Industrial Bread Composition: Potential Implications for Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Methods
2.1. Breads Screening
2.2. Ingredients Analysis
2.3. Literature Review
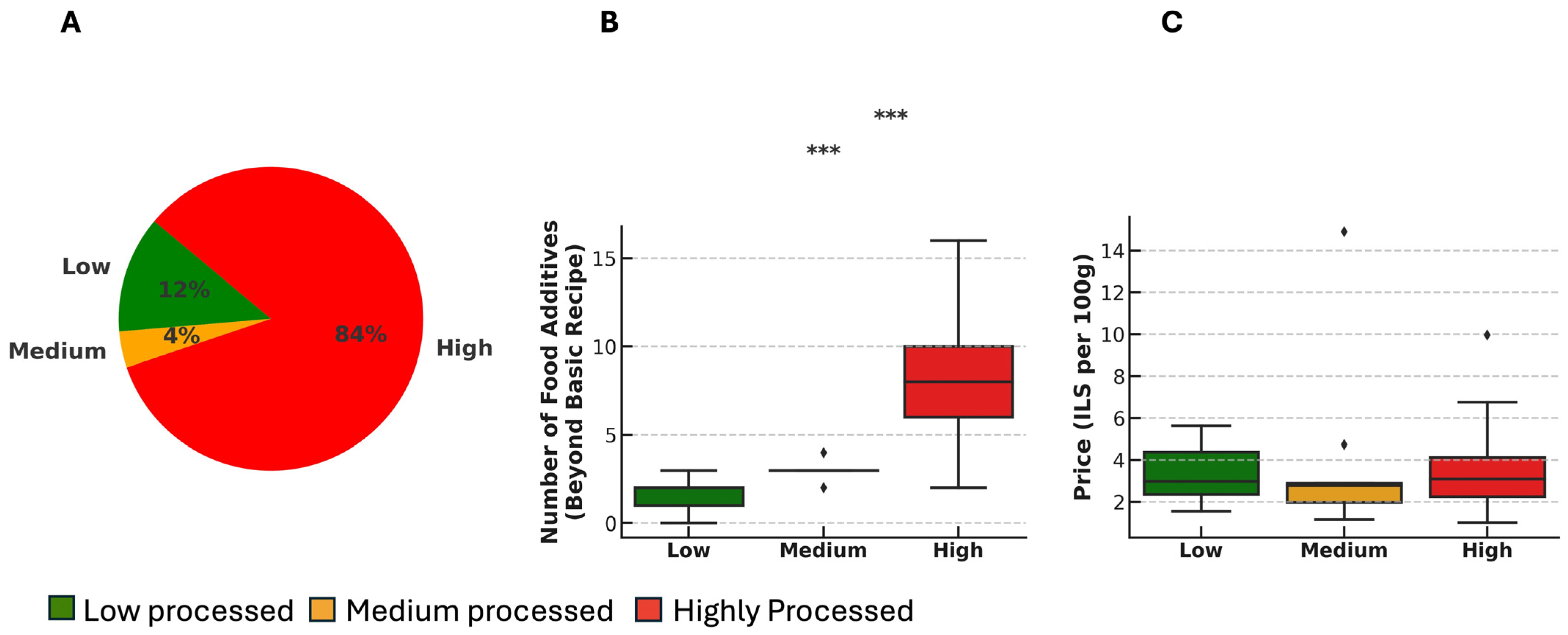
3. Results
3.1. Most Breads Are Highly Processed and Contain Multiple FAs
3.2. Preservatives and Emulsifiers Are Dominant in Industrial Breads
3.3. Prevalent FAs May Have Implications on Inflammation or Gut Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Stampfli, L.; Nersten, B. Emulsifiers in bread making. Food Chem. 1995, 52, 353–360. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Zhang, J. Diet fuelling inflammatory bowel diseases: Preclinical and clinical concepts. Gut 2022, 71, 2574–2586. [Google Scholar] [CrossRef]
- Sandall, A.; Smith, L.; Svensen, E.; Whelan, K. Emulsifiers in ultra-processed foods in the UK food supply. Public Health Nutr. 2023, 26, 2256–2270. [Google Scholar] [CrossRef]
- Sandall, A.M.; Cox, S.R.; Lindsay, J.O.; Gewirtz, A.T.; Chassaing, B.; Rossi, M.; Whelan, K. Emulsifiers Impact Colonic Length in Mice and Emulsifier Restriction is Feasible in People with Crohn’s Disease. Nutrients 2020, 12, 2827. [Google Scholar] [CrossRef]
- The Central Bureau of Statistics. Household Income and Expenses from the 2021 Household Expenditure Survey. 2023. Available online: www.cbs.gov.il (accessed on 26 May 2024).
