Association Between Follistatin and PAI-1 Levels in MASLD Subjects Undergoing a Plant-Based Dietary Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. MASLD Assessment
2.3. Anthropometric Parameters
2.4. Bioelectrical Impedance Analysis (BIA)
2.5. Biochemical Analyses
2.6. Variables of Exposure and Confounders
2.7. Statistical Methods
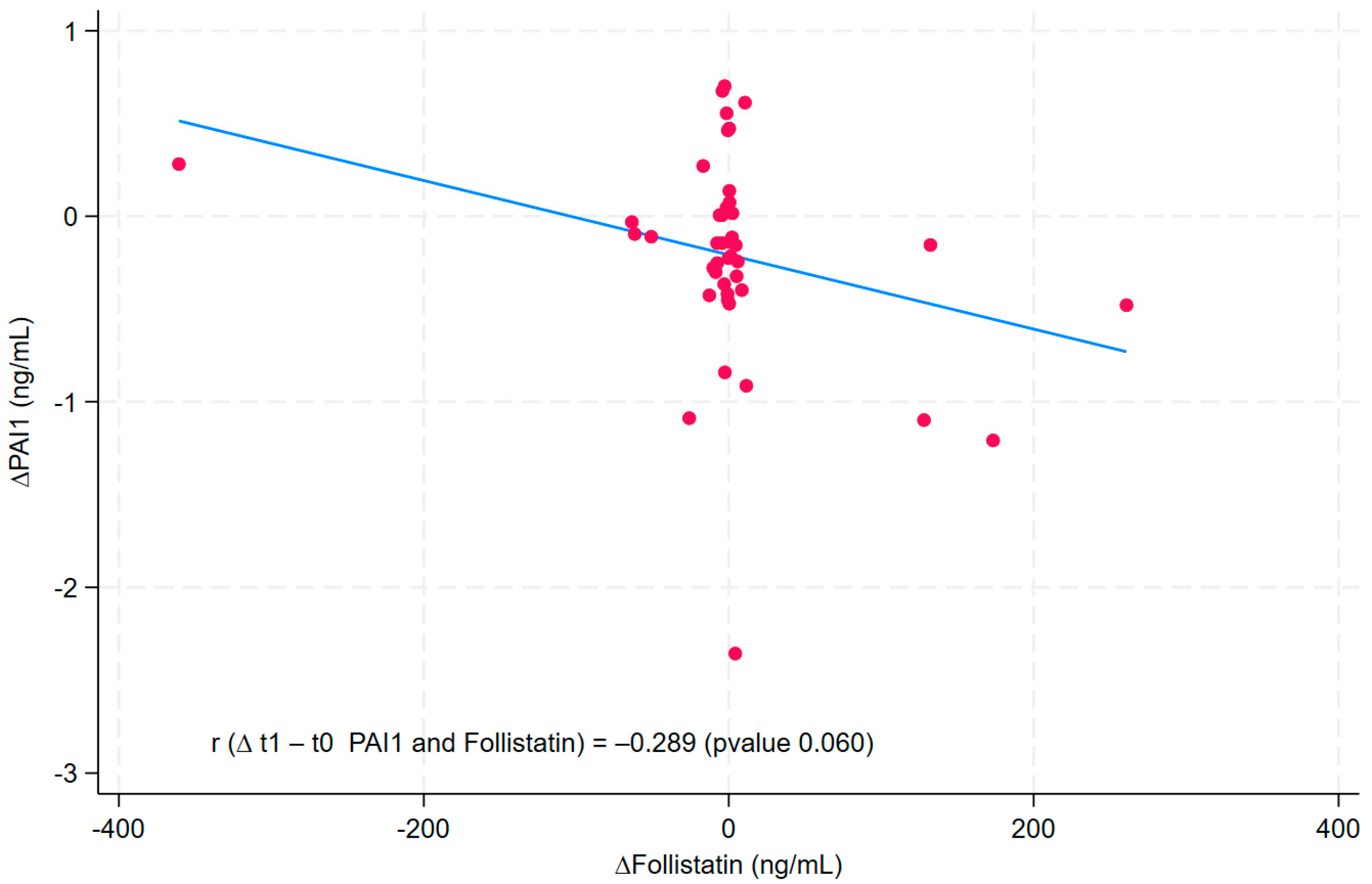
3. Results
4. Discussion
4.1. Follistatin as a Modulator of Inflammation and Fibrosis
4.2. PAI-1 as a Metabolic and Inflammatory Target
4.3. Role of Diet and Bioactive Compounds
4.4. Clinical Implications
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MASH | Metabolic Steatohepatitis |
| CVDs | Cardiovascular Diseases |
| BMI | Body Mass Index |
| WC | Waist Circumference |
| CAP | Controlled Attenuation Parameter |
| LSM | Liver Stiffness Measurement |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| FSG | Fasting Serum Glucose |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| HDL-C | High-Density Lipoprotein Cholesterol |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| γGT | Gamma-Glutaminyl Transferase |
| HbA1c | Glycated Hemoglobin |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| IPAQ | International Physical Activity Questionnaire |
| PREDIMED | Prevention with Mediterranean Diet |
| FM | Fat Mass |
| FFM | Fat-Free Mass |
| TBW | Total Body Water |
References
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- McMillin, S.; Ryan, A.S. Plasminogen Activator Inhibitor-1, Body Fat and Insulin Action in Aging Women. Ann. Gerontol. Geriatr. Res. 2014, 1, 1006. [Google Scholar] [PubMed] [PubMed Central]
- Alessi, M.C.; Juhan-Vague, I. PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Chen, S.; Wang, P.; Xu, Q.; Zhang, Y.; Luo, Y.; Wu, S.; Wang, A. Insulin resistance mediates obesity-related risk of cardiovascular disease: A prospective cohort study. Cardiovasc. Diabetol. 2022, 21, 289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lisco, G.; Guido, D.; Cerabino, N.; Di Chito, M.; Donvito, R.; Bonfiglio, C.; Shahini, E.; Zappimbulso, M.; Randazzo, C.; Barletta, D.; et al. Liver steatosis is positively associated with plasminogen activator inhibitor-1 in apparently healthy individuals with overweight and obesity: A FibroScan-Based Cross-Sectional study. J. Transl. Med. 2025, 23, 487. [Google Scholar] [CrossRef]
