High-Calorie Diet Exacerbates the Crosstalk Between Gestational Diabetes and Youth-Onset Diabetes in Female Offspring Through Disrupted Estrogen Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment Design
2.2. OGTT and Insulin Tolerance Test (ITT)
2.3. Immunohistochemistry (IHC)
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Real-Time Quantitative PCR (qPCR)
2.6. Western Blotting (WB)
2.7. Cell Culture and Treatment
2.8. Immunofluorescence (IF)
2.9. Quantitative and Statistical Analysis
3. Results
3.1. Maternal GDM Caused Glucose Metabolism Disorders in Female-Offspring Mice During Sexual Maturity
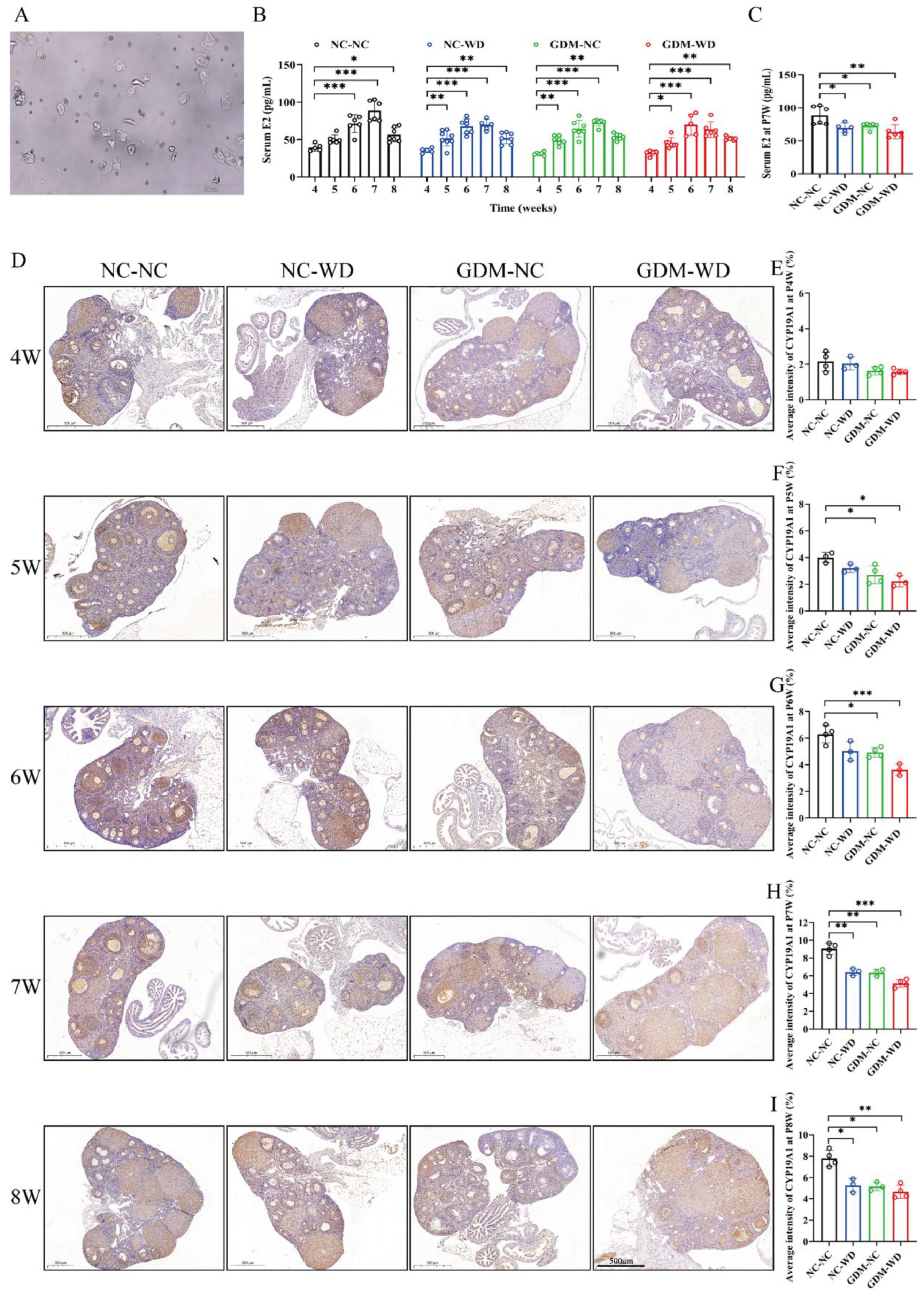
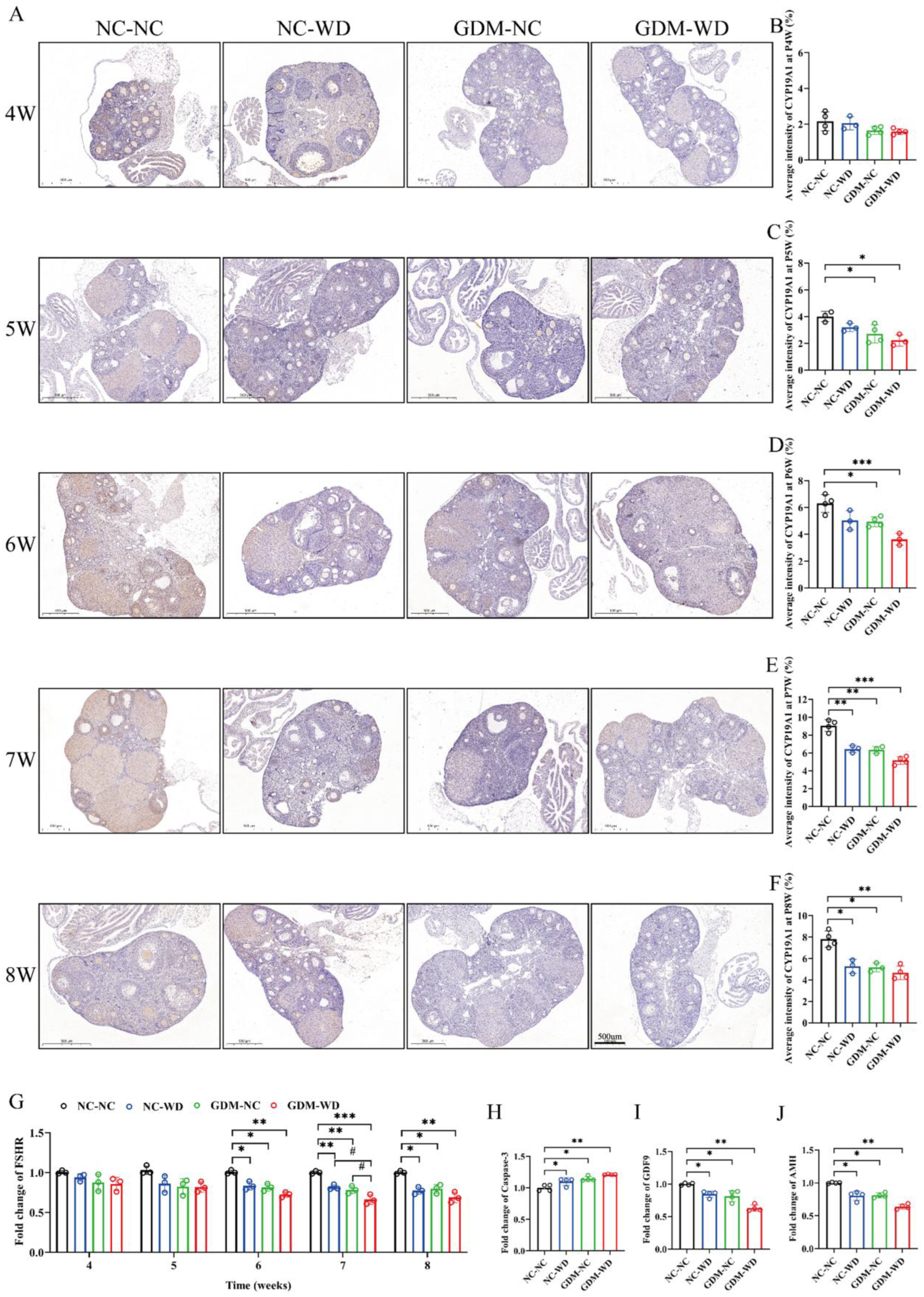
3.2. Maternal GDM Caused Sexual Maturation Disruption and Ovarian CYP19A1 Downregulation in Female-Offspring Mice
3.3. Maternal GDM Caused Changes in the Area and Number of Follicle Types During Sexual Maturity
3.4. Maternal GDM Caused the Atresia of Follicles in Female-Offspring Mice During Sexual Maturity
3.5. Maternal GDM Impaired the Estrogen Receptor and Insulin Signaling in Liver Tissue
3.6. The Interaction Between Estrogen and Insulin Signals In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| AMH | Anti-Müllerian hormone |
| Caspase-3 | Cysteine-aspartic protease 3 |
| CYP19A1 | Cytochrome p450 family 19 subfamily a member 1 |
| ELISA | Enzyme-linked immunosorbent assay |
| E2 | Estradiol |
| ERα | Estrogen receptor alpha |
| ERβ | Estrogen receptor beta |
| FHSR | Follicle-stimulating hormone receptor |
| GDF9 | Growth differentiation factor 9 |
| GDM | Gestational diabetes mellitus |
| GPER | G protein-coupled estrogen receptor |
| IRS-1 | Insulin receptor substrate 1 |
| ITT | Insulin tolerance test |
| OGTT | Oral glucose tolerance test |
| T2D | Type 2 diabetes |
| WD | Western diet |
| YOT2D | Youth-onset type 2 diabetes |
References
