Voghera Sweet Pepper Regulates Cell Death Pathways in an Aging In Vitro Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
2.2. Immunofluorescence Reactions
2.3. Fluorescence Microscopy
2.4. Immunofluorescence Analyses
2.5. Transmission Electron Microscopy (TEM)
2.6. Statistical Analysis
3. Results
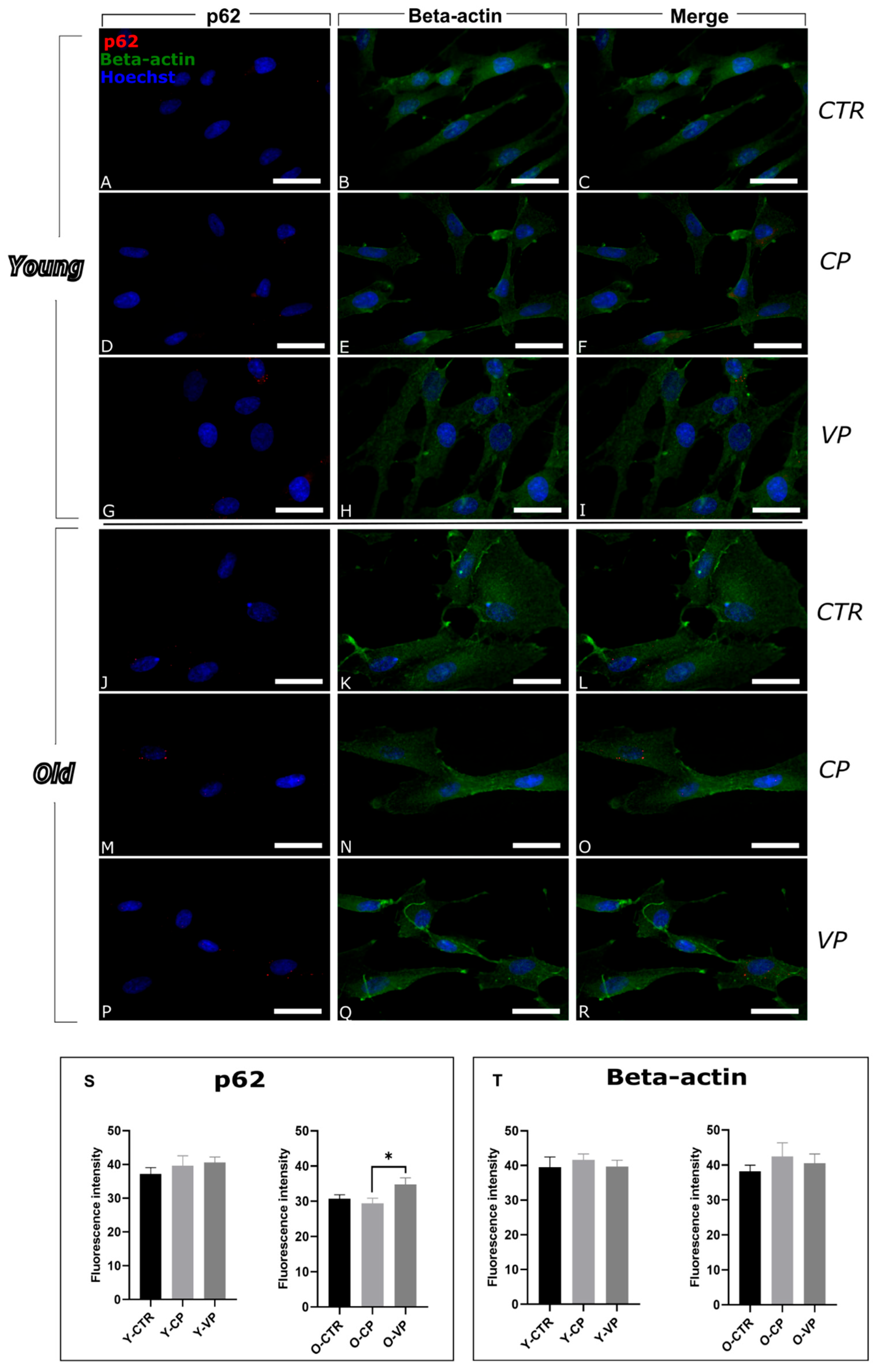
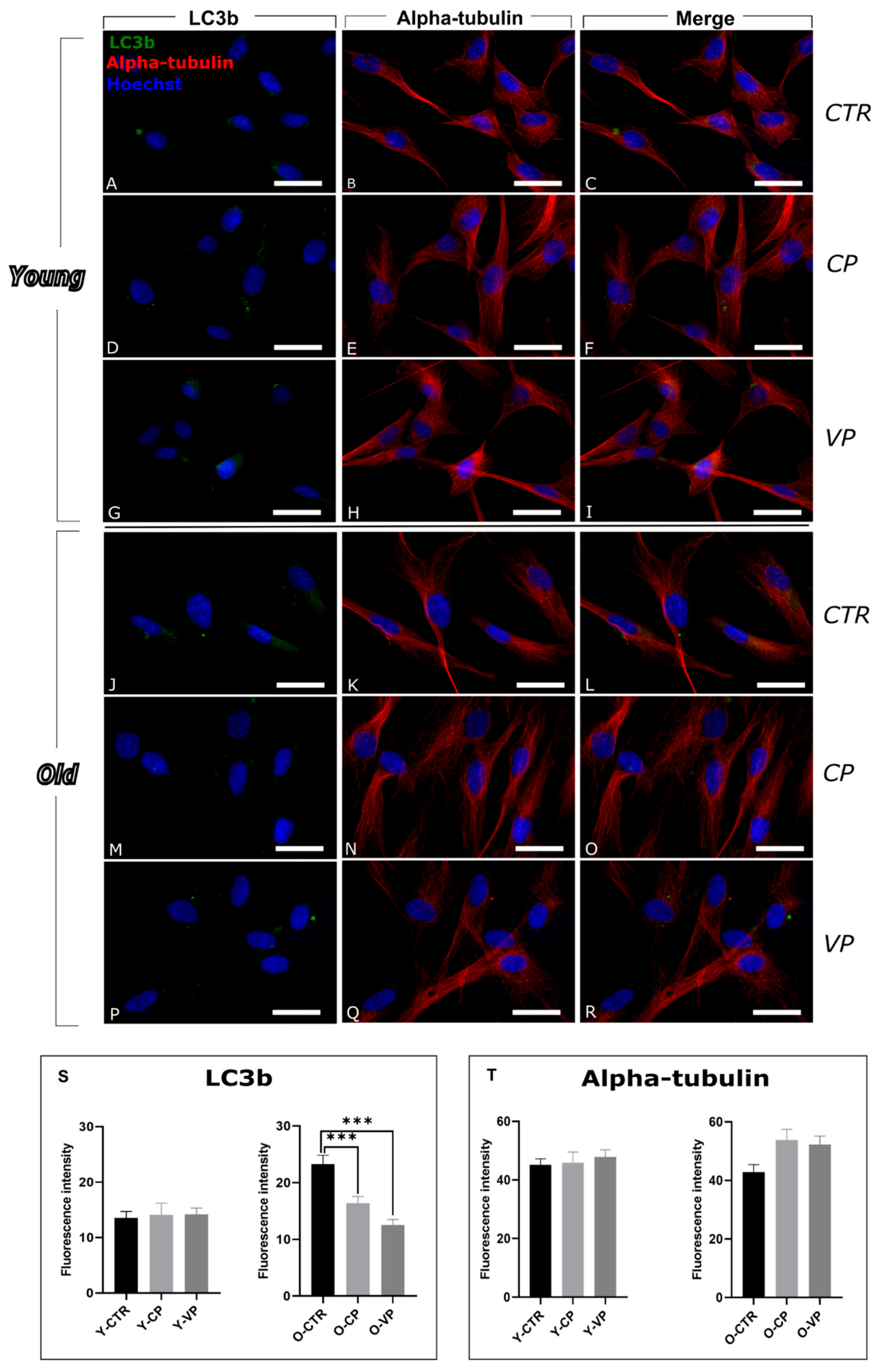
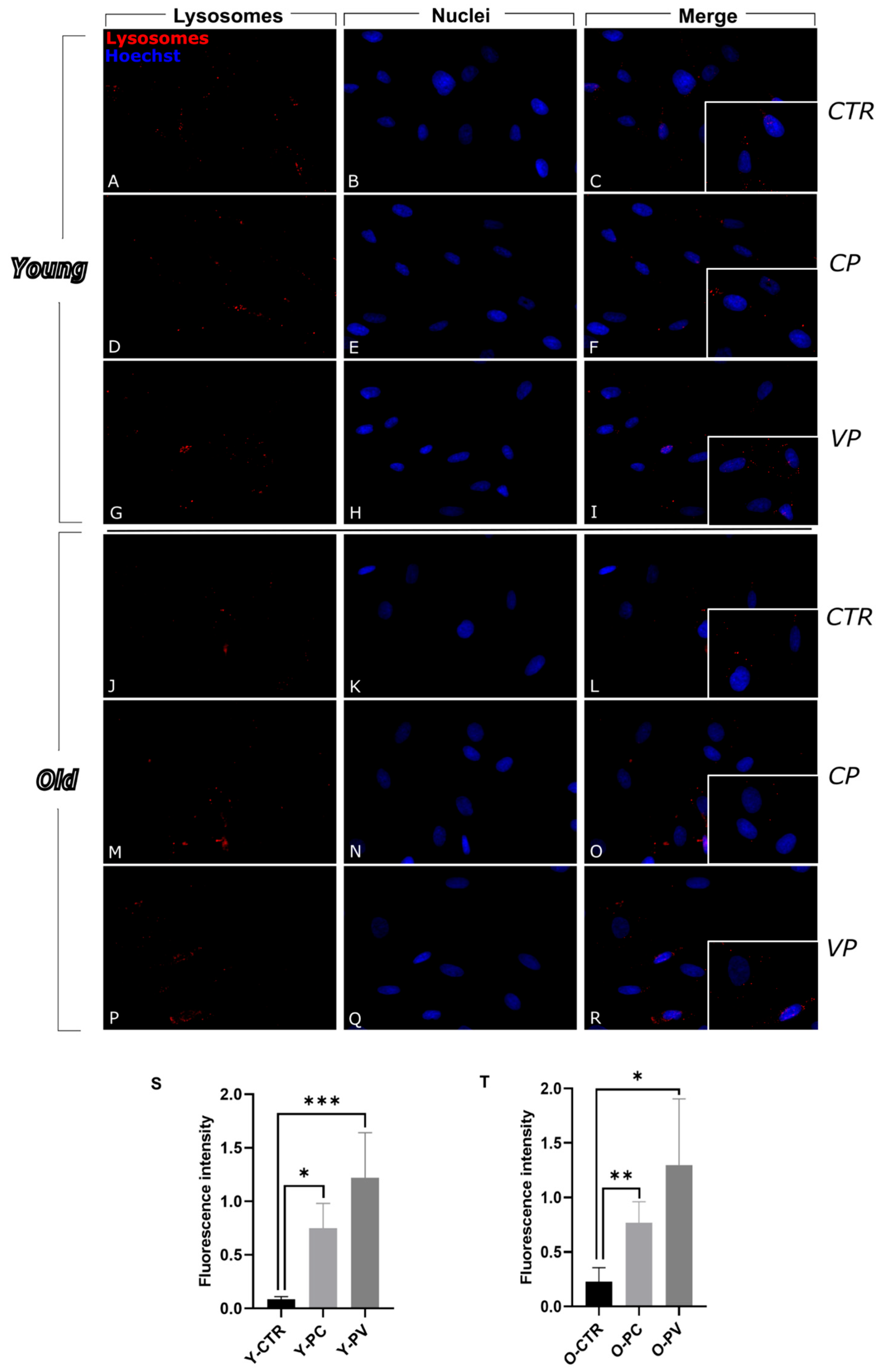
3.1. Modulation of Autophagy
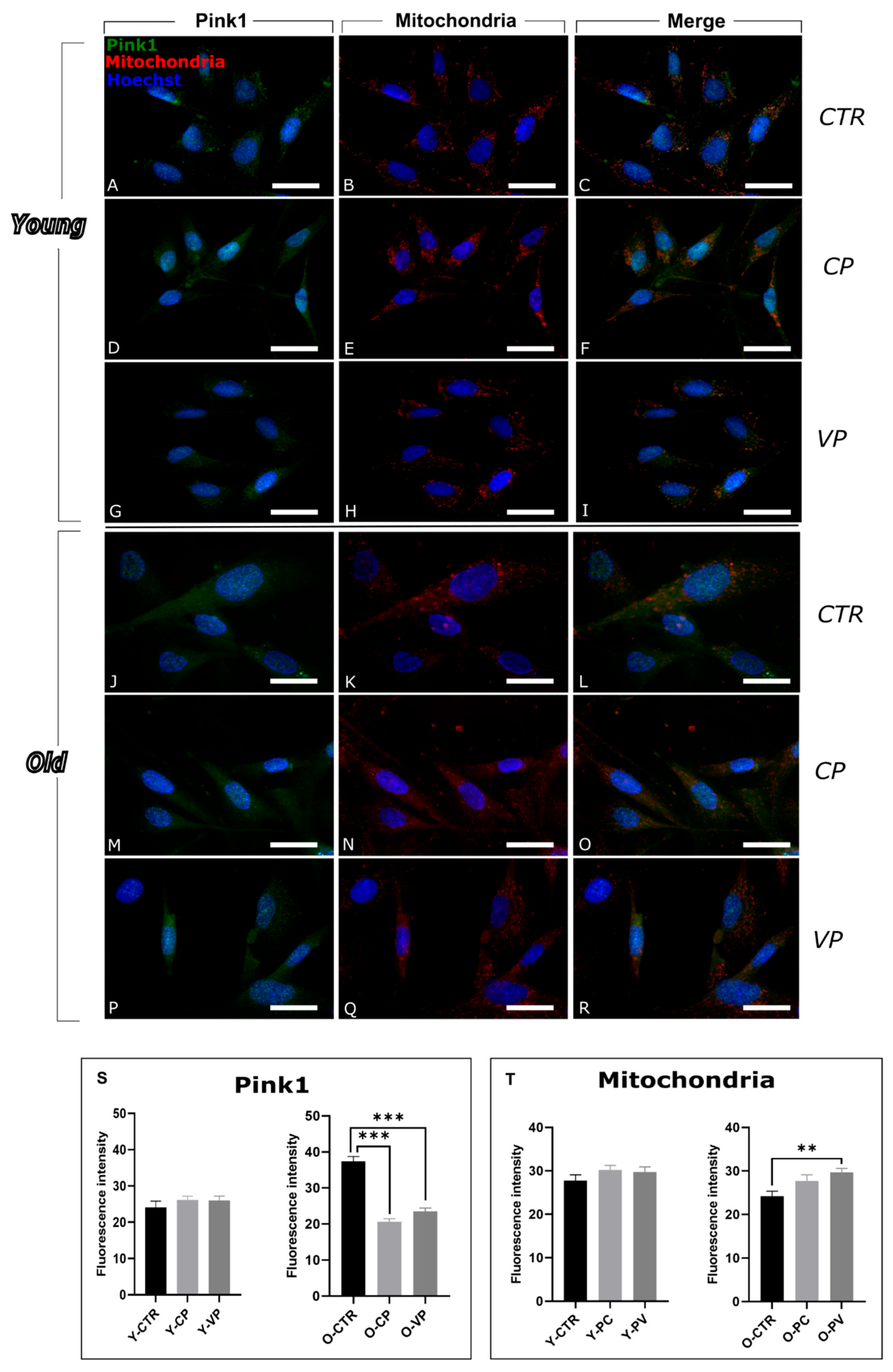
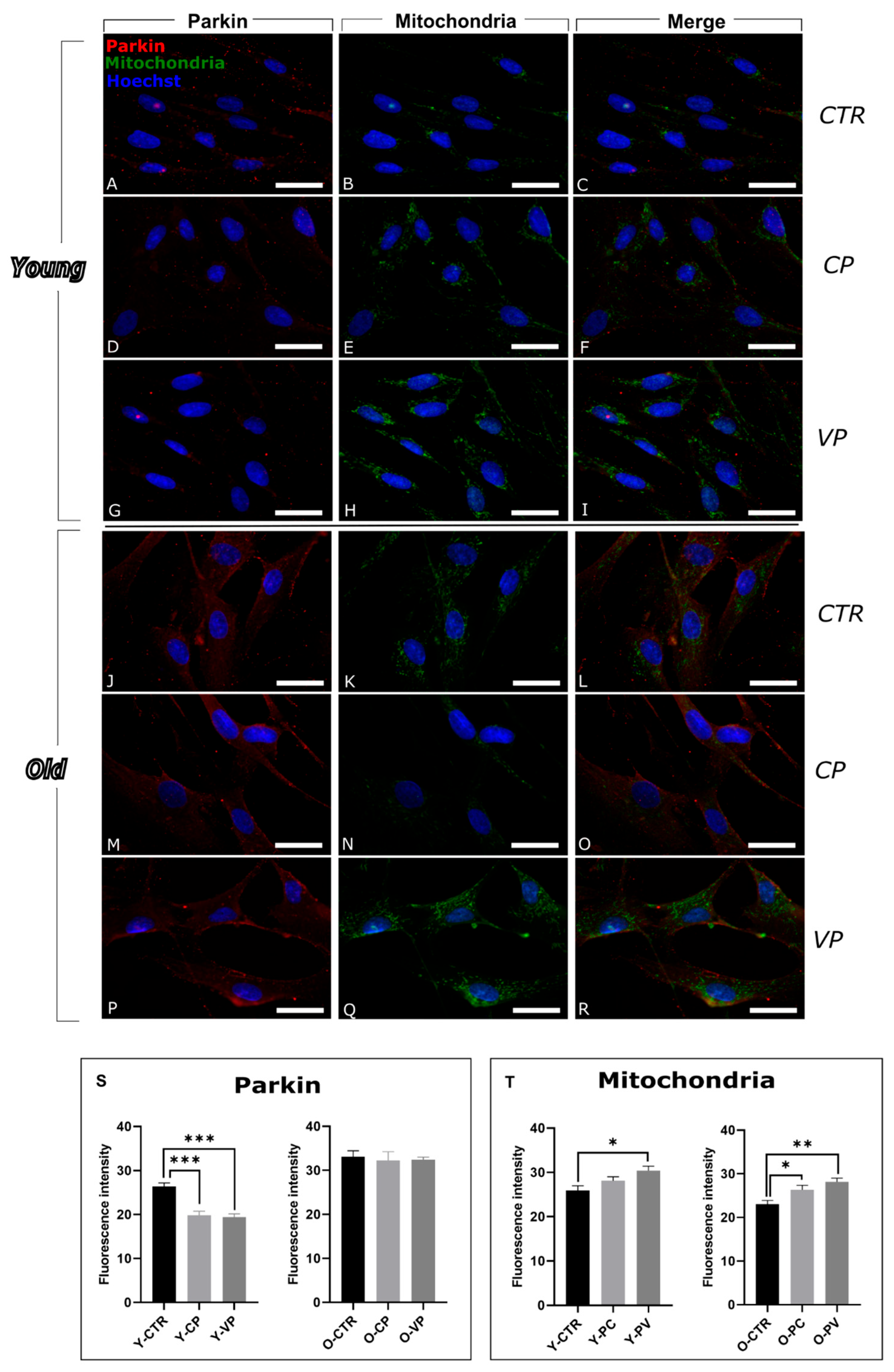
3.2. Modulation of Mitophagy
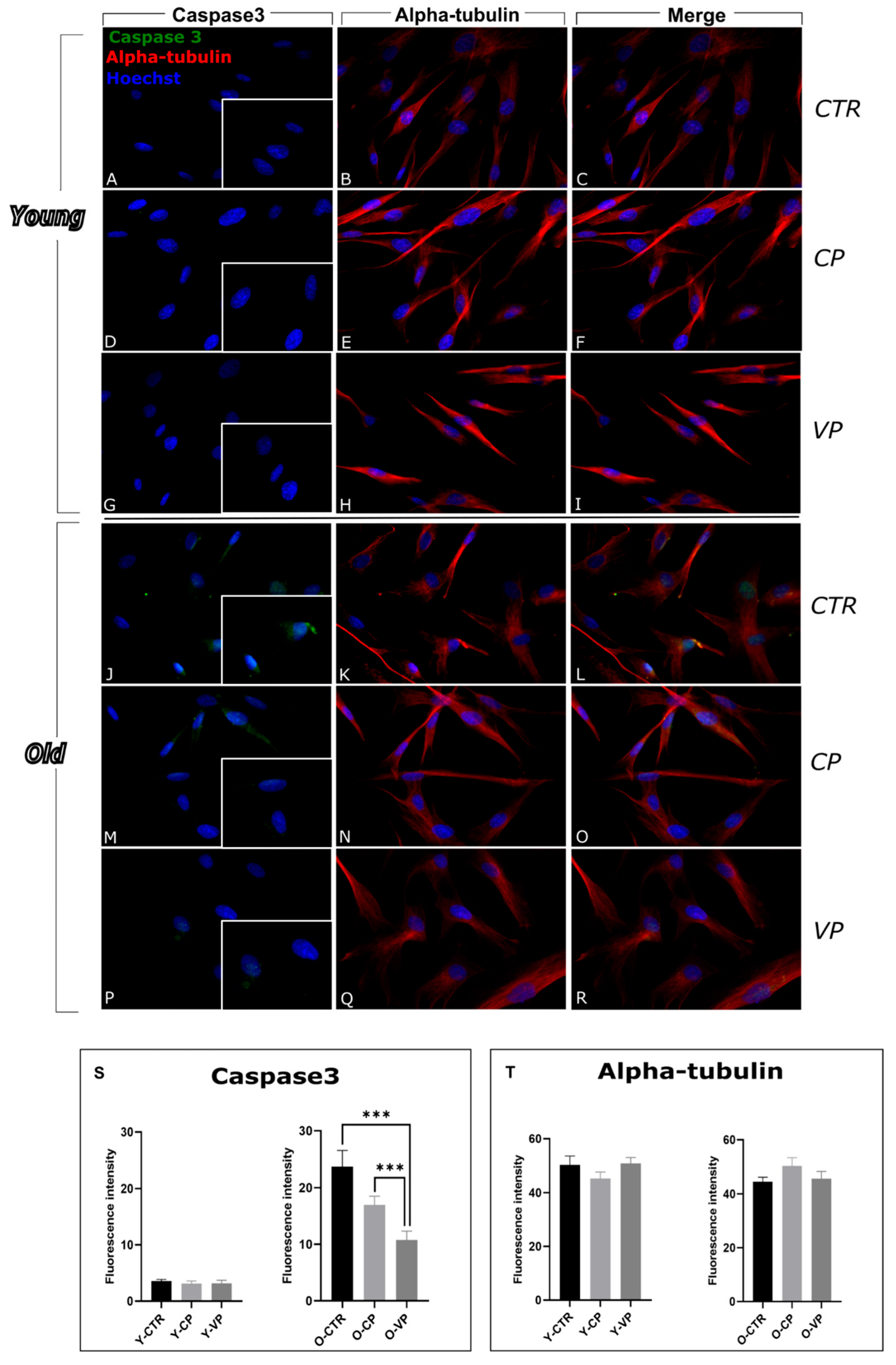
3.3. Inhibition of Apoptosis
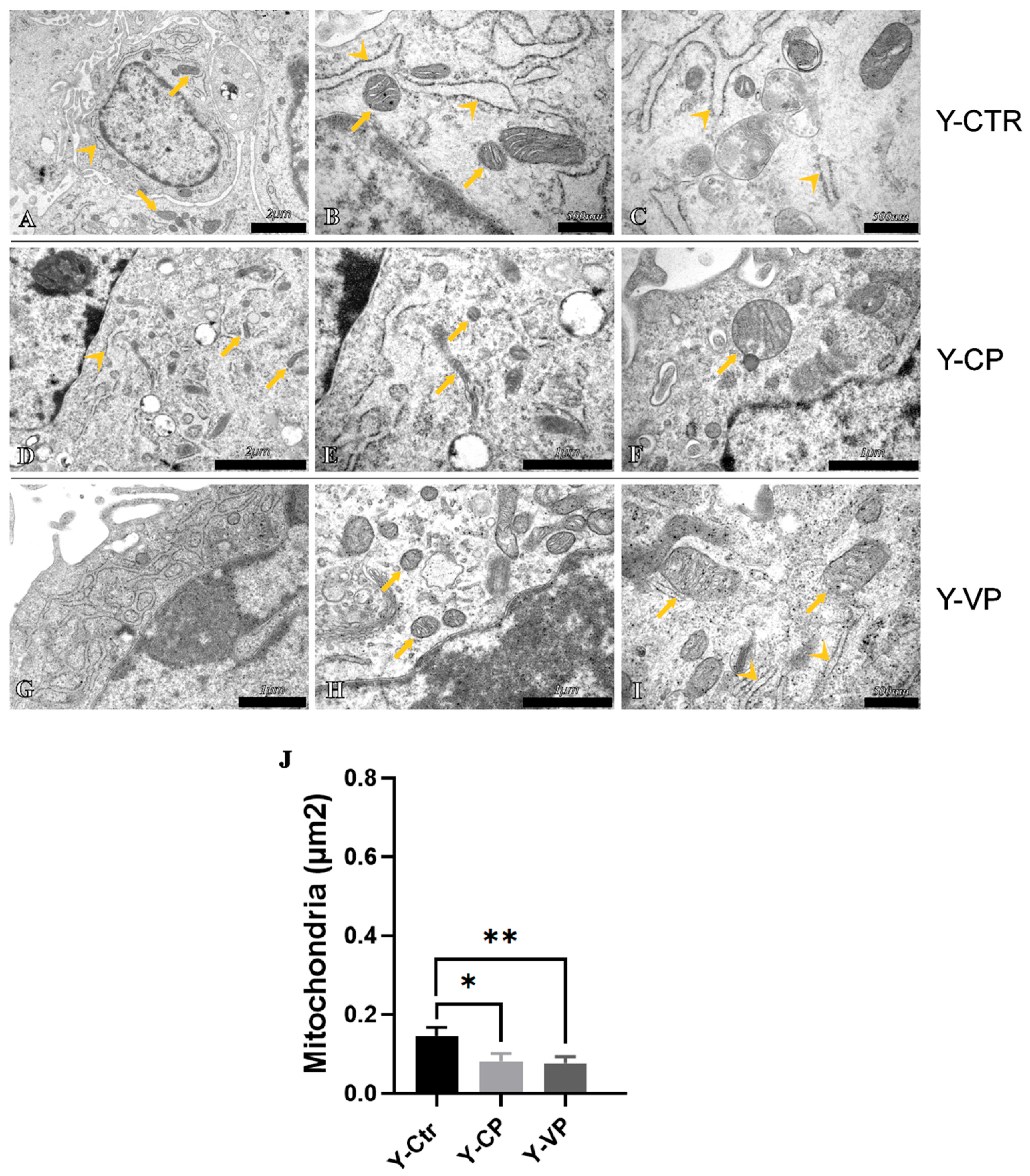
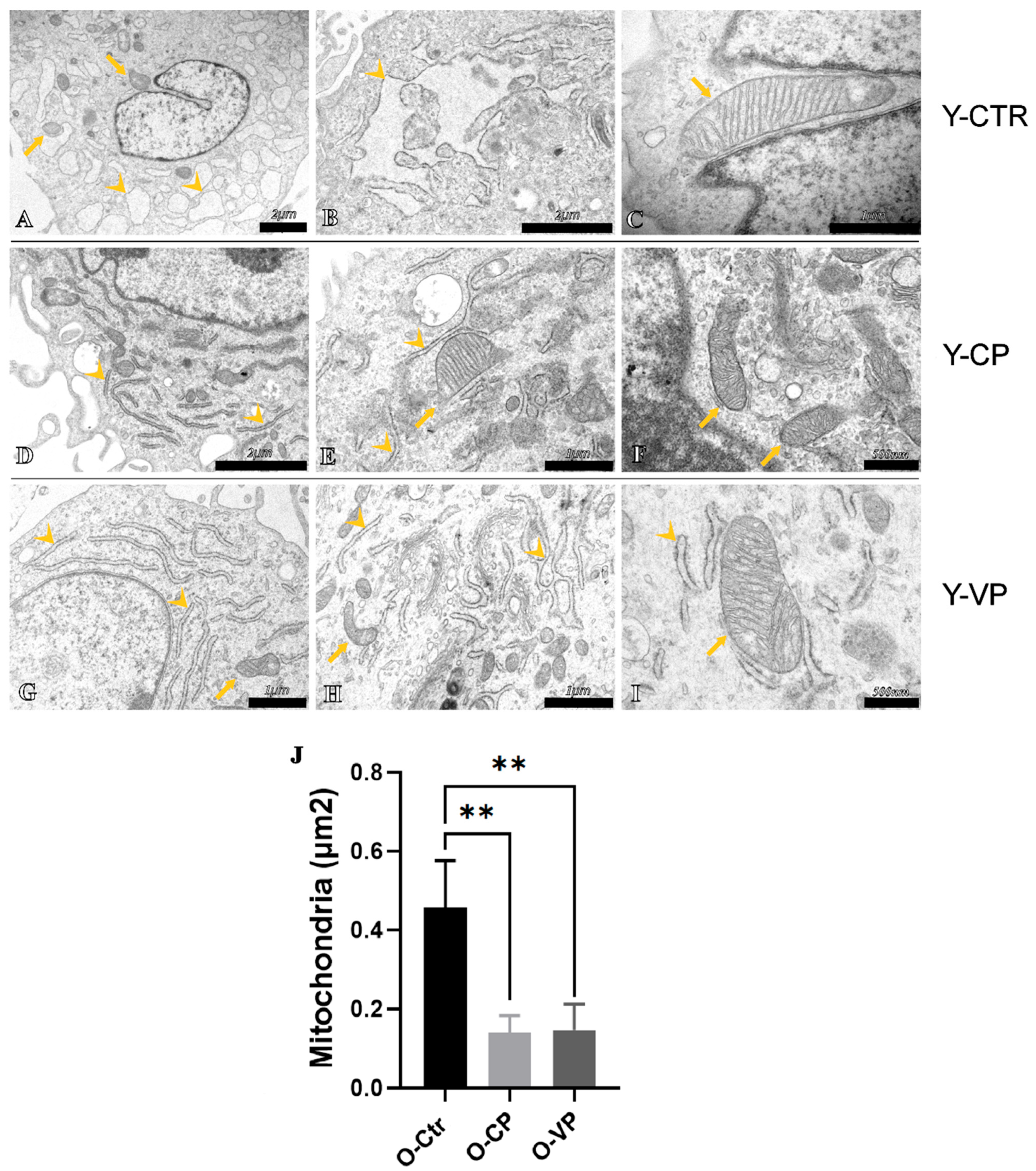
3.4. Ultrastructural Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; Cabo, R. Measuring Biological Aging in Humans: A Quest. Aging Cell 2020, 19, e13080. [Google Scholar] [CrossRef]
- Mechchate, H.; El Allam, A.; El Omari, N.; El Hachlafi, N.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Bouyahya, A. Vegetables and Their Bioactive Compounds as Anti-Aging Drugs. Molecules 2022, 27, 2316. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Roda, E.; Luca, F.; Locatelli, C.A.; Ratto, D.; Di Iorio, C.; Savino, E.; Bottone, M.G.; Rossi, P. From a Medicinal Mushroom Blend a Direct Anticancer Effect on Triple-Negative Breast Cancer: A Preclinical Study on Lung Metastases. Molecules 2020, 25, 5400. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Gillet, G.; Popgeorgiev, N. Caspases in the Developing Central Nervous System: Apoptosis and Beyond. Front. Cell Dev. Biol. 2021, 9, 702404. [Google Scholar] [CrossRef]
- Li, X.L.; Xu, G.; Chen, T.; Wong, Y.S.; Zhao, H.L.; Fan, R.R.; Gu, X.M.; Tong, P.C.; Chan, J.C. Phycocyanin Protects INS-1E Pancreatic Beta Cells against Human Islet Amyloid Polypeptide-Induced Apoptosis through Attenuating Oxidative Stress and Modulating JNK and p38 Mitogen-Activated Protein Kinase Pathways. Int. J. Biochem. Cell Biol. 2009, 41, 1526–1535. [Google Scholar] [CrossRef]
- Seo, J.Y.; Masumune, A.; Shimosegawa, T.; Kim, H. Protective Effect of Lycopene on Oxidative Stress-Induced Cell Death of Pancreatic Acinar Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 570–575. [Google Scholar] [CrossRef]
- Xu, S.; Yang, P.; Qian, K.; Li, Y.; Guo, Q.; Wang, P.; Meng, R.; Wu, J.; Cao, J.; Cheng, Y. Modulating Autophagic Flux via ROS-Responsive Targeted Micelles to Restore Neuronal Proteostasis in Alzheimer’s Disease. Bioact. Mater. 2022, 11, 300–316. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, B.; Sun, J.; Song, J.; Nie, L.; Zhu, K. Fraxetin Suppresses Reactive Oxygen Species-Dependent Autophagy by the PI3K/AKT Pathway to Inhibit Isoflurane-Induced Neurotoxicity in Hippocampal Neuronal Cells. J. Appl. Toxicol. 2022, 42, 617–628. [Google Scholar] [CrossRef]
