Safety and Tolerance of Bifidobacterium longum subsp. Infantis YLGB-1496 in Toddlers with Respiratory Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes and Measurements
2.2.1. Questionnaires
- (a)
- Sociodemographic questionnaire.
- (b)
- Stool characteristics: Stool consistency (evaluated using the Bristol Stool Scale (BSS)) and frequency.
- (c)
- Gastrointestinal health: Frequency of adverse gastrointestinal symptoms (poor appetite, nausea, vomiting, stomachache, anal discomfort, diarrhea, and dehydration).
- (d)
- Dietary intake: Consumption rates of breast milk, formula, yogurt, other dairy products, grains and tubers, vegetables, fruits, protein sources (meat, eggs, and seafood), beverages, snacks, and various nutritional supplements.
- (e)
- Adverse events (AEs) and serious adverse events (SAEs).
2.2.2. Body Measurements
2.2.3. Sample Collection and Analysis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Growth and Development
3.3. Defecation Characteristics
3.4. Gastrointestinal Symptoms
3.5. Biochemical Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, I.; Schofield, Z.; Hall, L.J. Exploring the role of the microbiota member Bifidobacterium in modulating immune-linked diseases. Emerg. Top. Life Sci. 2017, 1, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Bajorek, S.; Duar, R.M.; Corrigan, M.; Matrone, C.; Winn, K.A.; Norman, S.; Mitchell, R.D.; Cagney, O.; Aksenov, A.A.; Melnik, A.V.; et al. B. infantis EVC001 Is Well-Tolerated and Improves Human Milk Oligosaccharide Utilization in Preterm Infants in the Neonatal Intensive Care Unit. Front. Pediatr. 2021, 9, 795970. [Google Scholar] [CrossRef] [PubMed]
- Chichlowski, M.; Shah, N.; Wampler, J.L.; Wu, S.S.; Vanderhoof, J.A. Bifidobacterium longum Subspecies infantis (B. infantis) in Pediatric Nutrition: Current State of Knowledge. Nutrients 2020, 12, 1581. [Google Scholar] [CrossRef]
- Moreno-Muñoz, J.A.; Martín-Palomas, M.; Jiménez López, J. Bifidobacterium longum subsp. infantis CECT 7210 (B. infantis IM-1®) shows activity against intestinal pathogens. Nutr. Hosp. 2022, 39, 65–68. [Google Scholar] [CrossRef]