- Moon, J.H.; Jang, H.C. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab. J. 2022, 46, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Chao, L.C.; Cree-Green, M.; Dart, A.B.; King, M.; Looker, H.C.; Magliano, D.J.; Nadeau, K.J.; Pinhas-Hamiel, O.; Shah, A.S.; et al. Youth-onset type 2 diabetes mellitus: An urgent challenge. Nat. Rev. Nephrol. 2022, 19, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; Allen, N.B.; Patton, G.C. Puberty, Developmental Processes, and Health Interventions. In Child and Adolescent Health and Development; Bundy, D.A.P., Silva, N.D., Horton, S., Jamison, D.T., Patton, G.C., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Wu, H.; Patterson, C.C.; Zhang, X.; Ghani, R.B.A.; Magliano, D.J.; Boyko, E.J.; Ogle, G.D.; Luk, A.O.Y. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res. Clin. Pract. 2022, 185, 109785. [Google Scholar] [CrossRef]
- Xie, J.; Wang, M.; Long, Z.; Ning, H.; Li, J.; Cao, Y.; Liao, Y.; Liu, G.; Wang, F.; Pan, A. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: Systematic analysis of the Global Burden of Disease Study 2019. BMJ 2022, 379, e072385. [Google Scholar] [CrossRef]
- Lynch, J.L.; Barrientos-Pérez, M.; Hafez, M.; Jalaludin, M.Y.; Kovarenko, M.; Rao, P.V.; Weghuber, D. Country-Specific Prevalence and Incidence of Youth-Onset Type 2 Diabetes: A Narrative Literature Review. Ann. Nutr. Metab. 2020, 76, 289–296. [Google Scholar] [CrossRef]
- La Grasta Sabolic, L.; Marusic, S.; Cigrovski Berkovic, M. Challenges and pitfalls of youth-onset type 2 diabetes. World J. Diabetes 2024, 15, 876–885. [Google Scholar] [CrossRef]
- Dabelea, D.; Stafford, J.M.; Mayer-Davis, E.J.; D’Agostino, R., Jr.; Dolan, L.; Imperatore, G.; Linder, B.; Lawrence, J.M.; Marcovina, S.M.; Mottl, A.K.; et al. Association of Type 1 Diabetes vs. Type 2 Diabetes Diagnosed During Childhood and Adolescence with Complications During Teenage Years and Young Adulthood. JAMA 2017, 317, 825–835. [Google Scholar] [CrossRef]
- Candler, T.P.; Mahmoud, O.; Lynn, R.M.; Majbar, A.A.; Barrett, T.G.; Shield, J.P.H. Continuing rise of Type 2 diabetes incidence in children and young people in the UK. Diabet. Med. 2018, 35, 737–744. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, Y.; Li, Y.Y.; Liu, X.M.; Wang, X.X.; Zhang, C.L.; Hao, C.F.; Deng, S.L. Regulation of follicular development and differentiation by intra-ovarian factors and endocrine hormones. Front. Biosci. 2019, 24, 983–993. [Google Scholar] [CrossRef]