- Demirovic, D.; Nizard, C.; Rattan, S.I. Basal Level of Autophagy Is Increased in Aging Human Skin Fibroblasts In Vitro, but Not in Old Skin. PLoS ONE 2015, 10, e0126546. [Google Scholar] [CrossRef]
- Bakac, E.R.; Percin, E.; Gunes-Bayir, A.; Dadak, A. A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases. Int. J. Mol. Sci. 2023, 24, 15199. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources. Biomolecules 2023, 13, 1600. [Google Scholar] [CrossRef]
- De Luca, F.; Roda, E.; Rossi, P.; Bottone, M.G. Medicinal Mushrooms in Metastatic Breast Cancer: What Is Their Therapeutic Potential as Adjuvant in Clinical Settings? Curr. Issues Mol. Biol. 2024, 46, 7577–7591. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.R.; Pagé, B. The Genetics of Vitamin C Loss in Vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Shehata, W.F.; Hassan, U.; Shah, S.T.; Haleema, B.; Jalal, A.; Amin, R.; Khalid, M.A.; et al. Ascorbic Acid Enhances Growth and Yield of Sweet Peppers (Capsicum annuum) by Mitigating Salinity Stress. Gesunde Pflanz. 2022, 74, 423–433. [Google Scholar] [CrossRef]
- Fratianni, F.; d’Acierno, A.; Cozzolino, A.; Spigno, P.; Riccardi, R.; Raimo, F.; Pane, C.; Zaccardelli, M.; Tranchida Lombardo, V.; Tucci, M.; et al. Biochemical Characterization of Traditional Varieties of Sweet Pepper (Capsicum annuum L.) of the Campania Region, Southern Italy. Antioxidants 2020, 9, 556. [Google Scholar] [CrossRef]