- Barratt, M.J.; Nuzhat, S.; Ahsan, K.; Frese, S.A.; Arzamasov, A.A.; Sarker, S.A.; Islam, M.M.; Palit, P.; Islam, M.R.; Hibberd, M.C.; et al. Bifidobacterium infantis treatment promotes weight gain in Bangladeshi infants with severe acute malnutrition. Sci. Transl. Med. 2022, 14, eabk1107. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Lamas, A.; Del Carmen Mondragón, A.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic Effects against Virus Infections: New Weapons for an Old War. Foods 2021, 10, 130. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Diaz, H.; Meddings, L.; Diederichs, B.; Dmytrash, A.; Backer, J.; Looijer-van Langen, M.; Madsen, K.L. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1025–G1034. [Google Scholar] [CrossRef]
- Delacour, D.; Salomon, J.; Robine, S.; Louvard, D. Plasticity of the brush border—The yin and yang of intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 161–174. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Castanet, M.; Costalos, C.; Haiden, N.; Hascoet, J.M.; Berger, B.; Sprenger, N.; Grathwohl, D.; Brüssow, H.; De Groot, N.; Steenhout, P.; et al. Early Effect of Supplemented Infant Formulae on Intestinal Biomarkers and Microbiota: A Randomized Clinical Trial. Nutrients 2020, 12, 1481. [Google Scholar] [CrossRef]
- Henrick, B.M.; Chew, S.; Casaburi, G.; Brown, H.K.; Frese, S.A.; Zhou, Y.; Underwood, M.A.; Smilowitz, J.T. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr. Res. 2019, 86, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Ferre, N.; Gispert-Llaurado, M.; Luque, V.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Polanco, I.; Codoner, F.M.; Chenoll, E.; Morera, M.; et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: A randomized controlled trial. Pediatr. Res. 2018, 83, 1120–1128. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Moya, J.; Breck, M.A.; Cook, C.; Fineberg, A.; Angkustsiri, K.; Underwood, M.A. Safety and tolerability of Bifidobacterium longum subspecies infantis EVC001 supplementation in healthy term breastfed infants: A phase I clinical trial. BMC Pediatr. 2017, 17, 133. [Google Scholar] [CrossRef]
- Li, P.; Mageswary, U.; Ali, A.; Taib, F.; Koo, T.H.; Yusof, A.; Jiang, H.; Lan, H.; Hung, W.; Liong, M.T.; et al. Clinical effects of Bifidobacterium longum Subsp. infantis YLGB-1496 on children with respiratory symptoms. Front. Nutr. 2025, 12, 1537610. [Google Scholar] [CrossRef]
- Capeding, M.R.Z.; Phee, L.C.M.; Ming, C.; Noti, M.; Vidal, K.; Le Carrou, G.; Frézal, A.; Moll, J.M.; Vogt, J.K.; Myers, P.N.; et al. Safety, efficacy, and impact on gut microbial ecology of a Bifidobacterium longum subspecies infantis LMG11588 supplementation in healthy term infants: A randomized, double-blind, controlled trial in the Philippines. Front. Nutr. 2023, 10, 1319873. [Google Scholar] [CrossRef]