- Tsoulis, M.W.; Chang, P.E.; Moore, C.J.; Chan, K.A.; Gohir, W.; Petrik, J.J.; Vickers, M.H.; Connor, K.L.; Sloboda, D.M. Maternal High-Fat Diet-Induced Loss of Fetal Oocytes Is Associated with Compromised Follicle Growth in Adult Rat Offspring. Biol. Reprod. 2016, 94, 94. [Google Scholar] [CrossRef]
- Liu, L.; Hao, M.; Zhang, J.; Chen, Z.; Zhou, J.; Wang, C.; Zhang, H.; Wang, J. FSHR-mTOR-HIF1 signaling alleviates mouse follicles from AMPK-induced atresia. Cell Rep. 2023, 42, 113158. [Google Scholar] [CrossRef]
- Yang, L.; Du, X.; Wang, S.; Lin, C.; Li, Q.; Li, Q. A regulatory network controlling ovarian granulosa cell death. Cell Death Discov. 2023, 9, 70. [Google Scholar] [CrossRef]
- Sanfins, A.; Rodrigues, P.; Albertini, D.F. GDF-9 and BMP-15 direct the follicle symphony. J. Assist. Reprod. Genet. 2018, 35, 1741–1750. [Google Scholar] [CrossRef]
- Kanakatti Shankar, R.; Dowlut-McElroy, T.; Dauber, A.; Gomez-Lobo, V. Clinical Utility of Anti-Mullerian Hormone in Pediatrics. J. Clin. Endocrinol. Metab. 2022, 107, 309–323. [Google Scholar] [CrossRef]
- Sacharidou, A.; Chambliss, K.; Peng, J.; Barrera, J.; Tanigaki, K.; Luby-Phelps, K.; Özdemir, İ.; Khan, S.; Sirsi, S.R.; Kim, S.H.; et al. Endothelial ERα promotes glucose tolerance by enhancing endothelial insulin transport to skeletal muscle. Nat. Commun. 2023, 14, 4989. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Arterburn, J.B.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor GPER: Molecular Pharmacology and Therapeutic Applications. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 295–320. [Google Scholar] [CrossRef]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 2019, 68, 291–304. [Google Scholar] [CrossRef]