- Rossi, G.; Guzzon, F.; Canella, M.; Tazzari, E.R.; Cauzzi, P.; Bodino, S.; Ardenghi, N.M.G. Le varietà agronomiche lombarde tradizionali a rischio di estinzione o di erosione genetica. In Ortive e Cerealicole: Uno Sguardo D’insieme; Pavia University Press: Lombardia, Italy, 2019. [Google Scholar]
- Gola, F.; Gaiaschi, L.; Roda, E.; De Luca, F.; Ferulli, F.; Vicini, R.; Rossi, P.; Bottone, M.G. Voghera Sweet Pepper: A Potential Ally against Oxidative Stress and Aging. Int. J. Mol. Sci. 2023, 24, 3782. [Google Scholar] [CrossRef]
- De Luca, F.; Gola, F.; Azzalin, A.; Casali, C.; Gaiaschi, L.; Milanesi, G.; Vicini, R.; Rossi, P.; Bottone, M.G. A Lombard Variety of Sweet Pepper Regulating Senescence and Proliferation: The Voghera Pepper. Nutrients 2024, 16, 1681. [Google Scholar] [CrossRef]
- Somwong, P.; Kamkaen, N. Wound-healing activity and quantification of bioactive compounds from Derris scandens extract. J. Adv. Pharm. Technol. Res. 2022, 13, 38–43. [Google Scholar] [CrossRef]
- Sinjari, B.; Diomede, F.; Murmura, G.; Traini, T.; Merciaro, I.; Trubiani, O.; Caputi, S. A cytotoxic analysis of a sardinian plant extract cream on human oral primary cell cultures: An in vitro study. J. Biol. Regul. Homeost. Agents 2015, 29, 103–113. [Google Scholar] [PubMed]
- Ratto, D.; Ferrari, B.; Roda, E.; Brandalise, F.; Siciliani, S.; De Luca, F.; Priori, E.C.; Di Iorio, C.; Cobelli, F.; Veneroni, P.; et al. Squaring the Circle: A New Study of Inward- and Outward-Rectifying Potassium Currents in U251 GBM Cells. Cell. Mol. Neurobiol. 2020, 40, 813–828. [Google Scholar] [CrossRef]
