Are Nuts Safe in Diverticulosis? A Mixed-Methods Systematic Review of Available Evidence
Abstract
1. Introduction
2. Pathophysiology and Clinical Relevance of Diverticulosis
2.1. Definition and Etiology
2.2. Incidence and Clinical Relevance
2.2.1. Diverticulitis
2.2.2. Diverticular Bleeding
2.2.3. Complicated Diverticulitis
2.3. A Brief Overview of Management and Therapy
2.3.1. Diverticulosis and Uncomplicated Diverticulitis
2.3.2. Complicated Diverticulitis
2.3.3. Emerging and Adjunctive Therapies
3. Nut Consumption and Dietary Recommendations
3.1. Nuts and Seeds: The Myth
3.2. The Turning Point: The Emergence of Clinical Evidence
3.3. The Dietary Recommendations: Evidence-Based Clinical Studies
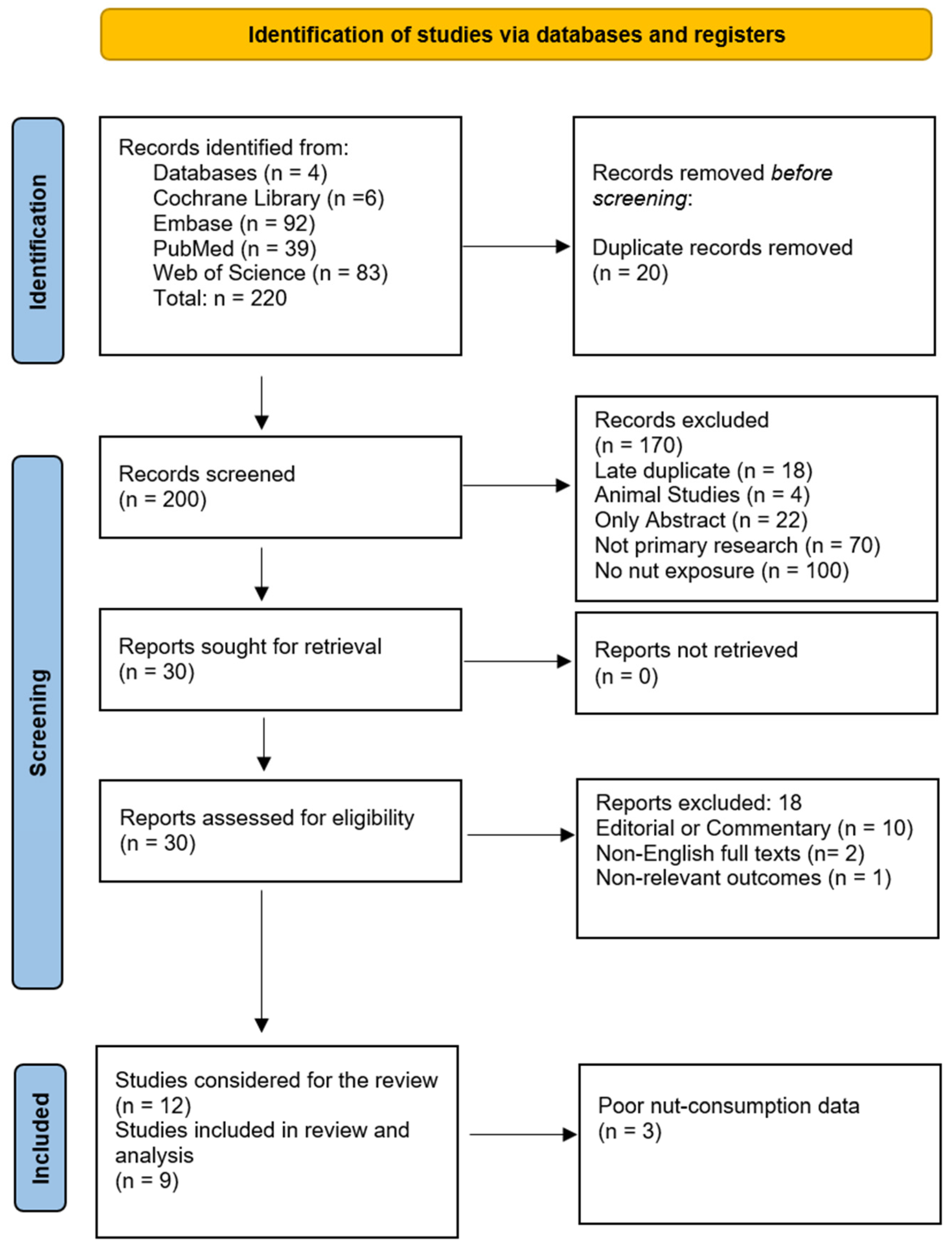
4. The Search for Available Evidence
4.1. Literature Search and Data Procurement
4.1.1. Study Selection
4.1.2. Data Sources and Search Strategy
4.1.3. Data Extraction
4.2. Qualitative Analyses
4.2.1. Risk of Bias Assessment
4.2.2. Certainty of Evidence
4.2.3. Narrative Synthesis
4.3. Quantitative Analyses
4.3.1. Outcome-Specific Analyses
4.3.2. Dose–Response Modeling
4.3.3. Influence, Robustness, and Influence Diagnostics
- Leave-one-out analysis: we repeated the analysis while omitting one study at a time and observing how the overall HR or OR, 95% CIs, and the I2 statistic changed [71].
- Baujat plot (prevalence model only): for the prevalence model, we also created a plot, which pinpoints which study contributes most to heterogeneity (Qi) and which exerts the greatest influence on the pooled result [72].
- Risk-of-bias-weighted pooling: recognizing that not all studies are of equal quality, we down-weighted any paper with a “serious” ROBINS-I rating by half and then re-ran our random-effects models, re-pooled estimates, and compared Qi [73].