- Lau, A.S.Y.; Yusoff, M.S.B.; Lee, Y.Y.; Choi, S.B.; Rashid, F.; Wahid, N.; Xiao, J.Z.; Liong, M.T.; School of Industrial Technology; Universiti Sains Malaysia Pulau Pinang Malaysia; et al. Development, translation and validation of questionnaires for diarrhea and respiratory-related illnesses during probiotic administration in children. Educ. Med. J. 2017, 9, 19–30. [Google Scholar] [CrossRef]
- Frese, S.A.; Hutton, A.A.; Contreras, L.N.; Shaw, C.A.; Palumbo, M.C.; Casaburi, G.; Xu, G.; Davis, J.C.C.; Lebrilla, C.B.; Henrick, B.M.; et al. Persistence of Supplemented Bifidobacterium longum subsp. infantis EVC001 in Breastfed Infants. mSphere 2017, 2, e00501–e00517. [Google Scholar] [CrossRef] [PubMed]
- Larke, J.A.; Kuhn-Riordon, K.; Taft, D.H.; Sohn, K.; Iqbal, S.; Underwood, M.A.; Mills, D.A.; Slupsky, C.M. Preterm Infant Fecal Microbiota and Metabolite Profiles Are Modulated in a Probiotic Specific Manner. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 535–542. [Google Scholar] [CrossRef]
- Ding, M.; Li, B.; Chen, H.; Liang, D.; Ross, R.P.; Stanton, C.; Zhao, J.; Chen, W.; Yang, B. Human breastmilk-derived Bifidobacterium longum subsp. infantis CCFM1269 regulates bone formation by the GH/IGF axis through PI3K/AKT pathway. Gut Microbes 2023, 16, 2290344. [Google Scholar] [CrossRef]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Stool microbiota and vaccine responses of infants. Pediatrics 2014, 134, e362–e372. [Google Scholar] [CrossRef]
- Guo, H.; Fan, M.; Hou, T.; Li, Y.; Wang, S.; Wang, X.; Peng, H.; Wang, M.; Wu, T.; Zhang, Y. Efficacy and Safety of Bifidobacterium longum Supplementation in Infants: A Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 4451. [Google Scholar] [CrossRef]
- Nuzhat, S.; Hasan, S.M.T.; Palit, P.; Islam, M.R.; Mahfuz, M.; Islam, M.M.; Alam, M.A.; Flannery, R.L.; Kyle, D.J.; Sarker, S.A.; et al. Effects of probiotic and synbiotic supplementation on ponderal and linear growth in severely malnourished young infants in a randomized clinical trial. Sci. Rep. 2023, 13, 1845. [Google Scholar] [CrossRef]
- Manzano, S.; De Andres, J.; Castro, I.; Rodriguez, J.M.; Jimenez, E.; Espinosa-Martos, I. Safety and tolerance of three probiotic strains in healthy infants: A multi-centre randomized, double-blind, placebo-controlled trial. Benef. Microbes 2017, 8, 569–578. [Google Scholar] [CrossRef]
