | 44.95 ± 4.52 | NA | NA | 2 (8.7%) | 0 (0%) | 3 (13.0%) | 3 (13.6%) | 6 (26.1%) | 8 (36.4%) | NA | NA | NA | NA | NA | NA |
| Kazzi 2021 [27] | 15 (42.8%) | 20 (57.2%) | 49.3 ± 13.7 | 46.6 ± 11.6 | 3 (20%) | 4 (25%) | NA | NA | 43.0 ± 7.0 | 49.2 ± 8.3 | 6.0 ± 0.7% | 6.0 ± 0.6% | 7 (46.7%) | 4 (20%) | 3 (20%) | 8 (40%) | 9 (60%) | 16 (80%) | 2 (13.3%) | 5 (25%) | NA | NA | NA | NA |
| Woodard 2009 [21] | 19 (46.3%) | 22 (53.7%) | 48.6 | 41.2 | 3 (15.8%) | 2 (9.1%) | 125.4 | 139.0 | 45.7 | 49.6 | NA | NA | NA | NA | 10 (52.6%) | 4 (18.2%) | 11 (57.9%) | 13 (59.1%) | NA | NA | NA | NA | NA | NA |
| Ramos 2021 [26] | 51 (50.5%) | 50 (49.5%) | 37.1 ± 11.1 | 43.8 ± 10.4 | 4 (10.5%) | 5 (15.2%) | 122.24 ± 23.7 | 120.36 ± 20.7 | 43.95 ± 5.43 | 44.31 ± 5.3 | ~5.1 ± 1.41 | ~5.3% ± 1.67 | 0 (0%) | 1 (3.0%) | 6 (15.8%) | 7 (21.2%) | 11 (28.9%) | 21 (63.6%) | 27 (71.1%) | 18 (54.4%) | NA | NA | 26 (68.4%) | 25 (75.8%) |
| Ramos 2022 [28] | 13 (44.8%) | 16 (55.2%) | 37.00 ± 12.88 | 47.00 ± 8.47 | 2 (15.4%) | 3 (18.8%) | NA | NA | 41.94 ± 6.47 | 45.13 ± 6.75 | NA | NA | 0 (0%) | 1 (6.2%) | 1 (7.7%) | 4 (25.0%) | 4 (30.8%) | 10 (62.5%) | 9 (69.2%) | 5 (31.2%) | NA | NA | 12 (92.3%) | 10 (62.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakab, M.S.; Rateb, R.M.; Maamoun, A.; Radwan, N.; Shubietah, A.; Manasrah, A.; Rajab, I.; Scichilone, G.; Tussing-Humphreys, L.; Mahmoud, A.M. Impact of Probiotic/Synbiotic Supplementation on Post-Bariatric Surgery Anthropometric and Cardiometabolic Outcomes: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2025, 17, 2193. https://doi.org/10.3390/nu17132193
Rakab MS, Rateb RM, Maamoun A, Radwan N, Shubietah A, Manasrah A, Rajab I, Scichilone G, Tussing-Humphreys L, Mahmoud AM. Impact of Probiotic/Synbiotic Supplementation on Post-Bariatric Surgery Anthropometric and Cardiometabolic Outcomes: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2025; 17(13):2193. https://doi.org/10.3390/nu17132193
Chicago/Turabian StyleRakab, Mohamed Saad, Rahma Mogahed Rateb, Alaa Maamoun, Nada Radwan, Abdalhakim Shubietah, AlMothana Manasrah, Islam Rajab, Giorgia Scichilone, Lisa Tussing-Humphreys, and Abeer M. Mahmoud. 2025. "Impact of Probiotic/Synbiotic Supplementation on Post-Bariatric Surgery Anthropometric and Cardiometabolic Outcomes: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 17, no. 13: 2193. https://doi.org/10.3390/nu17132193
APA StyleRakab, M. S., Rateb, R. M., Maamoun, A., Radwan, N., Shubietah, A., Manasrah, A., Rajab, I., Scichilone, G., Tussing-Humphreys, L., & Mahmoud, A. M. (2025). Impact of Probiotic/Synbiotic Supplementation on Post-Bariatric Surgery Anthropometric and Cardiometabolic Outcomes: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 17(13), 2193. https://doi.org/10.3390/nu17132193