- Ding, W.X.; Yin, X.M. Mitophagy: Mechanisms, Pathophysiological Roles, and Analysis. Biol. Chem. 2012, 393, 547–564. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and Medical Implications of Mammalian Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- He, C.; Klionsky, D.J. Regulation Mechanisms and Physiological Roles of Autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and Aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2013, 332, 1429–1433. [Google Scholar] [CrossRef]
- Tan, J.X.; Finkel, T. Lysosomes in Senescence and Aging. EMBO Rep. 2023, 24, e57265. [Google Scholar] [CrossRef]
- Minami, S.; Yamamoto, T.; Yamamoto-Imoto, H.; Isaka, Y.; Hamasaki, M. Autophagy in Kidney Aging. Prog. Biophys. Mol. Biol. 2023, 179, 10–15. [Google Scholar] [CrossRef]
- Kroemer, G.; Jaattela, M. Lysosomes and Autophagy in Cell Death Control. Nat. Rev. Cancer 2005, 5, 886–897. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms of Mitochondria. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, Mitochondria, and Oxidative Stress: Cross-Talk and Redox Signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 Network Regulates Autophagy and Apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Gao, X.M.; Dong, W.H.; Xia, C.L.; Ma, Z.Y.; Wang, Y.; Sarra, S.; Xu, H.M.; Qi, W.Y. Centranthera grandiflore Alleviates Alcohol-Induced Oxidative Stress and Cell Apoptosis. Chin. J. Nat. Med. 2022, 20, 572–579. [Google Scholar] [CrossRef]