- Eicher-Miller, H.; Boushey, C. How Often and How Much? Differences in Dietary Intake by Frequency and Energy Contribution Vary among U.S. Adults in NHANES 2007–2012. Nutrients 2017, 9, 86. [Google Scholar] [CrossRef]
- Godny, L.; Elial-Fatal, S.; Arrouasse, J.; Fischler, T.S.; Reshef, L.; Kutukov, Y.; Cohen, S.; Pfeffer-Gik, T.; Barkan, R.; Shakhman, S.; et al. Mechanistic Implications of the Mediterranean Diet in Patients with Newly Diagnosed Crohn’s Disease: Multiomic Results From a Prospective Cohort. Gastroenterology 2025, 168, 952–964.e2. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-H.; Khandpur, N.; Rossato, S.L.; Lochhead, P.; Lopes, E.W.; Burke, K.E.; Richter, J.M.; Song, M.; Korat, A.V.A.; Sun, Q.; et al. Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, e1323–e1337. [Google Scholar] [CrossRef] [PubMed]
- Sarbagili-Shabat, C.; Zelber-Sagi, S.; Isakov, N.F.; Hirsch, A.; Ron, Y.; Grinshpan, L.S.; Cohen, N.A.; Leibovitzh, H.; Thurm, T.; Maharshak, N. High ultra-processed food consumption is associated with clinical exacerbation in patients with Crohn’s disease in remission—A prospective cohort study. Dig. Dis. 2025, 1–17. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.; Moubarac, J.C.; Jaime, P.; Martins, A.P.; Canella, D.; Louzada, M.; Parra, D. NOVA. The star shines bright. World Nutr. 2016, 7, 28–38. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Laura Da Costa Louzada, M.; Machado, P.P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System. 2019. Available online: http://www.wipo.int/amc/en/mediation/rules (accessed on 4 July 2024).
- Food and Agriculture Organization of the United Nations, World Health Organization. General Standard for Food Additives. 1995. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/en/ (accessed on 2 July 2024).
- Israel Ministry of Health; Public Health Services; Food Control Services. Food Additives Approved for Use in Israel—List and Conditions. 2022. Available online: https://www.gov.il/BlobFolder/reports/fcs_list/he/files_publications_food_food-addictive-libarary_fcs_list.pdf (accessed on 2 July 2024).
- Cauvain, S. Technology of Breadmaking; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Katsoudas, N.; Tavakoli, P.; Wu, N.; Shapiro, A.; Leach, S.T.; Williams, A.-J.; Paramsothy, R.; Ghaly, S.; Connor, S.J.; Samocha-Bonet, D.; et al. Dietary Emulsifier Exposure in People With Inflammatory Bowel Disease Compared with Healthy Controls: Is There a Cause for Concern? Inflamm. Bowel Dis. 2024, 30, 1241–1250. [Google Scholar] [CrossRef]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef]
- Elmén, L.; Zlamal, J.E.; Scott, D.A.; Lee, R.B.; Chen, D.J.; Colas, A.R.; Rodionov, D.A.; Peterson, S.N. Dietary Emulsifier Sodium Stearoyl Lactylate Alters Gut Microbiota in vitro and Inhibits Bacterial Butyrate Producers. Front. Microbiol. 2020, 11, 892. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, M.; Zhang, H.; Li, Y.; Liu, M.; Feng, F. Antimicrobial Emulsifier–Glycerol Monolaurate Induces Metabolic Syndrome, Gut Microbiota Dysbiosis, and Systemic Low-Grade Inflammation in Low-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1700547. [Google Scholar] [CrossRef]
- Swidsinski, A.; Ung, V.; Sydora, B.C.; Loening-Baucke, V.; Doerffel, Y.; Verstraelen, H.; Fedorak, R.N. Bacterial Overgrowth and Inflammation of Small Intestine After Carboxymethylcellulose Ingestion in Genetically Susceptible Mice. Inflamm. Bowel Dis. 2009, 15, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Bretin, A.; Dubé, P.E.; Maue, A.C.; Dauriat, C.J.; Barnich, N.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifiers Directly Impact Adherent-Invasive E. coli Gene Expression to Drive Chronic Intestinal Inflammation. Cell Rep. 2020, 33, 108229. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Merlin, D.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifier-Induced Low-Grade Inflammation Promotes Colon Carcinogenesis. Cancer Res. 2017, 77, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhakim, Y.M.; Hashem, M.M.; Abo-El-Sooud, K.; Ali, H.A.; Anwar, A.; El-Metwally, A.E.; Mahmoud, E.A.; Moustafa, G.G. Involvement of tumor necrosis factor-α, interferon gamma-γ, and interleukins 1β, 6, and 10 in immunosuppression due to long-term exposure to five common food preservatives in rats. Gene 2020, 742, 144590. [Google Scholar] [CrossRef]
- Nagpal, R.; Indugu, N.; Singh, P. Distinct Gut Microbiota Signatures in Mice Treated with Commonly Used Food Preservatives. Microorganisms 2021, 9, 2311. [Google Scholar] [CrossRef]
- Hrncirova, L.; Hudcovic, T.; Sukova, E.; Machova, V.; Trckova, E.; Krejsek, J.; Hrncir, T. Human gut microbes are susceptible to antimicrobial food additives in vitro. Folia Microbiol. 2019, 64, 497–508. [Google Scholar] [CrossRef]
- Xiao, N.; Ruan, S.; Mo, Q.; Zhao, M.; Liu, T.; Feng, F. Effects of potassium sorbate on systemic inflammation and gut microbiota in normal mice: A comparison of continuous intake and washout period. Food Chem. Toxicol. 2024, 184, 114443. [Google Scholar] [CrossRef]
- Fabia, R.; Willen, R.; Ar’Rajab, A.; Andersson, R.; Ahren, B.; Bengmark, S. Acetic Acid-Induced Colitis in the Rat: A Reproducible Experimental Model for Acute Ulcerative Colitis. Eur. Surg. Res. 1992, 24, 211–225. [Google Scholar] [CrossRef]
- Deleu, S.; Arnauts, K.; Deprez, L.; Machiels, K.; Ferrante, M.; Huys, G.R.B.; Thevelein, J.M.; Raes, J.; Vermeire, S. High Acetate Concentration Protects Intestinal Barrier and Exerts Anti-Inflammatory Effects in Organoid-Derived Epithelial Monolayer Cultures from Patients with Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 768. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; Hashem, M.M.; Anwar, A.; El-Metwally, A.E.; Abo-El-Sooud, K.; Moustafa, G.G.; Mouneir, S.M.; Ali, H.A. Effects of the food additives sodium acid pyrophosphate, sodium acetate, and citric acid on hemato-immunological pathological biomarkers in rats: Relation to PPAR-α, PPAR-γ and tnfα signaling pathway. Environ. Toxicol. Pharmacol. 2018, 62, 98–106. [Google Scholar] [CrossRef]
- Daniel, N.; Wu, G.D.; Walters, W.; Compher, C.; Ni, J.; Delaroque, C.; Albenberg, L.; Ley, R.E.; Patterson, A.D.; Lewis, J.D.; et al. Human Intestinal Microbiome Determines Individualized Inflammatory Response to Dietary Emulsifier Carboxymethylcellulose Consumption. Cell Mol. Gastroenterol. Hepatol. 2024, 17, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.L.; Nestor, M.; Onyewadume, L.; de Silva, P.S.; Korzenik, J.R.; Aguilar, H.; Bailen, L.; Berman, A.; Bhaskar, S.K.; Brown, M.; et al. High Dietary Intake of Specific Fatty Acids Increases Risk of Flares in Patients with Ulcerative Colitis in Remission During Treatment with Aminosalicylates. Clin. Gastroenterol. Hepatol. 2017, 15, 1390–1396.e1. [Google Scholar] [CrossRef]
- Tomassen, M.M.M.; Govers, C.; Vos, A.P.; de Wit, N.J.W. Dietary fat induced chylomicron-mediated LPS translocation in a bicameral Caco-2cell model. Lipids Health Dis. 2023, 22, 4. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Tian, M.; Chen, J.; Chen, F.; Guan, W. Different Sources of High Fat Diet Induces Marked Changes in Gut Microbiota of Nursery Pigs. Front. Microbiol. 2020, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Altomare, A.; Cocca, S.; Solida, E.; Ribolsi, M.; Carotti, S.; Rainer, A.; Francesconi, M.; Morini, S.; Cicala, M.; et al. Palmitic Acid Affects Intestinal Epithelial Barrier Integrity and Permeability In Vitro. Antioxidants 2020, 9, 417. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Clement, R.A.; Stearrett, N.; Issa, N.T.; Dore, F.J.; Mazumder, R.; King, C.H.; Hubal, M.J.; Walter, P.J.; Cai, H.; et al. Consumption of sucralose- and acesulfame-potassium-containing diet soda alters the relative abundance of microbial taxa at the species level: Findings of two pilot studies. Appl. Physiol. Nutr. Metab. 2024, 49, 125–134. [Google Scholar] [CrossRef]