- Dimitratos, S.M.; Brown, H.; Shafizadeh, T.; Kazi, S.; Altmann, T.; Ostrer, B. Symptomatic relief from at-home use of activated Bifidobacterium infantis EVC001 probiotic in infants: Results from a consumer survey on the effects on diaper rash, colic symptoms, and sleep. Benef. Microbes 2021, 12, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Rivero, M.; Grillon, C.; Belaroussi, N.; Kalindjian, A.; Marin, V. Alpha-lactalbumin-enriched and probiotic-supplemented infant formula in infants with colic: Growth and gastrointestinal tolerance. Eur. J. Clin. Nutr. 2010, 64, 765–767. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561, quiz 1546, 1562. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Goldenberg, J.Z.; Humphrey, C.; El Dib, R.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2019, 4, Cd004827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Zhang, J.; Sun, F.; Duan, L. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 859967. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, J.; Stanton, C.; Ross, R.P.; Zhao, J.; Yang, B.; Chen, W. Bifidobacterium longum subsp. infantis FJSYZ1M3 ameliorates DSS-induced colitis by maintaining the intestinal barrier, regulating inflammatory cytokines, and modifying gut microbiota. Food Funct. 2023, 14, 354–368. [Google Scholar] [CrossRef]
- Li, Y.; Hintze, K.J.; Ward, R.E. Effect of supplemental prebiotics, probiotics and bioactive proteins on the microbiome composition and fecal calprotectin in C57BL6/j mice. Biochimie 2021, 185, 43–52. [Google Scholar] [CrossRef]
- Fornai, M.; Pellegrini, C.; Benvenuti, L.; Tirotta, E.; Gentile, D.; Natale, G.; Ryskalin, L.; Colucci, R.; Piccoli, E.; Ghelardi, E.; et al. Protective effects of the combination Bifidobacterium longum plus lactoferrin against NSAID-induced enteropathy. Nutrition 2020, 70, 110583. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, M.; Pont-Beltran, A.; Lamelas, A.; Llobregat, L.; Martinez-Blanch, J.F.; Rojas, A.; Álvarez, B.; López Plaza, B.; Arcos Castellanos, L.; Chenoll, E.; et al. Effect of Postbiotic Bifidobacterium longum CECT 7347 on Gastrointestinal Symptoms, Serum Biochemistry, and Intestinal Microbiota in Healthy Adults: A Randomised, Parallel, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2024, 16, 3952. [Google Scholar] [CrossRef]