- Pérez, M.; Avila, J. The Role of the Cytoskeleton in Neurodegenerative Diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1102–1115. [Google Scholar]
- Georgakopoulou, E.A.; Tsimaratou, K.; Evangelou, K.; Havaki, S. Central Role of the Cytoskeleton in Cellular Senescence and Aging. Biogerontology 2013, 14, 109–129. [Google Scholar]
- Li, A.; Shami, G.J.; Griffiths, L.; Lal, S.; Irving, H.; Braet, F. Giant Mitochondria in Cardiomyocytes: Cellular Architecture in Health and Disease. Basic Res. Cardiol. 2023, 118, 39. [Google Scholar] [CrossRef]
- Cheville, N.F. Ultrastructural Pathology: An Introduction to Interpretation, 1st ed.; Wiley: New York, NY, USA, 1994; pp. 129–133. [Google Scholar]
- Shang, Y.; Li, Z.; Cai, P.; Li, W.; Xu, Y.; Zhao, Y.; Xia, S.; Shao, Q.; Wang, H. Megamitochondria Plasticity: Function Transition from Adaption to Disease. Mitochondrion 2023, 71, 64–75. [Google Scholar] [CrossRef]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the Autophagy-Inflammation-Cell Death Axis in Organismal Aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef]
- Frank, M.; Duvezin-Caubet, S.; Koob, S.; Occhipinti, A.; Jagasia, R.; Petcherski, A.; Ruonala, M.O.; Priault, M.; Salin, B.; Reichert, A.S. Mitophagy Is Triggered by Mild Oxidative Stress in a Mitochondrial Fission-Dependent Manner. Biochim. Biophys. Acta 2012, 1823, 2297–2310. [Google Scholar] [CrossRef]