- Hanawa, Y.; Higashiyama, M.; Kurihara, C.; Tanemoto, R.; Ito, S.; Mizoguchi, A.; Nishii, S.; Wada, A.; Inaba, K.; Sugihara, N.; et al. Acesulfame potassium induces dysbiosis and intestinal injury with enhanced lymphocyte migration to intestinal mucosa. J. Gastroenterol. Hepatol. 2021, 36, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Higashimura, Y.; Ushiroda, C.; Mizushima, K.; Ohashi, Y.; Yasukawa, Z.; Ozeki, M.; Tokunaga, M.; Okubo, T.; et al. Partially hydrolysed guar gum ameliorates murine intestinal inflammation in association with modulating luminal microbiota and SCFA. Br. J. Nutr. 2016, 116, 1199–1205. [Google Scholar] [CrossRef]
- Wu, X.; Huang, X.; Ma, W.; Li, M.; Wen, J.; Chen, C.; Liu, L.; Nie, S. Bioactive polysaccharides promote gut immunity via different ways. Food Funct. 2023, 14, 1387–1400. [Google Scholar] [CrossRef]
- Dia, V.P.; Gonzalez de Mejia, E. Differential gene expression of RAW 264.7 macrophages in response to the RGD peptide lunasin with and without lipopolysaccharide stimulation. Peptides 2011, 32, 1979–1988. [Google Scholar] [CrossRef]
- Karner, M.; Kocjan, A.; Stein, J.; Schreiber, S.; von Boyen, G.; Uebel, P.; Schmidt, C.; Kupcinskas, L.; Dina, I.; Zuelch, F.; et al. First Multicenter Study of Modified Release Phosphatidylcholine “LT-02” in Ulcerative Colitis: A Randomized, Placebo-Controlled Trial in Mesalazine-Refractory Courses. Am. J. Gastroenterol. 2014, 109, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.H.; Kim, J.S.; Kim, J.M.; Kim, N.; Jung, H.C.; Song, I.S. Plant sterol guggulsterone inhibits nuclear factor-κB signaling in intestinal epithelial cells by blocking IκB kinase and ameliorates acute murine colitis. Inflamm. Bowel Dis. 2006, 12, 1152–1161. [Google Scholar] [CrossRef]
- Velde, A.A.T.; Brüll, F.; Heinsbroek, S.E.M.; Meijer, S.L.; Lütjohann, D.; Vreugdenhil, A.; Plat, J. Effects of Dietary Plant Sterols and Stanol Esters with Low- and High-Fat Diets in Chronic and Acute Models for Experimental Colitis. Nutrients 2015, 7, 8518–8531. [Google Scholar] [CrossRef]
- Hu, P.; Yuan, M.; Guo, B.; Lin, J.; Yan, S.; Huang, H.; Chen, J.-L.; Wang, S.; Ma, Y. Citric Acid Promotes Immune Function by Modulating the Intestinal Barrier. Int. J. Mol. Sci. 2024, 25, 1239. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, H.; Zhang, X.; Li, X.; Yu, J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Clin. Exp. Med. 2015, 8, 20245–20253. [Google Scholar]
- Peter, K.; Rehli, M.; Singer, K.; Renner-Sattler, K.; Kreutz, M. Lactic acid delays the inflammatory response of human monocytes. Biochem. Biophys. Res. Commun. 2015, 457, 412–418. [Google Scholar] [CrossRef]
- Wang, Y.; Jian, C.; Salonen, A.; Dong, M.; Yang, Z. Designing healthier bread through the lens of the gut microbiota. Trends Food Sci. Technol. 2023, 134, 13–28. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohns Colitis. 2021, 15, 1068–1079. [Google Scholar] [CrossRef]
- Narula, N.; Chang, N.H.; Mohammad, D.; Wong, E.C.; Ananthakrishnan, A.N.; Chan, S.S.; Carbonnel, F.; Meyer, A. Food Processing and Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2483–2495.e1. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Garzón, R.; Hernando, I.; Llorca, E.; Rosell, C.M. Understanding the effect of emulsifiers on bread aeration during breadmaking. J. Sci. Food Agric. 2018, 98, 5494–5502. [Google Scholar] [CrossRef]