- Bryzgalova, G.; Gao, H.; Ahren, B.; Zierath, J.R.; Galuska, D.; Steiler, T.L.; Dahlman-Wright, K.; Nilsson, S.; Gustafsson, J.A.; Efendic, S.; et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia 2006, 49, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jiang, W.; Liao, W.; Yan, H.; Ai, W.; Pan, Q.; Brashear, W.A.; Xu, Y.; He, L.; Guo, S. An estrogen receptor α-derived peptide improves glucose homeostasis during obesity. Nat. Commun. 2024, 15, 3410. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; Hançer, N.J.; Qiu, W.; White, M.F. Serine 302 Phosphorylation of Mouse Insulin Receptor Substrate 1 (IRS1) Is Dispensable for Normal Insulin Signaling and Feedback Regulation by Hepatic S6 Kinase. J. Biol. Chem. 2016, 291, 8602–8617. [Google Scholar] [CrossRef] [PubMed]
- Pyle, L.; Kelsey, M.M. Youth-onset type 2 diabetes: Translating epidemiology into clinical trials. Diabetologia 2021, 64, 1709–1716. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Andersen, N.H.; Christin-Maitre, S.; Davis, S.M.; Duijnhouwer, A.; Gawlik, A.; Maciel-Guerra, A.T.; Gutmark-Little, I.; Fleischer, K.; Hong, D.; et al. Clinical practice guidelines for the care of girls and women with Turner syndrome. Eur. J. Endocrinol. 2024, 190, G53–G151. [Google Scholar] [CrossRef]
- Xu, C.; Zou, L.; Wang, L.; Lv, W.; Cao, X.; Jia, X.; Wang, Y.; Jiang, G.; Ji, L. Gestational diabetes mellitus-derived miR-7-19488 targets PIK3R2 mRNA to stimulate the abnormal development and maturation of offspring-islets. Life Sci. 2025, 363, 123369. [Google Scholar] [CrossRef]
- Wei, W.; Qin, F.; Gao, J.; Chang, J.; Pan, X.; Jiang, X.; Che, L.; Zhuo, Y.; Wu, D.; Xu, S. The effect of maternal consumption of high-fat diet on ovarian development in offspring. Anim. Reprod. Sci. 2023, 255, 107294. [Google Scholar] [CrossRef]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef]
- Ko, S.-H.; Jung, Y. Energy Metabolism Changes and Dysregulated Lipid Metabolism in Postmenopausal Women. Nutrients 2021, 13, 4556. [Google Scholar] [CrossRef]
- Camon, C.; Garratt, M.; Correa, S.M. Exploring the effects of estrogen deficiency and aging on organismal homeostasis during menopause. Nat. Aging 2024, 4, 1731–1744. [Google Scholar] [CrossRef]