- Hedger, M.P.; De Kretser, D.M. The activins and their binding protein, follistatin-Diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine Growth Factor Rev. 2013, 24, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J. Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 2004, 20, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Borné, Y.; Gao, R.; López Rodriguez, M.; Roell, W.C.; Wilson, J.M.; Regmi, A.; Luan, C.; Aly, D.M.; Peter, A.; et al. Elevated circulating follistatin associates with an increased risk of type 2 diabetes. Nat. Commun. 2021, 12, 6486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braga, M.; Reddy, S.T.; Vergnes, L.; Pervin, S.; Grijalva, V.; Stout, D.; David, J.; Li, X.; Tomasian, V.; Reid, C.B.; et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J. Lipid Res. 2014, 55, 375–384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hansen, J.; Brandt, C.; Nielsen, A.R.; Hojman, P.; Whitham, M.; Febbraio, M.A.; Pedersen, B.K.; Plomgaard, P. Exercise induces a marked increase in plasma follistatin: Evidence that follistatin is a contraction-induced hepatokine. Endocrinology 2011, 152, 164–171, Erratum in Endocrinology 2015, 156, 1200. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Rutti, S.; Arous, C.; Clemmesen, J.O.; Secher, N.H.; Drescher, A.; Gonelle-Gispert, C.; Halban, P.A.; Pedersen, B.K.; Weigert, C.; et al. Circulating Follistatin Is Liver-Derived and Regulated by the Glucagon-to-Insulin Ratio. J. Clin. Endocrinol. Metab. 2016, 101, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, R.; Kaji, K.; Namisaki, T.; Moriya, K.; Kawaratani, H.; Kitade, M.; Takaya, H.; Aihara, Y.; Douhara, A.; Asada, K.; et al. Novel oral plasminogen activator inhibitor-1 inhibitor TM5275 attenuates hepatic fibrosis under metabolic syndrome via suppression of activated hepatic stellate cells in rats. Mol. Med. Rep. 2020, 22, 2948–2956. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kisseleva, T.; Brenner, D.A. Mechanisms of fibrogenesis. Exp. Biol. Med. 2008, 233, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.K.; Blake, M.C.; Moses, H.L. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-beta-induced physical and functional interactions between smads and Sp1. J. Biol. Chem. 2000, 275, 40014–40019. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Mansell, A.; Patella, S.; Scott, B.J.; Hedger, M.P.; de Kretser, D.M.; Phillips, D.J. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc. Natl. Acad. Sci. USA 2007, 104, 16239–16244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DiPietro, L.; Buchner, D.M.; Marquez, D.X.; Pate, R.R.; Pescatello, L.S.; Whitt-Glover, M.C. New scientific basis for the 2018 U.S. Physical Activity Guidelines. J. Sport Health Sci. 2019, 8, 197–200. [Google Scholar] [CrossRef]