- Tirosh, A.; Calay, E.S.; Tuncman, G.; Claiborn, K.C.; Inouye, K.E.; Eguchi, K.; Alcala, M.; Rathaus, M.; Hollander, K.S.; Ron, I.; et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci. Transl. Med. 2019, 11, eaav0120. [Google Scholar] [CrossRef] [PubMed]
- Adler, G.K.; Hornik, E.S.; Murray, G.; Bhandari, S.; Yadav, Y.; Heydarpour, M.; Basu, R.; Garg, R.; Tirosh, A. Acute effects of the food preservative propionic acid on glucose metabolism in humans. BMJ Open Diabetes Res. Care 2021, 9, e002336. [Google Scholar] [CrossRef]
- Deol, P.; Ruegger, P.; Logan, G.D.; Shawki, A.; Li, J.; Mitchell, J.D.; Yu, J.; Piamthai, V.; Radi, S.H.; Hasnain, S.; et al. Diet high in linoleic acid dysregulates the intestinal endocannabinoid system and increases susceptibility to colitis in Mice. Gut Microbes. 2023, 15, 2229945. [Google Scholar] [CrossRef]
- Whelan, K.; Bancil, A.S.; Lindsay, J.O.; Chassaing, B. Ultra-processed foods and food additives in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 406–427. [Google Scholar] [CrossRef]
- Martini, G.R.; Tikhonova, E.; Rosati, E.; DeCelie, M.B.; Sievers, L.K.; Tran, F.; Lessing, M.; Bergfeld, A.; Hinz, S.; Nikolaus, S.; et al. Selection of cross-reactive T cells by commensal and food-derived yeasts drives cytotoxic TH1 cell responses in Crohn’s disease. Nat. Med. 2023, 29, 2602–2614. [Google Scholar] [CrossRef]
- Wine, E. Could Gut and Food-Derived Yeast Be Responsible for Activating T Cells in Crohn’s Disease? Gastroenterology 2024, 166, 351–352. [Google Scholar] [CrossRef]
- Zimmermann, J.; De Fazio, L.; Kaden-Volynets, V.; Hitzmann, B.; Bischoff, S.C. Consumption of Yeast-Fermented Wheat and Rye Breads Increases Colitis and Mortality in a Mouse Model of Colitis. Dig. Dis. Sci. 2022, 67, 4422–4433. [Google Scholar] [CrossRef]
- Da Ros, A.; Polo, A.; Rizzello, C.G.; Acin-Albiac, M.; Montemurro, M.; Di Cagno, R.; Gobbetti, M.; Howell, K.S. Feeding with Sustainably Sourdough Bread Has the Potential to Promote the Healthy Microbiota Metabolism at the Colon Level. Microbiol. Spectr. 2021, 9, e00494-21. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L.; Wahbeh, G.; Gregory, N.; Vendettuoli, H.; Christie, D. Nutritional Therapy in Pediatric Crohn Disease: The Specific Carbohydrate Diet. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 87–91. [Google Scholar] [CrossRef]
- Halmos, E.P.; Godny, L.; Vanderstappen, J.; Sarbagili-Shabat, C.; Svolos, V. Role of diet in prevention versus treatment of Crohn’s disease and ulcerative colitis. Frontline Gastroenterol. 2024, 15, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin Induces an Increase in Intestinal Permeability and Zonulin Release by Binding to the Chemokine Receptor CXCR3. Gastroenterology 2008, 135, 194–204.e3. [Google Scholar] [CrossRef]
- Mohan, M.; Chow, C.-E.T.; Ryan, C.N.; Chan, L.S.; Dufour, J.; Aye, P.P.; Blanchard, J.; Moehs, C.P.; Sestak, K. Dietary Gluten-Induced Gut Dysbiosis Is Accompanied by Selective Upregulation of microRNAs with Intestinal Tight Junction and Bacteria-Binding Motifs in Rhesus Macaque Model of Celiac Disease. Nutrients 2016, 8, 684. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Araya, M.; Roncoroni, L.; Doneda, L.; Elli, L. Dietary Gluten as a Conditioning Factor of the Gut Microbiota in Celiac Disease. Adv. Nutr. 2020, 11, 160–174. [Google Scholar] [CrossRef]
- Armstrong, H.; Mander, I.; Zhang, Z.; Armstrong, D.; Wine, E. Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front. Pediatr. 2021, 8, 620189. [Google Scholar] [CrossRef]