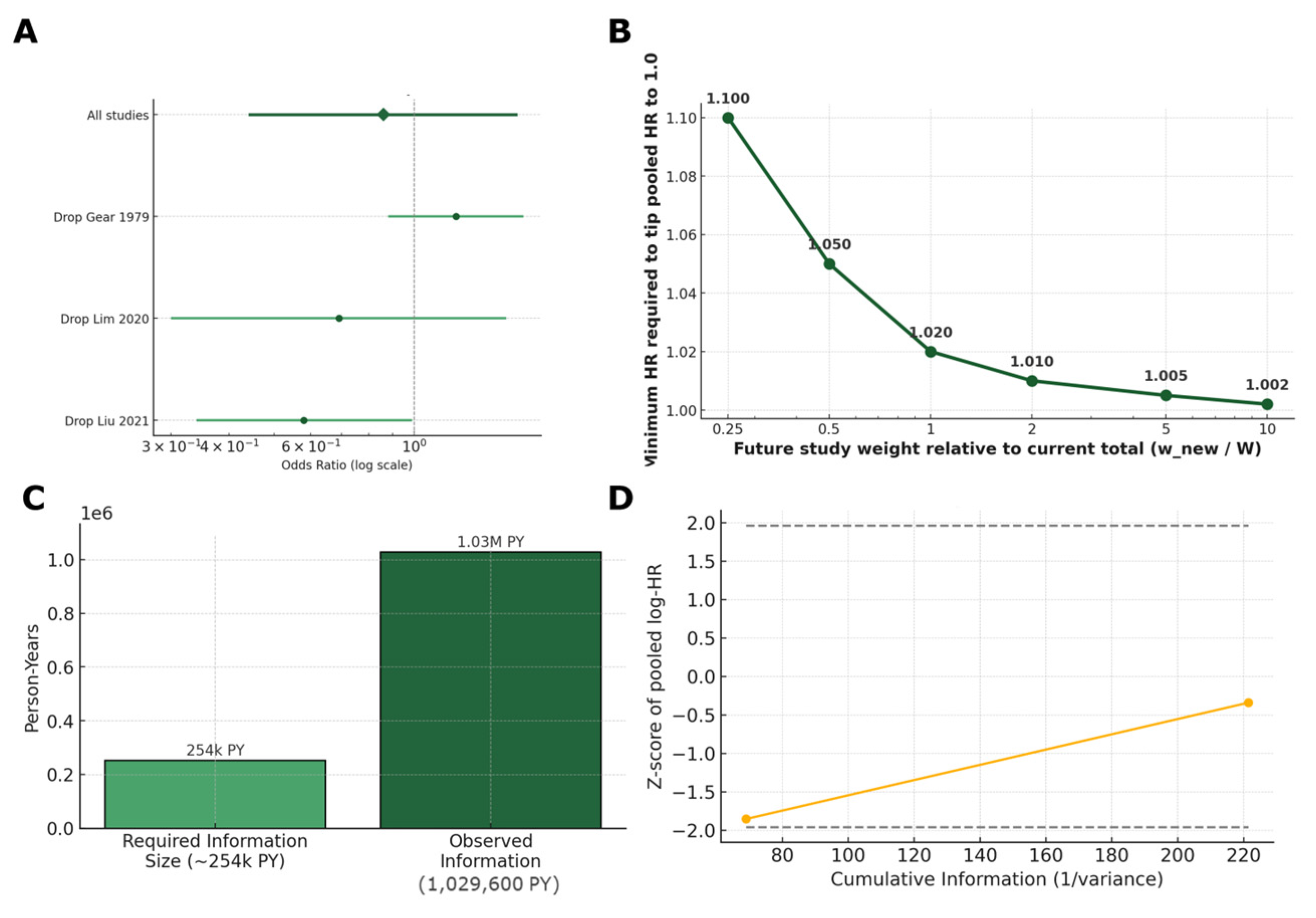
- Tipping-point analysis: we hypothesized that new studies are bound to be published in the future. We sought to investigate how large and how harmful a new study would need to be to alter our combined HR to exactly 1.0. By varying the hypothetical study’s relative size, we calculated the minimum HR required to nullify the current pooled HR, illustrating result robustness [74].
- E-value for unmeasured confounding: to assess how robust our pooled HR is to hidden biases, we computed the E-value for the point estimate and the confidence bound nearest to no effect. The E-value, a metric introduced by Van Der Weele & Ding (2017) [75], represents the minimum strength of association an unmeasured confounder would need to have with both nut intake and diverticulitis, beyond the measured covariates, to fully explain away the observed hazard ratio (HR). For protective effects (HR < 1) the E-value is computed as HR + √[HR × (HR* − 1)]**, where HR* = 1/HR [75].
4.3.4. Sequential Monitoring and Stability Checks
4.3.5. Absolute and Public-Health Metrics
5. Results
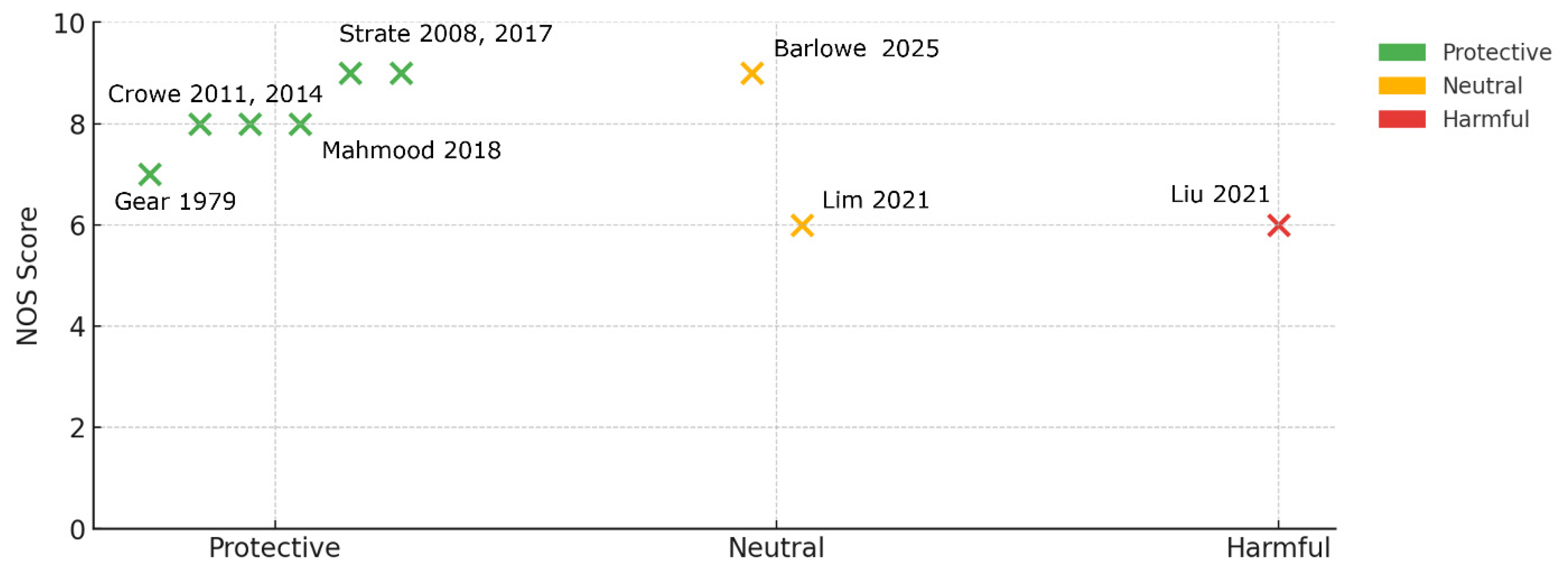
5.1. Investigated Studies
5.2. Qualitative Analysis
5.2.1. Risk of Bias
5.2.2. Certainty of Evidence
5.2.3. Narrative Synthesis
5.3. Quantitative Analysis
5.3.1. Outcome-Specific Analyses
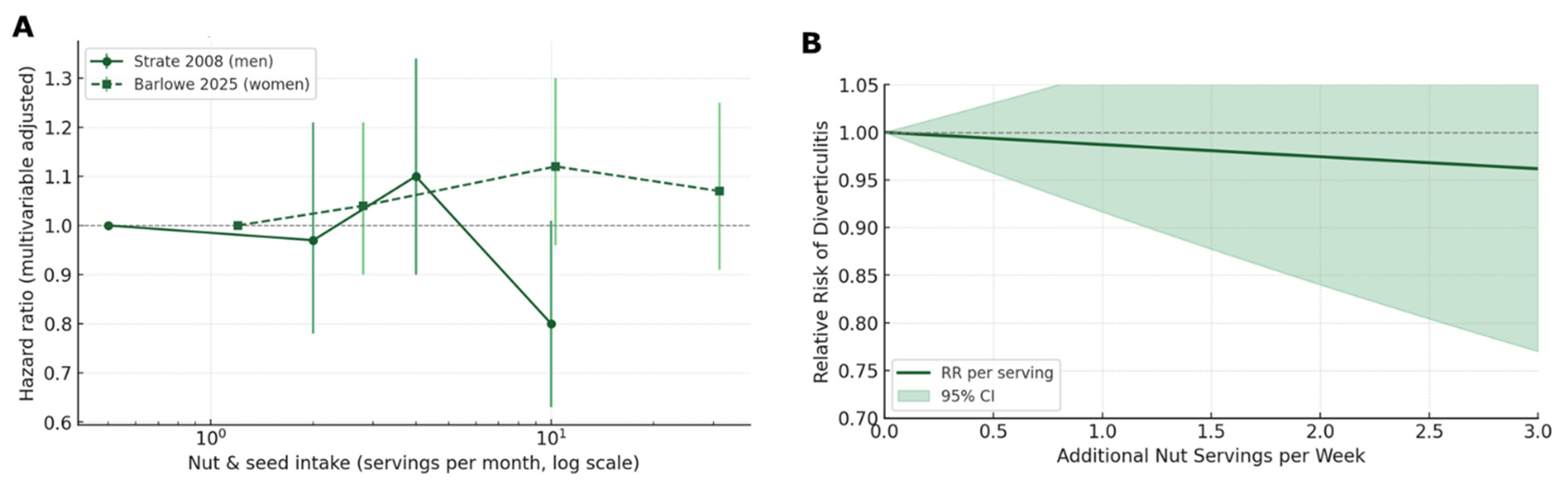
5.3.2. Dose–Response Modelling
5.3.3. Influence, Robustness, and Influence Diagnostics
5.3.4. Sequential Monitoring and Stability Checks
5.3.5. Absolute and Public-Health Metrics
6. Discussion
6.1. Principal Findings
6.2. Comparison with Guidelines
6.3. Mechanistic Insights and Clinical Points of View
6.4. Strengths and Limitations
6.5. The Need for Future Studies
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HR | Hazard ratio |
| HPFS | Health Professionals Follow-up Study |
| I2 | Percentage of the variability in effect estimates that is due to heterogeneity |
| IPD | Individual participant data |
| LOO | Leave-one-out (influence) analysis |
| NHANES III | Third National Health and Nutrition Examination Survey |
| NOS | Newcastle–Ottawa Scale |
| NNT | Number needed to treat |
| OR | Odds ratio |
| PAF | Population attributable fraction |
| PR | Prevalence ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomised controlled trial |
| RIS | Required information size |
| RoB | Risk of Bias |
| ROBINS-I | Risk Of Bias In Non-randomized Studies—of Interventions |
| RR | Relative risk |
| TSA | Trial sequential analysis |
| α | Probability of a Type I error |
| β | Probability of a Type II error |
| k | Number of studies |
| τ2 | Between-study variance in random-effects meta-analysis |
Appendix A
| # | First Author (Year) | Country | Design | Sample (n) | Nut Exposure | Outcome | Use in Analyses |
|---|---|---|---|---|---|---|---|
| 1 | Gear, J.S. (1979) [81] | UK | Cross-section | 376 | Lifelong vegetarian status (proxy for high nuts/seeds) | Prevalent diverticulosis | Reported narratively Prevalence meta-analysis RoB-weighted model Baujat plot |
| 2 | Strate, L.L. (2008) [47] | USA | Cohort (18 years) | 47,228 | Peanuts, walnuts, other nuts, popcorn (servings wk−1) | Incident diverticulitis | Reported narratively Core diverticulitis meta-analysis Dose–response analysis Threshold meta-analysis Tipping-point analysis E-value |
| 3 | Crowe, F.L. (2011) [48] | UK | Cohort (11.6 years) | ~690,000 | Total dietary fiber (nut component not isolated) | Hospitalized diverticular disease | Reported narratively (not meta-pooled) |
| 4 | Crowe, F.L. (2014) [52] | UK | Cohort (6 years) | 690,075 | Change in fiber intake including nuts | Hospitalized diverticular disease | Reported narratively (not meta-pooled) |
| 5 | Strate, L.L. (2017) [67] | USA | Cohort 26 years | 46,295 | “Prudent” dietary-pattern score (rich in nuts) | Incident diverticulitis | Reported narratively Sensitivity meta-analysis (only labelled to avoid double-counting) |
| 6 | Mahmood, W. (2018) [54] | Nordic | Cohort (7 years) | ~80,000 | Fruit-/veg-fiber (includes nut residues) | Hospital diverticular disease | Narrative only (not meta-pooled) |