- Tatsuta, T.; Langer, T. Quality Control of Mitochondria: Protection against Neurodegeneration and Aging. EMBO J. 2008, 27, 306–314. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Nie, M.M.; Liu, C.Q.; Jiang, N.; Liu, C.J.; Li, D.J. Citrus Flavanones Enhance beta-Carotene Uptake in Vitro Experiment Using Caco-2 Cell: Structure-Activity Relationship and Molecular Mechanisms. J. Agric. Food Chem. 2019, 67, 4280–4288. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; McGarvey, D.J.; Truscott, T.G. Beta-carotene with vitamins E and C offers synergistic cell protection against NOx. FEBS Lett. 1998, 436, 387–389. [Google Scholar] [CrossRef]
- Niki, E.; Noguchi, N.; Tsuchihashi, H.; Gotoh, N. Interaction among vitamin C, vitamin E, and beta-carotene. Am. J. Clin. Nutr. 1995, 62 (Suppl. S6), 1322S–1326S. [Google Scholar] [CrossRef]
| Antigen | Primary Antibody | Dilution |
|---|---|---|
| P62 | Mouse monoclonal anti-SQQTM1/p62 (Abcam, Cambridge, UK) | 1:250 |
| LC3B | Rabbit polyclonal anti-LC3B (Cell Signaling Technology, Danvers, MA, USA) | 1:400 |
| Pink1 | Rabbit polyclonal anti-PINK1 (Abcam, Cambridge, UK) | 1:500 |
| Parkin | Rabbit polyclonal anti-Parkin (Abcam, Cambridge, UK) | 1:500 |
| Cleaved-caspase3 | Mouse monoclonal anti-Casp3 (Cell Signaling Technology, Danvers, MA, USA) | 1:200 |