- Armstrong, H.K.; Bording-Jorgensen, M.; Santer, D.M.; Zhang, Z.; Valcheva, R.; Rieger, A.M.; Kim, J.S.-H.; Dijk, S.I.; Mahmood, R.; Ogungbola, O.; et al. Unfermented β-fructan Fibers Fuel Inflammation in Select Inflammatory Bowel Disease Patients. Gastroenterology 2023, 164, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.A.; Gibson, P.R.; Taylor, K.M.; Halmos, E.P. Development of Novel High and Low Emulsifier Diets Based upon Emulsifier Distribution in the Australian Food Supply for Intervention Studies in Crohn’s Disease. Nutrients 2024, 16, 1922. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Godny, L.; Reshef, L.; Pfeffer-Gik, T.; Goren, I.; Yanai, H.; Tulchinsky, H.; Gophna, U.; Dotan, I. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 2020, 59, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Godny, L.; Dotan, I. Is the Mediterranean Diet in Inflammatory Bowel Diseases Ready for Prime Time? J. Can. Assoc. Gastroenterol. 2024, 7, 97–103. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Yanai, H.; Fischler, T.S.; Goren, I.; Eran-Banai, H.; Ollech, E.J.; Snir, Y.; Broitman, Y.; Barkan, R.; Pfeffer-Gik, T.; Godny, L.; et al. A Real-World Prospective Cohort Study of Patients with Newly Diagnosed Crohn’s Disease Treated by a Multidisciplinary Team: 1-Year Outcomes. Crohns Colitis 2023, 360, otad064. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, A.L.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J. Crohns Colitis. 2023, 17, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults With Crohn’s Disease. Gastroenterology 2021, 161, 837–852.e9. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, C.; Cenni, S.; Serra, M.R.; Dolce, P.; Martinelli, M.; Staiano, A.; Miele, E. Effectiveness of Mediterranean Diet’s Adherence in Children with Inflammatory Bowel Diseases. Nutrients 2020, 12, 3206. [Google Scholar] [CrossRef]
- Godny, L.; Reshef, L.; Fischler, T.S.; Elial-Fatal, S.; Pfeffer-Gik, T.; Raykhel, B.; Rabinowitz, K.; Levi-Barda, A.; Perets, T.; Barkan, R.; et al. Increasing adherence to the Mediterranean diet and lifestyle is associated with reduced fecal calprotectin and intra-individual changes in microbial composition of healthy subjects. Gut Microbes. 2022, 14, 2120749. [Google Scholar] [CrossRef]
- Martinez-Steele, E.; Khandpur, N.; Batis, C.; Bes-Rastrollo, M.; Bonaccio, M.; Cediel, G.; Huybrechts, I.; Juul, F.; Levy, R.B.; Louzada, M.L.d.C.; et al. Best practices for applying the Nova food classification system. Nat. Food. 2023, 4, 445–448. [Google Scholar] [CrossRef]
- Dangarembizi, R.; Erlwanger, K.; Rummel, C.; Roth, J.; Madziva, M.; Harden, L. Brewer’s yeast is a potent inducer of fever, sickness behavior and inflammation within the brain. Brain Behav. Immun. 2018, 68, 211–223. [Google Scholar] [CrossRef]
- Lindberg, E.; Magnusson, K.E.; Tysk, C.; Jarnerot, G. Antibody (IgG, IgA, and IgM) to baker’s yeast (Saccharomyces cerevisiae), yeast mannan, gliadin, ovalbumin and betalactoglobulin in monozygotic twins with inflammatory bowel disease. Gut 1992, 33, 909–913. [Google Scholar] [CrossRef]
- Wang, W.; Xie, R.; Cao, Q.; Ye, H.; Zhang, C.; Dong, Z.; Feng, D.; Zuo, J. Effects of glucose oxidase on growth performance, clinical symptoms, serum parameters, and intestinal health in piglets challenged by enterotoxigenic Escherichia coli. Front. Microbiol. 2022, 13, 994151. [Google Scholar] [CrossRef]
- Paradis, M.-E.; Couture, P.; Gigleux, I.; Marin, J.; Vohl, M.-C.; Lamarche, B. Impact of systemic enzyme supplementation on low-grade inflammation in humans. PharmaNutrition 2015, 3, 83–88. [Google Scholar] [CrossRef]