| 7 | Lim, Y.K. (2020) [83] | Korea | Cross-section | 3864 | “Snack” dietary-pattern factor (contains nuts) | Right-colonic diverticulosis | Reported narratively Prevalence meta-analysis RoB-weighted model Baujat plot |
| 8 | Liu, Y.-H. (2021) [82] | Taiwan | Cross-section | 5586 | Betel-nut (areca) chewing (freq. categories) | Any diverticulosis | Reported narratively Prevalence meta-analysis RoB-weighted model Baujat plot |
| 9 | Barlowe, T. (2025) [66] | USA | Cohort (6 years) | 29,916 | Total nuts + seeds (quartiles, g d−1) | Incident diverticulitis | Reported narratively Core diverticulitis meta-analysis Prevalence meta-analysis Dose–response analysis Threshold meta-analysis Tipping-point analysis RoB-weighted model Baujat plot E-value |
References
- Barbaro, M.R.; Cremon, C.; Fuschi, D.; Marasco, G.; Palombo, M.; Stanghellini, V.; Barbara, G. Pathophysiology of Diverticular Disease: From Diverticula Formation to Symptom Generation. Int. J. Mol. Sci. 2022, 23, 6698. [Google Scholar] [CrossRef]
- Tursi, A. Diverticulosis Today: Unfashionable and Still under-Researched. Ther. Adv. Gastroenterol. 2016, 9, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Ali, S.; Stollman, N. Diverticular Disease: An Update on Pathogenesis and Management. Gut Liver 2018, 12, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Lembcke, B. Diagnosis, Differential Diagnoses, and Classification of Diverticular Disease. Visz. Gastrointest. Med. Surg. 2015, 31, 95–102. [Google Scholar] [CrossRef]
- Strate, L.L.; Morris, A.M. Epidemiology, Pathophysiology, and Treatment of Diverticulitis. Gastroenterology 2019, 156, 1282–1298.e1. [Google Scholar] [CrossRef]
- Stollman, N.; Smalley, W.; Hirano, I. American Gastroenterological Association Institute Guideline on the Management of Acute Diverticulitis. Gastroenterology 2015, 149, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.; McGrath, J.W.; Graham, R.; McMullan, G. Food for Thought-The Link between Clostridioides Difficile Metabolism and Pathogenesis. PLoS Pathog. 2023, 19, e1011034. [Google Scholar] [CrossRef]
- Zullo, A. Medical Hypothesis: Speculating on the Pathogenesis of Acute Diverticulitis. Ann. Gastroenterol. 2018, 31, 747–749. [Google Scholar] [CrossRef]
- Munie, S.T.; Nalamati, S.P.M. Epidemiology and Pathophysiology of Diverticular Disease. Clin. Colon Rectal Surg. 2018, 31, 209–213. [Google Scholar] [CrossRef]
- Teke, E.; Ciyiltepe, H.; Bulut, N.E.; Gunes, Y.; Fersahoglu, M.M.; Ergin, A.; Karip, B.; Memisoglu, K. Management of Acute Uncomplicated Diverticulitis: Inpatient or Outpatient. Sisli Etfal Hastan. Tip Bul. 2022, 56, 503–508. [Google Scholar] [CrossRef]
- Thomas, S.; Peel, R.L.; Evans, L.E.; Haarer, K.A. Giant Colonic Diverticulum. RadioGraphics 2006, 26, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Mcquade, K.L.; Foreman, M.L. Giant Colonic Diverticulum. Bayl. Univ. Med. Cent. Proc. 2008, 21, 25. [Google Scholar] [CrossRef]
- Custer, T.J.; Blevins, D.V.; Vara, T.M. Giant Colonic Diverticulum: A Rare Manifestation of a Common Disease. J. Gastrointest. Surg. 1999, 3, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Nigri, G.; Petrucciani, N.; Giannini, G.; Aurello, P.; Magistri, P.; Gasparrini, M.; Ramacciato, G. Giant Colonic Diverticulum: Clinical Presentation, Diagnosis and Treatment: Systematic Review of 166 Cases. World J. Gastroenterol. 2015, 21, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, R.; Cargiolli, M.; Cassarano, S.; Carabotti, M.; Annibale, B. Treatment of Diverticular Disease, Targeting Symptoms or Underlying Mechanisms. Curr. Opin. Pharmacol. 2018, 43, 124–131. [Google Scholar] [CrossRef]
- Granlund, J.; Svensson, T.; Olén, O.; Hjern, F.; Pedersen, N.L.; Magnusson, P.K.E.; Thelin Schmidt, P. The Genetic Influence on Diverticular Disease—A Twin Study. Aliment. Pharmacol. Ther. 2012, 35, 1103–1107. [Google Scholar] [CrossRef]
- Ma, W.; Nguyen, L.H.; Song, M.; Jovani, M.; Liu, P.H.; Cao, Y.; Tam, I.; Wu, K.; Giovannucci, E.L.; Strate, L.L.; et al. Intake of Dietary Fiber, Fruits, and Vegetables and Risk of Diverticulitis. Am. J. Gastroenterol. 2019, 114, 1531–1538. [Google Scholar] [CrossRef]