| Lysosomes | Human autoimmune serum recognizing lysosomal proteins | 1:400 |
| Mitochondria | Human autoimmune serum recognizing the 70 kDa E2 subunit of pyruvate dehydrogenase complex | 1:300 |
| Beta-actin | Rabbit polyclonal anti-β-actin (GeneTex, Irvine, CA, USA) | 1:300 |
| Alpha-tubulin | Mouse monoclonal anti-α-tubulin (Cell Signaling Technology, Danvers, MA, USA) | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gola, F.; Casali, C.; Gaiaschi, L.; Roda, E.; Milanesi, G.; De Luca, F.; Bottone, M.G. Voghera Sweet Pepper Regulates Cell Death Pathways in an Aging In Vitro Model. Nutrients 2025, 17, 2147. https://doi.org/10.3390/nu17132147
Gola F, Casali C, Gaiaschi L, Roda E, Milanesi G, De Luca F, Bottone MG. Voghera Sweet Pepper Regulates Cell Death Pathways in an Aging In Vitro Model. Nutrients. 2025; 17(13):2147. https://doi.org/10.3390/nu17132147
Chicago/Turabian StyleGola, Federica, Claudio Casali, Ludovica Gaiaschi, Elisa Roda, Gloria Milanesi, Fabrizio De Luca, and Maria Grazia Bottone. 2025. "Voghera Sweet Pepper Regulates Cell Death Pathways in an Aging In Vitro Model" Nutrients 17, no. 13: 2147. https://doi.org/10.3390/nu17132147
APA StyleGola, F., Casali, C., Gaiaschi, L., Roda, E., Milanesi, G., De Luca, F., & Bottone, M. G. (2025). Voghera Sweet Pepper Regulates Cell Death Pathways in an Aging In Vitro Model. Nutrients, 17(13), 2147. https://doi.org/10.3390/nu17132147