- Bjorck, S.; Bosaeus, I.; Ek, E.; Jennische, E.; Lönnroth, I.; Johansson, E.; Lange, S. Food induced stimulation of the antisecretory factor can improve symptoms in human inflammatory bowel disease: A study of a concept. Gut 2000, 46, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.C.; Fedirko, V.; Baron, J.A.; Barry, E.L.; Flanders, W.D.; McCullough, M.L.; Yacoub, R.; Raavi, T.; Rutherford, R.E.; Seabrook, M.E.; et al. Inflammation Modulation by Vitamin D and Calcium in the Morphologically Normal Colorectal Mucosa of Patients with Colorectal Adenoma in a Clinical Trial. Cancer Prev. Res. 2020, 14, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Peuranen, S.; Tiihonen, K.; Apajalahti, J.; Kettunen, A.; Saarinen, M.; Rautonen, N. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. Br. J. Nutr. 2004, 91, 905–914. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Nichols, B.; McGowan, M.; Svolos, V.; Papadopoulou, R.; Kokkorou, M.; Rebull, M.; Gonzalez, T.B.; Hansen, R.; Russell, R.K.; et al. The Effects of Commonly Consumed Dietary Fibres on the Gut Microbiome and Its Fibre Fermentative Capacity in Adults with Inflammatory Bowel Disease in Remission. Nutrients 2022, 14, 1053. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Y.; Liao, A.; Pan, L.; Yang, S.; Liu, Y.; Wang, J.; Xue, Y.; Zhang, M.; Zhu, Z.; et al. Integrated Analysis of Gut Microbiome and Adipose Transcriptome Reveals Beneficial Effects of Resistant Dextrin from Wheat Starch on Insulin Resistance in Kunming Mice. Biomolecules 2024, 14, 186. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Nie, Q.; Hu, J.; Li, Y.; Shi, Z.; Ji, H.; Zhang, H.; Zhao, M.; Chen, C.; et al. Effects of four food hydrocolloids on colitis and their regulatory effect on gut microbiota. Carbohydr. Polym. 2023, 323, 121368. [Google Scholar] [CrossRef]
- Kim, C.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.; Mine, Y. L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2009, 1790, 1161–1169. [Google Scholar] [CrossRef]
- Richie, T.G.; Heeren, L.; Kamke, A.; Monk, K.; Pogranichniy, S.; Summers, T.; Wiechman, H.; Ran, Q.; Sarkar, S.; Plattner, B.L.; et al. Limitation of amino acid availability by bacterial populations during enhanced colitis in IBD mouse model. mSystems 2023, 8, e0070323. [Google Scholar] [CrossRef]
- Nickerson, K.P.; Homer, C.R.; Kessler, S.P.; Dixon, L.J.; Kabi, A.; Gordon, I.O.; Johnson, E.E.; de la Motte, C.A.; McDonald, C.; Kufer, T.A. The Dietary Polysaccharide Maltodextrin Promotes Salmonella Survival and Mucosal Colonization in Mice. PLoS ONE 2014, 9, e101789. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress–Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef]
- Nickerson, K.P.; McDonald, C.; Mizoguchi, E. Crohn’s Disease-Associated Adherent-Invasive Escherichia coli Adhesion Is Enhanced by Exposure to the Ubiquitous Dietary Polysaccharide Maltodextrin. PLoS ONE 2012, 7, e52132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Huang, S.; Wu, Z.; Pang, J.; Wu, Y.; Wang, J.; Han, D.; Mostarica-Stojković, M. Resistant Maltodextrin Alleviates Dextran Sulfate Sodium-Induced Intestinal Inflammatory Injury by Increasing Butyric Acid to Inhibit Proinflammatory Cytokine Levels. BioMed Res. Int. 2020, 2020, 7694734. [Google Scholar] [CrossRef]
- Ray, S.C.; Baban, B.; Tucker, M.A.; Seaton, A.J.; Chang, K.C.; Mannon, E.C.; Sun, J.; Patel, B.; Wilson, K.; Musall, J.B.; et al. Oral NaHCO3 Activates a Splenic Anti-Inflammatory Pathway: Evidence That Cholinergic Signals Are Transmitted via Mesothelial Cells. J. Immunol. 2018, 200, 3568–3586. [Google Scholar] [CrossRef] [PubMed]

| Low Processed | Medium Processed | Highly Processed |
|---|---|---|
| Baking soda | Added fiber | Acidity regulators |
| Canola oil | Enzymes | Added gluten |
| Olive oil | L-cysteine | Anticaking agents |
| Sourdough | Malt | Baking improver |
| Sugar | Soy flour | Emulsifiers |
| Vitamin C | Flavorings | |
| Yeast * | Palm oil, soy oil, or unspecified vegetable oil | |
| Preservatives | ||
| Stabilizers | ||
| Sweeteners: acesulfame K, maltodextrin, dextrose | ||
| Wheat starch or corn starch |
| Group | Food Additive | E-Number | Prevalence (in Screened Breads) (%) | Model | Effect on Microbiome | Effect on Inflammation | Ref |