- Carabotti, M.; Falangone, F.; Cuomo, R.; Annibale, B. Role of Dietary Habits in the Prevention of Diverticular Disease Complications: A Systematic Review. Nutrients 2021, 13, 1288. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Corrente, V.; Tana, C.; Di Mario, F.; Meschi, T. Diverticular Disease: A Gut Microbiota Perspective. J. Gastrointest. Liver Dis. 2019, 28, 327–337. [Google Scholar] [CrossRef]
- Tursi, A.; Papa, A. The Role of Gut Microbiota in the Pathogenesis of Diverticular Disease: Where Are We Now? Genome Med. 2024, 16, 153. [Google Scholar] [CrossRef]
- Peery, A.F.; Keku, T.O.; Martin, C.F.; Eluri, S.; Runge, T.; Galanko, J.A.; Sandler, R.S. Distribution and Characteristics of Colonic Diverticula in a United States Screening Population. Clin. Gastroenterol. Hepatol. 2016, 14, 980–985.e1. [Google Scholar] [CrossRef] [PubMed]
- Azhar, N.; Aref, H.; Brorsson, A.; Lydrup, M.L.; Jörgren, F.; Schultz, J.K.; Buchwald, P. Management of Acute Uncomplicated Diverticulitis without Antibiotics: Compliance and Outcomes—A Retrospective Cohort Study. BMC Emerg. Med. 2022, 22, 28. [Google Scholar] [CrossRef]
- Rodríguez-Cerrillo, M.; Poza-Montoro, A.; Fernandez-Diaz, E.; Matesanz-David, M.; Iñurrieta Romero, A. Treatment of Elderly Patients with Uncomplicated Diverticulitis, Even with Comorbidity, at Home. Eur. J. Intern. Med. 2013, 24, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A. Advances in the Management of Colonic Diverticulitis. CMAJ Can. Med. Assoc. J. 2012, 184, 1470–1476. [Google Scholar] [CrossRef][Green Version]
- Ahmed, A.M.; Moahammed, A.T.; Mattar, O.M.; Mohamed, E.M.; Faraag, E.A.E.; AlSafadi, A.M.; Hirayama, K.; Huy, N.T. Surgical Treatment of Diverticulitis and Its Complications: A Systematic Review and Meta-Analysis of Randomized Control Trials. Surgeon 2018, 16, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Minordi, L.M.; Larosa, L.; Berte, G.; Pecere, S.; Manfredi, R. CT of the Acute Colonic Diverticulitis: A Pictorial Essay. Diagn. Interv. Radiol. 2020, 26, 546–551. [Google Scholar] [CrossRef]
- Daniels, L.; Ünlü, Ç.; de Korte, N.; van Dieren, S.; Stockmann, H.B.; Vrouenraets, B.C.; Consten, E.C.; van der Hoeven, J.A.; Eijsbouts, Q.A.; Faneyte, I.F.; et al. Randomized Clinical Trial of Observational versus Antibiotic Treatment for a First Episode of CT-Proven Uncomplicated Acute Diverticulitis. Br. J. Surg. 2017, 104, 52–61. [Google Scholar] [CrossRef]
- Kishnani, S.; Ottaviano, K.; Rosenberg, L.; Arker, S.H.; Lee, H.; Schuster, M.; Tadros, M.; Valerian, B. Diverticular Disease—An Updated Management Review. Gastroenterol. Insights 2022, 13, 326–339. [Google Scholar] [CrossRef]
- Dichman, M.L.; Rosenstock, S.J.; Shabanzadeh, D.M. Antibiotics for Uncomplicated Diverticulitis. Cochrane Database Syst. Rev. 2022, 2022, CD009092. [Google Scholar] [CrossRef]
- Peery, A.F.; Shaukat, A.; Strate, L.L. AGA Clinical Practice Update on Medical Management of Colonic Diverticulitis: Expert Review. Gastroenterology 2021, 160, 906–911.e1. [Google Scholar] [CrossRef]
- Isacson, D.; Smedh, K.; Nikberg, M.; Chabok, A. Long-Term Follow-up of the AVOD Randomized Trial of Antibiotic Avoidance in Uncomplicated Diverticulitis. Br. J. Surg. 2019, 106, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, S.T.; Daniels, L.; Ünlü, Ç.; de Korte, N.; van Dieren, S.; Stockmann, H.B.; Vrouenraets, B.C.; Consten, E.C.; van der Hoeven, J.A.; Eijsbouts, Q.A.; et al. Long-Term Effects of Omitting Antibiotics in Uncomplicated Acute Diverticulitis. Am. J. Gastroenterol. 2018, 113, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Hardiman, K.; Lee, S.; Lightner, A.; Stocchi, L.; Paquette, I.M.; Steele, S.R.; Feingold, D.L. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Left-Sided Colonic Diverticulitis. Dis. Colon Rectum 2020, 63, 728–747. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.H.; Kaiser, A.M. Update on the Management of Sigmoid Diverticulitis. World J. Gastroenterol. 2021, 27, 760–781. [Google Scholar] [CrossRef] [PubMed]
- Mohamedahmed, A.Y.; Zaman, S.; Das, N.; Kakaniaris, G.; Vakis, S.; Eccersley, J.; Thomas, P.; Husain, N. Systematic Review and Meta-Analysis of the Management of Acute Uncomplicated Diverticulitis: Time to Change Traditional Practice. Int. J. Color. Dis. 2024, 39, 47. [Google Scholar] [CrossRef]