- Babio, N.; Bulló, M.; Basora, J.; Martínez-González, M.A.; Fernández-Ballart, J.; Márquez-Sandoval, F.; Molina, C.; Salas-Salvadó, J. Adherence to the Mediterranean diet and risk of metabolic syndrome and its components. Nutr. Metab. Cardiovasc. Dis. 2016, 19, 563–570. [Google Scholar] [CrossRef]
- De Nucci, S.; Rinaldi, R.; Di Chito, M.; Donghia, R.; Giannuzzi, V.; Shahini, E.; Cozzolongo, R.; Pesole, P.L.; Coletta, S.; De Pergola, G.; et al. The Replacement of Only One Portion of Starchy Carbohydrates with Green Leafy Vegetables Regresses Mid and Advanced Stages of NAFLD: Results from a Prospective Pilot Study. Nutrients 2023, 15, 2289. [Google Scholar] [CrossRef]
- Bacil, G.P.; Cogliati, B.; Cardoso, D.R.; Barbisan, L.F.; Romualdo, G.R. Are isothiocyanates and polyphenols from Brassicaceae vegetables emerging as preventive/therapeutic strategies for NAFLD? The landscape of recent preclinical findings. Food Funct. 2022, 13, 8348–8362. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wai-Sun Wong, V.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.H.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.; Wai-Sun Wong, V.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2022, 5, 362–373. [Google Scholar] [CrossRef]
- Beaudart, C.; Bruyère, O.; Geerinck, A.; Hajaoui, M.; Scafoglieri, A.; Perkisas, S.; Bautmans, I.; Gielen, E.; Reginster, J.-Y.; Buckinx, F. Equation models developed with bioelectric impedance analysis tools to assess muscle mass: A systematic review. Clin. Nutr. ESPEN 2020, 35, 47–62. [Google Scholar] [CrossRef]
- Ward, L.C. Bioelectrical impedance analysis for body composition assessment: Reflections on accuracy, clinical utility, and standardization. Eur. J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef]
- Hu, H.; Nakagawa, T.; Honda, T.; Yamamoto, S.; Mizoue, T. Should insulin resistance (HOMA-IR), insulin secretion (HOMA-β), and visceral fat area be considered for improving the performance of diabetes risk prediction models. BMJ Open Diabetes Res. Care 2024, 12, e003680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.-Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Wainwright, M. Statistical Learning with Sparsity: The Lasso and Generalizations, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Campanella, A.; Iacovazzi, P.A.; Misciagna, G.; Bonfiglio, C.; Mirizzi, A.; Franco, I.; Bianco, A.; Sorino, P.; Caruso, M.G.; Cisternino, A.M.; et al. The Effect of Three Mediterranean Diets on Remnant Cholesterol and Non-Alcoholic Fatty Liver Disease: A Secondary Analysis. Nutrients 2020, 12, 1674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugiyama, H.; Kobayashi, Y.; Sumida, Y.; Wada, S.; Tani, M.; Shizukawa, Y.; Shirota, K.; Sasai, Y.; Suzuki, T.; Aoi, W.; et al. A nutritional intervention that promotes increased vegetable intake in Japanese with non-alcoholic fatty liver disease: A six-month trial. J. Clin. Biochem. Nutr. 2022, 70, 46–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. 2011, 28, 549–559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Parameter | Value |
|---|---|
| N | 44 |
| Age * (years) | 46.5 (10.4) |
| Gender (%) | |
| Female | 22 (50) |
| Male | 22 (50) |
| Smoking habit (%) | |
| Never | 39 (95) |
| Current | 2 (5) |
| Physical activity (%) | |
| <30 min | 8 (20) |
| >30 min | 26 (63) |
| Sports person | 7 (17) |
| Education (%) | |
| Secondary School | 11 (27%) |
| High School | 22 (54%) |
| Graduate | 8 (20%) |
| Snoring (%) | |
| No | 10 (24%) |
| Yes | 31 (76%) |
| GERD symptoms (%) | |
| No | 25 (61%) |
| Yes | 16 (39%) |
| Sleepiness (%) | |
| No | 26 (63%) |
| Yes | 15 (37%) |
| Parameters | Pre-Diet | Post-Diet | p-Value ¥ |
|---|---|---|---|
| N | 44 | 44 | |
| Mean (SD) | Mean (SD) | ||
| Outcome variable: | |||
| PAI1 (ng/mL) | 3.58 (0.90) | 3.35 (0.80) | <0.001 |
| Molecule | |||
| Follistatin (ng/mL) | 43.6 (108.7) | 45.3 (80.5) | 0.392 |
| Ultrasonographic measures of liver steatosis and fibrosis | |||
| FibroScan CAP (dB/m) | 313.8 (47.0) | 278.5 (48.9) | <0.001 |
| FibroScan LSM (kPa) | 6.6 (3.0) | 6.4 (4.1) | 0.064 |
| Anthropometric and clinical parameters | |||
| SBP (mmHg) | 133.1 (13.0) | 124.5 (8.6) | 0.001 |
| DBP (mmHg) | 80.6 (11.4) | 76.2 (8.1) | <0.001 |
| PREDIMED questionnaire | 8.0 (7.0, 9.0) | 11.0 (10.0, 12.0) | <0.001 |
| BMI (kg/m2) | 36.7 (4.2) | 34.8 (4.1) | <0.001 |
| Waist circumference (cm) | 113.7 (11.9) | 107.4 (12.5) | <0.001 |
| Fat mass (kg) | 40.9 (10.2) | 36.0 (9.4) | <0.001 |
| Free-fat mass (kg) | 63.5 (11.8) | 62.8 (11.6) | 0.125 |
| Body cell mass | 35.8 (7.8) | 35.6 (7.9) | 0.338 |
| Blood tests: | |||
| Glucose (mg/dL) | 94.5 (8.9) | 94.8 (8.4) | 0.799 |
| Insulin (µIU/mL) | 19.4 (10.4) | 15.9 (8.4) | <0.001 |
| HOMA-IR | 4.6 (2.7) | 3.8 (2.1) | <0.001 |
| Hemoglobin A1C | 5.5 (0.4) | 5.4 (0.3) | <0.001 |
| Triglycerides (mg/dL) | 123.3 (59.5) | 101.5 (54.2) | <0.001 |
| Total cholesterol (mg/dL) | 192.4 (31.4) | 177.3 (29.5) | <0.001 |