- Viljanen, M.; Kuitunen, M.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Savilahti, E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2005, 16, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Oswari, H.; Prayitno, L.; Dwipoerwantoro, P.G.; Firmansyah, A.; Makrides, M.; Lawley, B.; Kuhn-Sherlock, B.; Cleghorn, G.; Tannock, G.W. Comparison of stool microbiota compositions, stool alpha1-antitrypsin and calprotectin concentrations, and diarrhoeal morbidity of Indonesian infants fed breast milk or probiotic/prebiotic-supplemented formula. J. Paediatr. Child Health 2013, 49, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- El-Saied, S.; Amar, A.; Kaplan, D.M.; Shitrit, R.; Kaminer, B.M.; Keshet, A.; Lewis, E.C. Local Alpha1-Antitrypsin Accelerates the Healing of Tympanic Membrane Perforation in Mice. Laryngoscope 2024, 134, 3802–3806. [Google Scholar] [CrossRef]
- Schukfeh, N.; Sivaraman, K.; Schmidt, A.; Vieten, G.; Dingemann, J.; Weidner, J.; Olmer, R.; Janciauskiene, S. Alpha-1-antitrypsin improves anastomotic healing in intestinal epithelial cells model. Pediatr. Surg. Int. 2024, 40, 258. [Google Scholar] [CrossRef]

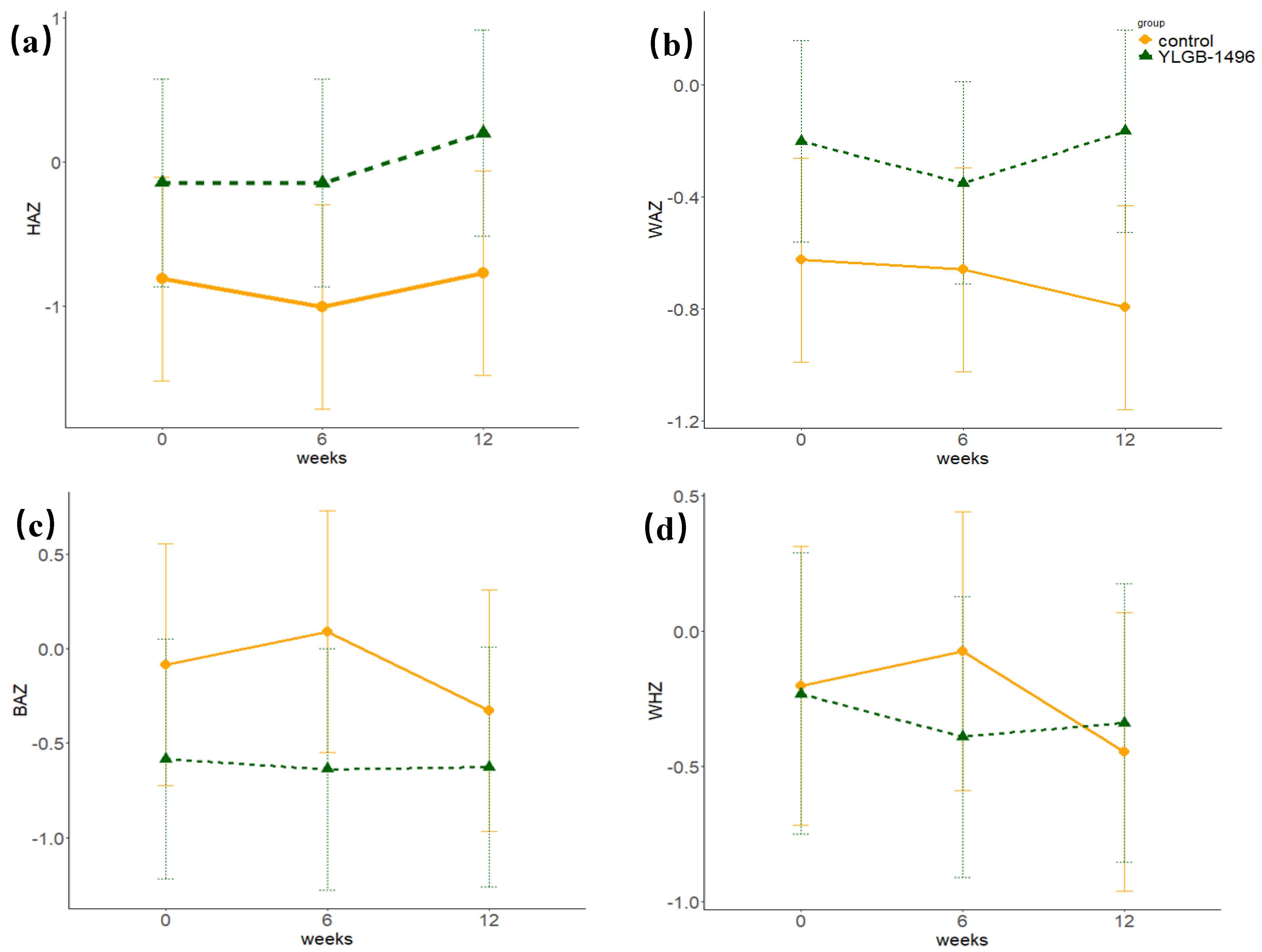

| Variables | Control | YLGB-1496 | p a | Model b | p c | ||
|---|---|---|---|---|---|---|---|
| Week | Group | Week × Group | |||||
| HAZ | |||||||
| Baseline | −0.81 ± 2.78 | −0.14 ± 2.49 | 0.184 | 1 | 0.007 | 0.165 | 0.820 |
| 6 weeks | −1.00 ± 2.91 | −0.14 ± 2.48 | 0.096 | 2 | 0.007 | 0.075 | |
| 12 weeks | −0.77 ± 3.01 | 0.20 ± 2.58 | 0.069 | 3 | 0.007 | 0.006 | |
| WAZ | |||||||
| Baseline | −0.63 ± 1.54 | −0.20 ± 1.48 | 0.135 | 1 | 0.194 | 0.162 | 0.029 |
| 6 weeks | −0.66 ± 1.37 | −0.35 ± 1.38 | 0.230 | 2 | 0.194 | 0.243 | |
| 12 weeks | −0.80 ± 1.35 | −0.17 ± 1.24 | 0.010 | 3 | 0.194 | 0.329 | |
| BAZ | |||||||
| Baseline | −0.08 ± 2.12 | −0.58 ± 2.66 | 0.267 | 1 | 0.382 | 0.514 | 0.068 |
| 6 weeks | 0.09 ± 2.36 | −0.64 ± 2.52 | 0.116 | 2 | 0.379 | 0.414 | |
| 12 weeks | −0.33 ± 2.55 | −0.63 ± 2.46 | 0.524 | 3 | 0.380 | 0.194 | |
| WHZ | |||||||
| Baseline | −0.20 ± 1.89 | −0.23 ± 1.95 | 0.937 | 1 | 0.126 | 0.620 | 0.097 |
| 6 weeks | −0.07 ± 2.00 | −0.39 ± 1.92 | 0.397 | 2 | 0.126 | 0.530 | |
| 12 weeks | −0.44 ± 2.14 | −0.34 ± 1.84 | 0.781 | 3 | 0.126 | 0.293 |
| Variables | Control | YLGB-1496 | p a | Model b | p c | ||
|---|---|---|---|---|---|---|---|
| Week | Group | Week × Group | |||||
| Defecation frequency (times/day) | |||||||
| Baseline | 1.08 ± 0.82 | 1.37 ± 0.96 | 0.082 | 1 | 0.194 | 0.253 | 0.110 |
| 6 weeks | 1.01 ± 0.72 | 1.34 ± 0.83 | 0.026 | 2 | 0.194 | 0.448 | |