- Klarenbeek, B.R.; De Korte, N.; Van Der Peet, D.L.; Cuesta, M.A. Review of Current Classifications for Diverticular Disease and a Translation into Clinical Practice. Int. J. Color. Dis. 2011, 27, 207. [Google Scholar] [CrossRef]
- Mohammed Ilyas, M.I.; Szilagy, E.J. Management of Diverticular Bleeding: Evaluation, Stabilization, Intervention, and Recurrence of Bleeding and Indications for Resection after Control of Bleeding. Clin. Colon. Rectal Surg. 2018, 31, 243–250. [Google Scholar] [CrossRef]
- Sartelli, M.; Weber, D.G.; Kluger, Y.; Ansaloni, L.; Coccolini, F.; Abu-Zidan, F.; Augustin, G.; Ben-Ishay, O.; Biffl, W.L.; Bouliaris, K.; et al. 2020 Update of the WSES Guidelines for the Management of Acute Colonic Diverticulitis in the Emergency Setting. World J. Emerg. Surg. 2020, 15, 32. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Di Mario, F.; Lanas, A.; Scarpignato, C.; Bafutto, M.; Barbara, G.; Bassotti, G.; Binda, G.A.; Biondi, A.; et al. International Consensus on Diverticulosis and Diverticular Disease. Statements from the 3rd International Symposium on Diverticular Disease. J. Gastrointestin. Liver Dis. 2019, 28, 57–65. [Google Scholar] [CrossRef]
- Schultz, J.K.; Azhar, N.; Binda, G.A.; Barbara, G.; Biondo, S.; Boermeester, M.A.; Chabok, A.; Consten, E.C.J.; van Dijk, S.T.; Johanssen, A.; et al. European Society of Coloproctology: Guidelines for the Management of Diverticular Disease of the Colon. Color. Dis. 2020, 22, 5–28. [Google Scholar] [CrossRef]
- Tanabe, H.; Tanaka, K.; Goto, M.; Sato, T.; Sato, K.; Fujiya, M.; Okumura, T. Rare Case of Fecal Impaction Caused by a Fecalith Originating in a Large Colonic Diverticulum: A Case Report. World J. Clin. Cases 2021, 9, 416. [Google Scholar] [CrossRef]
- Solak, A.; Solak, I.; Genç, B.; Sahin, N.; Yalaz, S. Transverse Colon Diverticulitis with Calcified Fecalith. Eurasian J. Med. 2013, 45, 68. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Park, S.B. A Case of Retroperitoneal Abscess: A Rare Complication of Meckel’s Diverticulum. Int. J. Surg. Case Rep. 2017, 41, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Wisnik, C.A.; Abdel-Khalek, A.; Fleites, O.; Pelenyi, S.S.; Tariq, A.; Tiesenga, F. Peanut-Related Perforated Diverticulitis Before the Age of 60. Cureus 2021, 13, e19767. [Google Scholar] [CrossRef]
- Painter, N.S. Diverticular Disease of the Colon—A Disease of the Century. Lancet 1969, 2, 586–588. [Google Scholar] [CrossRef]
- Cummings, J.H.; Engineer, A. Denis Burkitt and the Origins of the Dietary Fibre Hypothesis. Nutr. Res. Rev. 2018, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Strate, L.L.; Liu, Y.L.; Syngal, S.; Aldoori, W.H.; Giovannucci, E.L. Nut, Corn, and Popcorn Consumption and the Incidence of Diverticular Disease. JAMA 2008, 300, 907–914. [Google Scholar] [CrossRef]
- Crowe, F.L.; Appleby, P.N.; Allen, N.E.; Key, T.J. Diet and Risk of Diverticular Disease in Oxford Cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): Prospective Study of British Vegetarians and Non-Vegetarians. BMJ 2011, 343, d4131. [Google Scholar] [CrossRef]
- Creedon, A.C.; Hung, E.S.; Berry, S.E.; Whelan, K. Nuts and Their Effect on Gut Microbiota, Gut Function and Symptoms in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 2347. [Google Scholar] [CrossRef]
- Campos, S.B.; de Oliveira Filho, J.G.; Salgaço, M.K.; De Jesus, M.H.; Egea, M.B. Effects of Peanuts and Pistachios on Gut Microbiota and Metabolic Syndrome: A Review. Foods 2023, 12, 4440. [Google Scholar] [CrossRef]
- Manousos, O.; Day, N.E.; Tzonou, A.; Papadimitriou, C.; Kapetanakis, A.; Polychronopoulou-Trichopoulou, A.; Trichopoulos, D. Diet and Other Factors in the Aetiology of Diverticulosis: An Epidemiological Study in Greece. Gut 1985, 26, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Balkwill, A.; Cairns, B.J.; Appleby, P.N.; Green, J.; Reeves, G.K.; Key, T.J.; Beral, V. Source of Dietary Fibre and Diverticular Disease Incidence: A Prospective Study of UK Women. Gut 2014, 63, 1450–1456. [Google Scholar] [CrossRef]