| HDL cholesterol (mg/dL) | 50.0 (11.4) | 47.2 (10.9) | 0.008 |
| LDL cholesterol (mg/dL) | 125.8 (28.5) | 110.9 (25.2) | <0.001 |
| AST (U/L) | 22.2 (10.7) | 19.2 (7.7) | <0.001 |
| ALT (U/L) | 29.9 (18.1) | 22.6 (12.5) | <0.001 |
| γGT (U/L) | 24.6 (14.4) | 20.2 (12.1) | <0.001 |
| Uric acid (mg/dL) | 5.4 (1.7) | 5.4 (1.3) | 0.543 |
| Creatinine (mg/dL) | 0.9 (0.2) | 0.8 (0.2) | 0.592 |
| hs-CRP (mg/dL) | 0.3 (0.2) | 0.4 (0.7) | 0.210 |
| 25-hydroxyvitamin D | 25.2 (8.0) | 22.2 (5.6) | 0.003 |
| TSH (µmU/mL) | 1.9 (0.9) | 1.9 (1.2) | 0.083 |
| FT3 (pg/mL) | 3.4 (0.5) | 3.1 (0.3) | 0.002 |
| FT4 (ng/dL) | 12.2 (1.2) | 11.8 (2.0) | 0.423 |
| PAI1 (ng/mL) | β | p-Value | 95% CI |
|---|---|---|---|
| Model a: | |||
| Pre-Diet | 0.00 | ||
| Post-Diet | −0.145 | 0.041 | −0.285; −0.005 |
| Model b: | |||
| Pre-Diet | 0.00 | ||
| Post-Diet | −0.146 | 0.040 | −0.285; −0.007 |
| Model c: | |||
| Pre-Diet | 0.00 | ||
| Post-Diet | −0.194 | 0.028 | −0.368; −0.021 |
| Follistatin | −0.002 | 0.002 | −0.003; −0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerabino, N.; Bonfiglio, C.; Di Chito, M.; Donvito, R.; Mongelli, F.P.; Pesole, P.L.; Stabile, D.; Shahini, E.; Zappimbulso, M.; Cozzolongo, R.; et al. Association Between Follistatin and PAI-1 Levels in MASLD Subjects Undergoing a Plant-Based Dietary Intervention. Nutrients 2025, 17, 2124. https://doi.org/10.3390/nu17132124
Cerabino N, Bonfiglio C, Di Chito M, Donvito R, Mongelli FP, Pesole PL, Stabile D, Shahini E, Zappimbulso M, Cozzolongo R, et al. Association Between Follistatin and PAI-1 Levels in MASLD Subjects Undergoing a Plant-Based Dietary Intervention. Nutrients. 2025; 17(13):2124. https://doi.org/10.3390/nu17132124
Chicago/Turabian StyleCerabino, Nicole, Caterina Bonfiglio, Martina Di Chito, Rosanna Donvito, Francesco Pio Mongelli, Pasqua Letizia Pesole, Dolores Stabile, Endrit Shahini, Marianna Zappimbulso, Raffaele Cozzolongo, and et al. 2025. "Association Between Follistatin and PAI-1 Levels in MASLD Subjects Undergoing a Plant-Based Dietary Intervention" Nutrients 17, no. 13: 2124. https://doi.org/10.3390/nu17132124
APA StyleCerabino, N., Bonfiglio, C., Di Chito, M., Donvito, R., Mongelli, F. P., Pesole, P. L., Stabile, D., Shahini, E., Zappimbulso, M., Cozzolongo, R., Giannelli, G., & De Pergola, G. (2025). Association Between Follistatin and PAI-1 Levels in MASLD Subjects Undergoing a Plant-Based Dietary Intervention. Nutrients, 17(13), 2124. https://doi.org/10.3390/nu17132124