| 12 weeks | 1.02 ± 0.71 | 1.25 ± 0.79 | 0.115 | 3 | 0.194 | 0.211 | |
| Bristol Stool Scale scores | |||||||
| Baseline | 4.07 ± 1.02 | 3.92 ± 0.85 | 0.377 | 1 | 0.214 | 0.018 | 0.942 |
| 6 weeks | 4.07 ± 0.94 | 3.74 ± 0.51 | 0.021 | 2 | 0.214 | 0.073 | |
| 12 weeks | 4.13 ± 1.02 | 3.79 ± 0.47 | 0.024 | 3 | 0.214 | 0.232 | |
| Variables | Weeks | Control | YLGB-1496 | IRR (95% CI) | p a | ||
|---|---|---|---|---|---|---|---|
| N | IR (SE) | N | IR (SE) | ||||
| Poor appetite | 0 | 43 | 0.754 (0.165) | 30 | 0.517 (0.128) | 0.686 (0.426, 1.088) | 0.113 |
| 6 | 43 | 0.754 (0.165) | 6 | 0.103 (0.053) | 0.137 (0.052, 0.298) | <0.001 | |
| 12 | 42 | 0.737 (0.165) | 0 | 0 (0) | —— | —— | |
| Nausea | 0 | 15 | 0.263 (0.073) | 13 | 0.224 (0.065) | 0.852 (0.399, 1.793) | 0.672 |
| 6 | 14 | 0.246 (0.058) | 4 | 0.069 (0.042) | 0.281 (0.080, 0.783) | 0.025 | |
| 12 | 13 | 0.228 (0.056) | 0 | 0 (0) | —— | —— | |
| Vomiting | 0 | 18 | 0.316 (0.076) | 19 | 0.328 (0.131) | 1.037 (0.542, 1.991) | 0.911 |
| 6 | 16 | 0.281 (0.060) | 6 | 0.103 (0.047) | 0.369 (0.132, 0.896) | 0.037 | |
| 12 | 15 | 0.263 (0.059) | 2 | 0.034 (0.024) | 0.131 (0.021, 0.464) | 0.007 | |
| Stomachache | 0 | 23 | 0.404 (0.082) | 24 | 0.414 (0.085) | 1.025 (0.577, 1.826) | 0.931 |
| 6 | 22 | 0.386 (0.078) | 9 | 0.155 (0.064) | 0.402 (0.176, 0.845) | 0.021 | |
| 12 | 20 | 0.351 (0.077) | 2 | 0.034 (0.024) | 0.098 (0.016, 0.336) | 0.002 | |
| Anal discomfort | 0 | 22 | 0.386 (0.105) | 14 | 0.241 (0.079) | 0.625 (0.313, 1.210) | 0.170 |
| 6 | 24 | 0.421 (0.109) | 2 | 0.034 (0.024) | 0.082 (0.013, 0.276) | 0.001 | |
| 12 | 24 | 0.421 (0.109) | 0 | 0 (0) | —— | —— | |
| Diarrhea | 0 | 48 | 0.842 (0.203) | 37 | 0.638 (0.125) | 0.758 (0.491, 1.160) | 0.204 |
| 6 | 63 | 1.105 (0.237) | 15 | 0.259 (0.100) | 0.234 (0.128, 0.399) | <0.001 | |
| 12 | 53 | 0.930 (0.222) | 7 | 0.121 (0.061) | 0.130 (0.054, 0.267) | <0.001 | |
| Dehydration | 0 | 42 | 0.737 (0.165) | 19 | 0.328 (0.111) | 0.445 (0.253, 0.753) | 0.003 |
| 6 | 42 | 0.737 (0.165) | 3 | 0.052 (0.038) | 0.070 (0.017, 0.193) | <0.001 | |
| 12 | 41 | 0.719 (0.166) | 0 | 0 (0) | —— | —— | |
| Poor Appetite | Nausea | Vomiting | Stomachache | Anal Discomfort | Diarrhea | Dehydration | |
|---|---|---|---|---|---|---|---|
| Model 1 | |||||||
| week | 0.463 | 0.371 | 0.425 | 0.219 | 0.777 | 0.126 | 0.666 |
| group | <0.001 | 0.005 | 0.002 | 0.001 | <0.001 | <0.001 | 0.001 |