- Aldoori, W.H.; Giovannucci, E.L.; Rimm, E.B.; Wing, A.L.; Trichopoulos, D.V.; Willett, W.C. A Prospective Study of Diet and the Risk of Symptomatic Diverticular Disease in Men. Am. J. Clin. Nutr. 1994, 60, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.W.; Abraham-Nordling, M.; Håkansson, N.; Wolk, A.; Hjern, F. High Intake of Dietary Fibre from Fruit and Vegetables Reduces the Risk of Hospitalisation for Diverticular Disease. Eur. J. Nutr. 2019, 58, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Ga, S.W. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Bezerra, C.; Grande, A.J.; Galvão, V.K.; Dos Santos, D.H.M.; Atallah, Á.N.; Silva, V. Assessment of the Strength of Recommendation and Quality of Evidence: GRADE Checklist. A Descriptive Study. São Paulo Med. J. 2022, 140, 829. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; Debeer, H.; et al. GRADE Guidelines: 1. Introduction-GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without Meta-Analysis (SWiM) in Systematic Reviews: Reporting Guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Barnett-Page, E.; Thomas, J. Methods for the Synthesis of Qualitative Research: A Critical Review. BMC Med. Res. Methodol. 2009, 9, 59. [Google Scholar] [CrossRef]
- Ogilvie, D.; Fayter, D.; Petticrew, M.; Sowden, A.; Thomas, S.; Whitehead, M.; Worthy, G. The Harvest Plot: A Method for Synthesising Evidence about the Differential Effects of Interventions. BMC Med. Res. Methodol. 2008, 8, 8. [Google Scholar] [CrossRef]
- McKenzie, J.E.; Brennan, S.E. Synthesizing and Presenting Findings Using Other Methods. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 321–347. [Google Scholar] [CrossRef]
- Guidance on the Conduct of Narrative Synthesis in Systematic Reviews Final Report. Available online: https://www.researchgate.net/publication/235608210_Guidance_on_the_Conduct_of_Narrative_Synthesis_in_Systematic_Reviews_Final_Report (accessed on 30 May 2025).
- Bramley, P.; López-López, J.A.; Higgins, J.P.T. Examining How Meta-analytic Methods Perform in the Presence of Bias: A Simulation Study. Res. Synth. Methods 2021, 12, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Röver, C.; Knapp, G.; Friede, T. Hartung-Knapp-Sidik-Jonkman Approach and Its Modification for Random-Effects Meta-Analysis with Few Studies. BMC Med. Res. Methodol. 2015, 15, 99. [Google Scholar] [CrossRef]
- Barlowe, T.; Anderson, C.; Nichols, H.B.; Salvador, A.C.; Sandler, R.S.; Sandler, D.P.; Peery, A.F. Diet and Risk for Incident Diverticulitis in Women. Ann. Intern. Med. 2025, 178, 788–795. [Google Scholar] [CrossRef]
- Strate, L.L.; Keeley, B.R.; Cao, Y.; Wu, K.; Giovannucci, E.L.; Chan, A.T. Western Dietary Pattern Increases, and Prudent Dietary Pattern Decreases, Risk of Incident Diverticulitis in a Prospective Cohort Study. Gastroenterology 2017, 152, 1023–1030.e2. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials Revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A Re-Evaluation of Random-Effects Meta-Analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Longnecker, M.P. Methods for Trend Estimation from Summarized Dose-Response Data, with Applications to Meta-Analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.L. Outlier and Influence Diagnostics for Meta-Analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Baujat, B.; Mahé, C.; Pignon, J.P.; Hill, C. A Graphical Method for Exploring Heterogeneity in Meta-Analyses: Application to a Meta-Analysis of 65 Trials. Stat. Med. 2002, 21, 2641–2652. [Google Scholar] [CrossRef]
- Turner, R.M.; Spiegelhalter, D.J.; Smith, G.C.S.; Thompson, S.G. Bias Modelling in Evidence Synthesis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 21–47. [Google Scholar] [CrossRef]
- Han, W.; Wang, Z.; Xiao, M.; He, Z.; Chu, H.; Lin, L. Tipping Point Analysis for the Between-Arm Correlation in an Arm-Based Evidence Synthesis. BMC Med. Res. Methodol. 2024, 24, 162. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef]
- De Cassai, A.; Pasin, L.; Boscolo, A.; Salvagno, M.; Navalesi, P. Trial Sequential Analysis: Plain and Simple. Korean J. Anesthesiol. 2020, 74, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ambrogi, F.; Bokov, A.F.; Gu, H.; de Beurs, E.; Eskaf, K. Estimate Risk Difference and Number Needed to Treat in Survival Analysis. Ann. Transl. Med. 2018, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Liu, L.; Stuart, E.A. Estimating Target Population Treatment Effects in Meta-Analysis with Individual Participant-Level Data. Stat. Methods Med. Res. 2025, 34, 355–368. [Google Scholar] [CrossRef]