|---|---|---|---|---|---|---|---|
| Emulsifiers | SSL | E-481 | 37% | In vitro—fecal microbiota | ↓ Clostridiaceae, Lachnospiraceae, Ruminococcaceae ↓ Butyrate ↑ Bacteroidaceae, Enterobacteriaceae ↑ Propionate ↑ LPS and flagellin | [24] | |
| Emulsifiers | MDGs | E-471 | 22% | Murine model—mice | Changes β-diversity and microbial composition ↓ Akkermansia, Bifidobacterium, Lactobacillus, Lupinus luteus ↑ Bacteroides acidifaciens, E. coli | ↑ LPS, IL-1β, IL-6, and TNF-α levels in serum | [25] |
| Emulsifiers | DATEM | E-472e | 16% | Human microbiota—MBRAs | ↓ Bacterial density ↓ Lactobacillales members, including Streptococcus genus ↓ Faecalibacterium | [23] | |
| Emulsifiers | CMC | E-466 | 2% | M-SHIME, murine model—mice | ↑ Bioactive flagellin-related gene expressions ↑ IL-6 expression | ↑ Intestinal inflammation | [22] |
| Emulsifiers | CMC | E-466 | 2% | MBRAs | ↓ Lactobacillales members, Streptococcus genus | [23] | |
| Emulsifiers | CMC | E-466 | 2% | Murine model—mice | ↑ Bacterial adherence ↑ Bacterial overgrowth | [26] | |
| Emulsifiers | CMC | E-466 | 2% | Murine model—mice | ↑ E. coli ability to adhere and invade IEC ↑ Expression of virulence factors | [27] | |
| Emulsifiers | CMC | E-466 | 2% | Murine model—mice | ↑ Bioactive fecal LPS and flagellin | ↑ Shortened colons ↑ Splenomegaly | [10,28] |
| Preservatives | Calcium propionate | E-282 | 48% | Murine model—rats | In serum: ↓ IgG and IgM ↑ IL-4 expression mRNA expression: ↑ TNF-α expression | [29] | |
| Preservatives | Potassium sorbate | E-202 | 18% | Murine model—rats | In serum: ↓ IgG and IgM ↑ IL-4 expression mRNA expression: ↑ TNF-α expression and IFNγ | [29] | |
| Preservatives | Potassium sorbate | E-202 | 18% | Murine model—mice | ↓ α-diversity ↑ Parabacteroides and Adlercreutzia | [30] | |
| Preservatives | Potassium sorbate | E-202 | 18% | In vitro—fecal microbiota | ↓ E. Coli | [31] | |
| Preservatives | Potassium sorbate | E-202 | 18% | Murine model—mice | ↓ Lachnospiraceae ↓ Isobutyric acid production | ↑ IL-1β levels in serum ↑ Inflammatory cell infiltration in the liver | [32] |
| Preservatives, Acidity regulators | Acetic acid | E-260 | 14% | Murine model—rats | ↑ Colitis | [33] | |
| Preservatives, Acidity regulators | Acetic acid | E-260 | 14% | Organoid-derived colonic epithelial monolayer culture | ↑ Improved epithelial resistance ↑ Regulation of HIF1α, MUC2, and MKI67 ↓ Expression of: IL8, TNFα, CLDN1 | [34] | |
| Preservatives, Acidity regulators | Sodium hydrogen acetate | E-262(ii) | 0.40% | Murine model—rats | ↓ IgG and IgM levels ↓ PPAR-α, PPAR-γ expression ↑ TNF-α expression | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakhman, S.; Pfeffer-Gik, T.; Elial-Fatal, S.; Broitman, Y.; Yanai, H.; Gophna, U.; Dotan, I.; Godny, L. Industrial Bread Composition: Potential Implications for Patients with Inflammatory Bowel Disease. Nutrients 2025, 17, 2120. https://doi.org/10.3390/nu17132120
Shakhman S, Pfeffer-Gik T, Elial-Fatal S, Broitman Y, Yanai H, Gophna U, Dotan I, Godny L. Industrial Bread Composition: Potential Implications for Patients with Inflammatory Bowel Disease. Nutrients. 2025; 17(13):2120. https://doi.org/10.3390/nu17132120
Chicago/Turabian StyleShakhman, Shelly, Tamar Pfeffer-Gik, Sarine Elial-Fatal, Yelena Broitman, Henit Yanai, Uri Gophna, Iris Dotan, and Lihi Godny. 2025. "Industrial Bread Composition: Potential Implications for Patients with Inflammatory Bowel Disease" Nutrients 17, no. 13: 2120. https://doi.org/10.3390/nu17132120
APA StyleShakhman, S., Pfeffer-Gik, T., Elial-Fatal, S., Broitman, Y., Yanai, H., Gophna, U., Dotan, I., & Godny, L. (2025). Industrial Bread Composition: Potential Implications for Patients with Inflammatory Bowel Disease. Nutrients, 17(13), 2120. https://doi.org/10.3390/nu17132120