| week × group | 1.000 | 0.999 | 0.246 | 0.094 | 0.999 | 0.233 | 0.910 |
| Model 2 | |||||||
| week | 0.464 | 0.371 | 0.425 | 0.253 | 0.777 | 0.126 | 0.666 |
| group | <0.001 | <0.001 | 0.004 | 0.007 | 0.001 | 0.001 | <0.001 |
| Model 3 | |||||||
| week | 0.464 | 0.371 | 0.489 | 0.182 | 0.777 | 0.130 | 0.666 |
| group | <0.001 | <0.001 | 0.016 | 0.010 | 0.004 | 0.001 | <0.001 |
| Variables | Control | YLGB-1496 | p a | Model b | p c | ||
|---|---|---|---|---|---|---|---|
| Week | Group | Week × Group | |||||
| pH | |||||||
| 0 | 6.26 ± 1.31 | 6.70 ± 1.15 | 0.066 | 1 | 0.476 | 0.072 | 0.246 |
| 6 | 6.54 ± 1.19 | 7.06 ± 0.91 | 0.009 | 2 | 0.476 | 0.117 | |
| 12 | 6.58 ± 1.09 | 6.87 ± 0.78 | 0.100 | 3 | 0.476 | 0.263 | |
| FC (mg/g) | |||||||
| 0 | 1.30 ± 0.71 | 1.33 ± 0.59 | 0.776 | 1 | 0.123 | 0.137 | 0.595 |
| 6 | 1.02 ± 0.74 | 1.48 ± 0.85 | 0.003 | 2 | 0.122 | 0.114 | |
| 12 | 1.42 ± 2.75 | 1.67 ± 1.44 | 0.537 | 3 | 0.122 | 0.225 | |
| AAT (mg/g) | |||||||
| 0 | 0.28 ± 1.03 | 0.41 ± 0.47 | 0.399 | 1 | 0.251 | <0.001 | 0.580 |
| 6 | 0.09 ± 0.11 | 0.42 ± 0.76 | 0.001 | 2 | 0.251 | <0.001 | |
| 12 | 0.11 ± 0.12 | 0.48 ± 0.62 | <0.001 | 3 | 0.251 | 0.008 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Uma Mageswary, M.; Taib, F.; Koo, T.H.; Yusof, A.; Hamid, I.J.A.; Jiang, H.; Liong, M.-T.; Ali, A.; Zhang, Y. Safety and Tolerance of Bifidobacterium longum subsp. Infantis YLGB-1496 in Toddlers with Respiratory Symptoms. Nutrients 2025, 17, 2127. https://doi.org/10.3390/nu17132127
Li P, Uma Mageswary M, Taib F, Koo TH, Yusof A, Hamid IJA, Jiang H, Liong M-T, Ali A, Zhang Y. Safety and Tolerance of Bifidobacterium longum subsp. Infantis YLGB-1496 in Toddlers with Respiratory Symptoms. Nutrients. 2025; 17(13):2127. https://doi.org/10.3390/nu17132127
Chicago/Turabian StyleLi, Pin, Mageswaran Uma Mageswary, Fahisham Taib, Thai Hau Koo, Azianey Yusof, Intan Juliana Abd Hamid, Hua Jiang, Min-Tze Liong, Adli Ali, and Yumei Zhang. 2025. "Safety and Tolerance of Bifidobacterium longum subsp. Infantis YLGB-1496 in Toddlers with Respiratory Symptoms" Nutrients 17, no. 13: 2127. https://doi.org/10.3390/nu17132127
APA StyleLi, P., Uma Mageswary, M., Taib, F., Koo, T. H., Yusof, A., Hamid, I. J. A., Jiang, H., Liong, M.-T., Ali, A., & Zhang, Y. (2025). Safety and Tolerance of Bifidobacterium longum subsp. Infantis YLGB-1496 in Toddlers with Respiratory Symptoms. Nutrients, 17(13), 2127. https://doi.org/10.3390/nu17132127