- Peery, A.F.; Barrett, P.R.; Park, D.; Rogers, A.J.; Galanko, J.A.; Martin, C.F.; Sandler, R.S. A High-Fiber Diet Does Not Protect against Asymptomatic Diverticulosis. Gastroenterology 2012, 142, 266–272.e1. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Gear, J.S.S.; Fursdon, P.; Nolan, D.J.; Ware, A.; Mann, J.I.; Brodribb, A.J.M.; Vessey, M.P. Symptomless Diverticular Disease and Intake of Dietary Fibre. Lancet 1979, 313, 511–514. [Google Scholar] [CrossRef]
- Liu, Y.H.; Chen, W.L. Association between Betel Nut and Presence of Diverticulum in Male: A Cross-Sectional Study. Biomed. Res. Int. 2021, 2021, 6669792. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, Y.S.; Lee, J.E.; Youn, J.; Chung, G.E.; Song, J.H.; Yang, S.Y.; Kim, J.S. Dietary Pattern and Its Association with Right-Colonic Diverticulosis. J. Gastroenterol. Hepatol. 2021, 36, 144–150. [Google Scholar] [CrossRef]
- Rajaram, S.; Damasceno, N.R.T.; Braga, R.A.M.; Martinez, R.; Kris-Etherton, P.; Sala-Vila, A. Effect of Nuts on Markers of Inflammation and Oxidative Stress: A Narrative Review. Nutrients 2023, 15, 1099. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Malik, V.S.; Keum, N.N.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S.; Bao, Y. Associations between Nut Consumption and Inflammatory Biomarkers. Am. J. Clin. Nutr. 2016, 104, 722. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Gao, H.K. Impact of Different Types of Tree Nut, Peanut, and Soy Nut Consumption on Serum C-Reactive Protein (CRP): A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Medicine 2016, 95, e5165. [Google Scholar] [CrossRef] [PubMed]
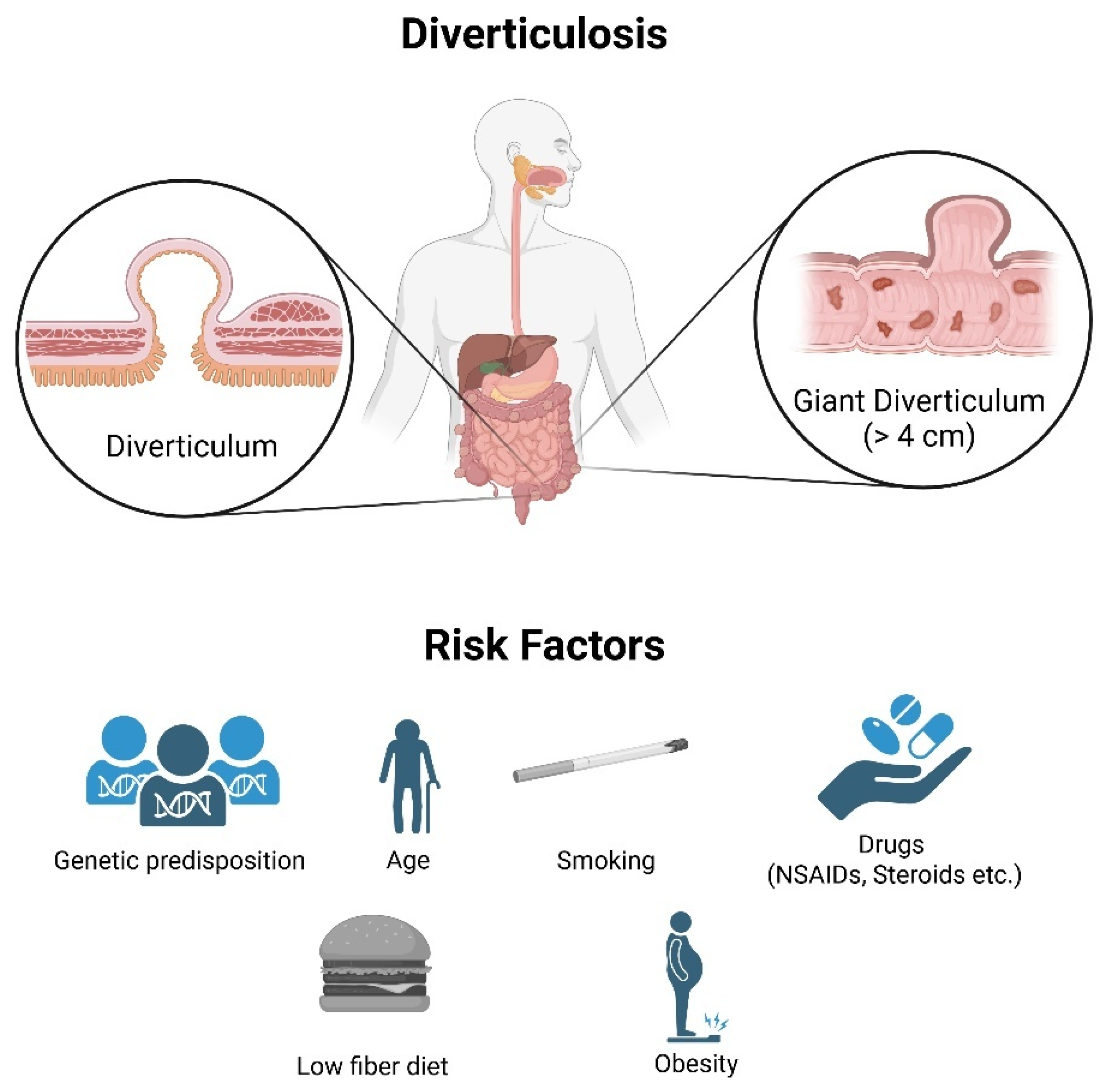




| Age | Sex Ratio | Prevalence |
|---|---|---|
| <40 years | Male > Female | 5–10% |
| 40–59 years | Male ≈ Female | 20–30% |
| 60–79 years | Male < Female | 50–60% |
| ≥80 years | Male < Female | 65–75% |
| Outcome and Comparison | Studies | Risk of Bias | Consistency | Directness | Precision | Publication Bias | GRADE Certainty |
|---|---|---|---|---|---|---|---|
| Incident diverticulitis high vs. low nut intake | Strate 2008 [47] Barlowe 2025 [66] | Not serious (both moderate ROBINS-I) | Serious (I2 = 91) | Direct (exposure, pop., outcome match PICO) | Serious (95% CI is wide and includes small harm) | Unlikely | Very Low |
| Incident diverticulitis, including prudent-diet proxy | Strate 2008 [47] Strate 2017 [67] Barlowe 2025 [66] | Not serious | Not serious (I2 = 68%) | Serious (diet pattern indirect for nuts) | Serious (CI still wide) | Unlikely | Very Low |
| Diverticulosis prevalence any nut-related exposure | Gear 1979 [81] Lim 2020 [83] Liu 2021 [82] | Serious (2 studies serious ROBINS-I) | Serious (I2 = 87%) | Serious (mixed proxies; betel ≠ culinary nuts) | Serious (CIs 0.18–4.06) | Unclear | Very Low |
| Hospital admission for diverticular disease vegetarian/nut-rich vs. non-vegetarian | Crowe 2011 [48] | Moderate (registry cohort) | N/A (single study) | Serious (vegetarianism only proxy for nuts) | Moderate (adequate events, but one estimate) | Unclear | Very Low |
| Acute complications nuts vs. low intake | Strate 2008 [47] | Serious (single cohort; residual confounding) | N/A | Direct (culinary nuts) | Very serious (sparse events) | Likely | Very Low |
| Study | Design | Exposure | Outcome | Main Strengths | Main Limitations |
|---|---|---|---|---|---|
| Gear, J.S. (1979) [81] | Cross-section (n = 376) | Lifelong Vegetarians (presumed high nut diet) | Prevalent diverticulosis | Radiologic outcome confirmation: validated questionnaire | Cross-sectional; nuts inferred indirectly; hospital selection bias |
| Strate, L.L. (2008) [47] | Cohort (18 years) (n = 47,228) | Peanuts, walnuts, and other nuts | Incident diverticulitis | Very large cohort; validated FFQ; chart-confirmed diverticulitis; long follow-up | Male health professionals only; residual confounding; observational design |
| Crowe, F.L. (2011) [48] | Cohort (11.6 years) (n = 690,000) | Total dietary fiber (presumed high nut diet) | Hospitalized Diverticular disease | Huge cohort; hospital-verified outcomes | Presumed nut diet nit measured older women only severe cases |