- Dittmer, J. Nuclear Mechanisms Involved in Endocrine Resistance. Front. Oncol. 2021, 11, 736597. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, J.; Chao, Y.; Bi, Y.; Zhang, W.; Zhang, Y.; Ji, T.; Fu, Y.; Chen, Q.; Zhang, Q.; et al. β-estradiol adjusts intestinal function via ERβ and GPR30 mediated PI3K/AKT signaling activation to alleviate postmenopausal dyslipidemia. Biochem. Pharmacol. 2020, 180, 114134. [Google Scholar] [CrossRef] [PubMed]
- Addington, K.S.; Kristiansen, M.; Hempler, N.F.; Frimodt-Møller, M.; Montori, V.M.; Kunneman, M.; Scheuer, S.H.; Diaz, L.J.; Andersen, G.S. Incidence of early-onset type 2 diabetes and sociodemographic predictors of complications: A nationwide registry study. J. Diabetes Its Complicat. 2025, 39, 108942. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; King, A.; Kattah, A.G.; Kuhle, C.L.; Sood, R.; Kling, J.M.; Mara, K.C.; Kapoor, E. Hypertensive disorders of pregnancy and menopausal symptoms: A cross-sectional study from the data registry on experiences of aging, menopause, and sexuality. Menopause 2021, 28, 25–31. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Cao, X.; Wang, Y.; Yang, S.; Ji, L. High-Calorie Diet Exacerbates the Crosstalk Between Gestational Diabetes and Youth-Onset Diabetes in Female Offspring Through Disrupted Estrogen Signaling. Nutrients 2025, 17, 2128. https://doi.org/10.3390/nu17132128
Jia X, Cao X, Wang Y, Yang S, Ji L. High-Calorie Diet Exacerbates the Crosstalk Between Gestational Diabetes and Youth-Onset Diabetes in Female Offspring Through Disrupted Estrogen Signaling. Nutrients. 2025; 17(13):2128. https://doi.org/10.3390/nu17132128
Chicago/Turabian StyleJia, Xinyu, Xiangju Cao, Yuan Wang, Shuai Yang, and Lixia Ji. 2025. "High-Calorie Diet Exacerbates the Crosstalk Between Gestational Diabetes and Youth-Onset Diabetes in Female Offspring Through Disrupted Estrogen Signaling" Nutrients 17, no. 13: 2128. https://doi.org/10.3390/nu17132128
APA StyleJia, X., Cao, X., Wang, Y., Yang, S., & Ji, L. (2025). High-Calorie Diet Exacerbates the Crosstalk Between Gestational Diabetes and Youth-Onset Diabetes in Female Offspring Through Disrupted Estrogen Signaling. Nutrients, 17(13), 2128. https://doi.org/10.3390/nu17132128