| Crowe, F.L. (2014) [52] | Cohort (17.6 years) (n = 690,000) | Total dietary fiber (presumed high nut diet | Hospitalized diverticular disease | Robust hospital linkage; sensitivity analyses | Presumed nut diet, Documented only severe cases |
| Strate, L.L. (2017) [67] | Cohort 26 years (n = 46,295) | Prudent diet (rich in nuts) | Incident diverticulitis | Extended follow-up; holistic diet pattern | Nuts not isolated; double use of HPFS person-years; pattern confounding |
| Mahmood, W. (2018) [54] | Cohort (7 years) (n = 80,000) | Vegetarians (high nut diet) | Hospital diverticular disease | Two large cohorts: national registers | Presumed nut diet |
| Lim, Y.K. (2020) [83] | Cross-section (n = 3864) | “Snack” dietary-pattern factor (contains nuts) | Right-colonic diverticulosis | Colonoscopic confirmation; structured FFQ | Cross-sectional; Single center; Pattern includes high-sugar foods |
| Liu, Y.-H. (2021) [82] | Cross-section (n = 5586) | Betel-nut | Any diverticulosis | Large sample; Colonoscopic outcome; adjusted for smoking/alcohol | Cross-sectional; male-only; Exposure to non-culinary nut |
| Barlowe, A. (2025) [66] | Cohort (6 years) (n = 29,916) | Peanuts, walnuts, other nuts | Incident diverticulitis | Prospective, validated diet tool; medical-record outcomes; female cohort | Moderate follow-up seeds pooled with nuts self-reported diet |
| Study | Exposure Category | Mid-Point * (Servings wk−1) | Outcome Ascertainment | Effect Measure | Effect Estimate | 95% CI (Lower–Upper) | Covariates |
|---|---|---|---|---|---|---|---|
| Strate, L.L. (2008) [47] | Culinary nuts (≥2 serv wk−1 vs. <1 mo−1) | ≈4 serv. wk−1 † | Chart-confirmed incident diverticulitis | HR | 0.80 | 0.63–1.01 | Age, BMI, total energy, smoking, alcohol, physical activity, red meat, fiber |
| Barlowe, A. (2025) [66] | Culinary nuts (Q4 vs. Q1) | ≈7 serv. wk−1 † | Medical-record incident diverticulitis | HR | 1.07 | 0.91–1.25 | Age, race/ethnicity, BMI, energy, smoking, alcohol, physical activity, hormone use |
| Strate, L.L. (2017) [67] | Nut rich diet Q5 vs. Q1 | N/A (pattern score) | Chart-confirmed incident diverticulitis | HR | 0.56 | 0.45–0.70 | Age, BMI, smoking, energy, alcohol, physical activity, red-meat pattern |
| Gear, J.S. (1979) [81] | Nut rich diet (nut-rich proxy vs. omnivore) | N/A | Barium-contrast X-ray diverticulosis prevalence | OR | 0.29 | 0.13–0.66 | Age (matching); other covariates not reported |
| Lim, Y.K. (2020) [83] | “Snack” dietary pattern (nuts + sweets) top vs. bottom tertile | N/A | Colonoscopy-verified right-sided diverticulosis | OR | 0.95 | 0.76–1.18 | Age, sex, BMI, smoking, alcohol, comorbidity score |
| Liu, Y.-H. (2021) [82] | Daily betel nut chewing vs. none | ≈14 “chews” wk−1 ‡ | Colonoscopy-verified any diverticulosis | OR | 1.65 | 1.12–2.44 | Age, BMI, smoking, alcohol, diabetes, hypertension |
| Analysis Set | k | Pooled Effect | 95% CI | I2 (%) | Prediction Interval |
|---|---|---|---|---|---|
| Incident diverticulitis (two nut-specific cohorts) | 2 | HR 0.89 | 0.71–1.12 | 91% | 0.43–1.84 |
| Incident diverticulitis (+prudent pattern for sensitivity) | 3 | HR 0.75 | 0.58–0.97 | 68% | 0.34–1.64 |
| Diverticulosis prevalence (mixed exposures) | 3 | OR 0.86 | 0.44–1.67 | 87% | 0.18–4.06 |
| Threshold analysis (≥2 servings weekly vs. <1 serving monthly) | 2 | HR 0.89 | 0.71–1.12 | 91% | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voniatis, C.; Csupor, T.; Szijártó, A. Are Nuts Safe in Diverticulosis? A Mixed-Methods Systematic Review of Available Evidence. Nutrients 2025, 17, 2122. https://doi.org/10.3390/nu17132122
Voniatis C, Csupor T, Szijártó A. Are Nuts Safe in Diverticulosis? A Mixed-Methods Systematic Review of Available Evidence. Nutrients. 2025; 17(13):2122. https://doi.org/10.3390/nu17132122
Chicago/Turabian StyleVoniatis, Constantinos, Timea Csupor, and Attila Szijártó. 2025. "Are Nuts Safe in Diverticulosis? A Mixed-Methods Systematic Review of Available Evidence" Nutrients 17, no. 13: 2122. https://doi.org/10.3390/nu17132122
APA StyleVoniatis, C., Csupor, T., & Szijártó, A. (2025). Are Nuts Safe in Diverticulosis? A Mixed-Methods Systematic Review of Available Evidence. Nutrients, 17(13), 2122. https://doi.org/10.3390/nu17132122