Non-Pharmacological Interventions Aimed at Changing the Gut Microbiota for Preventing the Progression of Diabetic Kidney Disease
Abstract
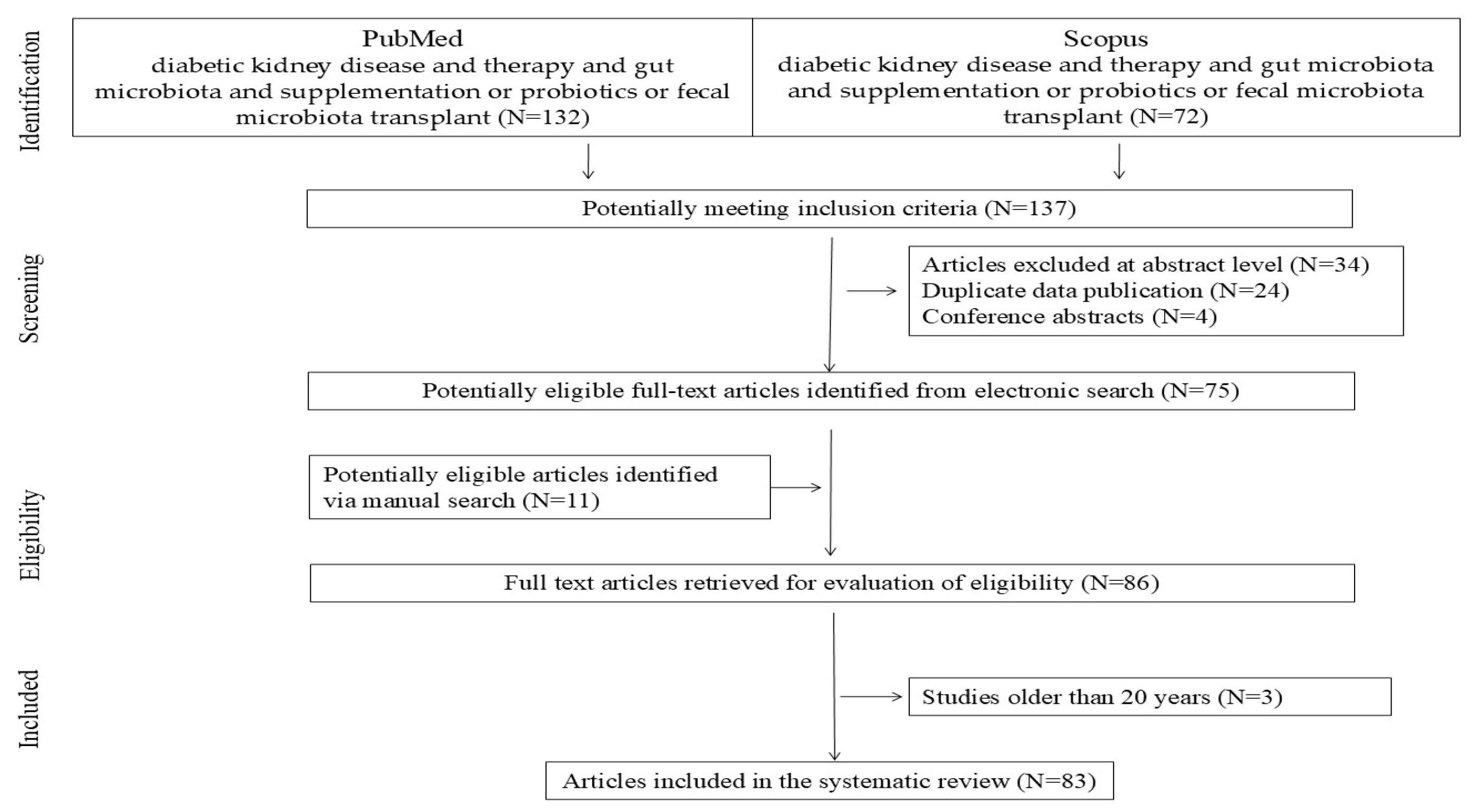
1. Introduction
2. Materials and Methods
3. Superfoods
3.1. Polyphenols and Anthocyanins
3.2. Compounds Found in Certain Plants and Bee Products
4. Sodium Butyrate
5. Probiotics
6. Synbiotics
7. Fecal Microbiota Transplantation
8. Low-Protein Diet—Impact of Limiting Animal Protein on Gut Microbiota
9. Research Directions
10. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Beaumont, M.; Pallister, T.; Villar, J.; Goodrich, J.K.; Clark, A.; Pascual, J.; Ley, R.E.; Spector, T.D.; Bell, J.T.; et al. Gut-Microbiota-Metabolite Axis in Early Renal Function Decline. PLoS ONE 2015, 10, e0134311. [Google Scholar] [CrossRef] [PubMed]
- Koye, D.N.; Shaw, J.E.; Reid, C.M.; Atkins, R.C.; Reutens, A.T.; Magliano, D.J. Incidence of chronic kidney disease among people with diabetes: A systematic review of observational studies. Diabet. Med. 2017, 34, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.T.; Xu, X.; Lim, P.S.; Hung, K.Y. Worldwide epidemiology of diabetes-related end-stage renal disease, 2000–2015. Diabetes Care 2021, 44, 89–97. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Bosch, C.C.S.; Soler, M.J.; Ortiz, A.; Fernandez-Fernandez, B. Tirzepatide and prevention of chronic kidney disease. Clin. Kidney J. 2022, 16, sfac274. [Google Scholar] [CrossRef]
- Naaman, S.C.; Bakris, G.L. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care 2023, 46, 1574–1586. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes Diabetes Working Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef]
- Caruso, I.; Giorgino, F. Renal effects of GLP-1 receptor agonists and tirzepatide in individuals with type 2 diabetes: Seeds of a promising future. Endocrine 2024, 84, 822–835. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Qin, Y.; Yu, Z.; Zhang, Y.; Ning, X.; Sun, S. The Specific Alteration of Gut Microbiota in Diabetic Kidney Diseases-A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 908219. [Google Scholar] [CrossRef]
- Li, Q.; Xie, S.; Liu, Y.; Yue, W.; Wang, L.; Liang, Y.; Chen, Y.; Yuan, H.; Yu, J. Gut microbiota profiling reflects the renal dysfunction and psychological distress in patients with diabetic kidney disease. Front. Endocrinol. 2024, 15, 1410295. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, M.; Pircalabioru, G.G.; Savu, O. Microbiota analysis in individuals with type two diabetes mellitus and end-stage renal disease: A pilot study. Exp. Ther. Med. 2024, 27, 211. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.; Alvarenga, L.; Esgalhado, M.; Cardozo, L.; Lindholm, B.; Stenvinkel, P. Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease. Nutrients 2019, 11, 496. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, Y.; Yang, M.; Jin, L.; Zhang, X.; Zhang, R.; Wu, Y.; Yan, C.; Gao, Y.; Zeng, M.; et al. Vitamin D is involved in the effects of the intestinal flora and its related metabolite TMAO on perirenal fat and kidneys in mice with DKD. Nutr. Diabetes 2024, 14, 42. [Google Scholar] [CrossRef]
- Zaky, A.; Glastras, S.J.; Wong, M.Y.W.; Pollock, C.A.; Saad, S. The Role of the Gut Microbiome in Diabetes and Obesity-Related Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9641. [Google Scholar] [CrossRef]
- Cai, K.; Ma, Y.; Cai, F.; Huang, X.; Xiao, L.; Zhong, C.; Ren, P.; Luo, Q.; Chen, J.; Han, F. Changes of gut microbiota in diabetic nephropathy and its effect on the progression of kidney injury. Endocrine 2022, 76, 294–303. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Xu, Q.; Shikov, A.N.; Pozharitskaya, O.N.; Flisyuk, E.V.; Liu, M.; Li, H.; Vargas-Murga, L.; Duez, P. Flavonoids and Saponins: What Have We Got or Missed? Phytomedicine 2023, 109, 154580. [Google Scholar] [CrossRef]
- Mafra, D.; Kalantar-Zadeh, K.; Moore, L.W. New Tricks for Old Friends: Treating Gut Microbiota of Patients with CKD. J. Ren. Nutr. 2021, 31, 433–437. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as Medicine: Targeting the Uraemic Phenotype in Chronic Kidney Disease. Nat. Rev. Nephrol. 2021, 17, 153–171. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Soulage, C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins 2018, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Alvarenga, L.; Cardozo, L.F.M.F.; Chermut, T.R.; Sequeira, J.; de Souza Gouveia Moreira, L.; Teixeira, K.T.R.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. From the Distinctive Smell to Therapeutic Effects: Garlic for Cardiovascular, Hepatic, Gut, Diabetes and Chronic Kidney Disease. Clin. Nutr. 2021, 40, 4807–4819. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.; Ediriweera, M.K.; Kim Cho, S. Interplay between Phytochemicals and the Colonic Microbiota. Nutrients 2023, 15, 1989. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Verediano, T.A.; Stampini Duarte Martino, H.; Dias Paes, M.C.; Tako, E. Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients 2021, 13, 1331. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; Melo, M.F.S.; Mota, J.C.; Mafra, D.; Guimarães, J.T.; Stockler-Pinto, M.B. Blueberry, Cranberry, Raspberry, and Strawberry as Modulators of the Gut Microbiota: Target for Treatment of Gut Dysbiosis in Chronic Kidney Disease? From Current Evidence to Future Possibilities. Nutr. Rev. 2023, 82, 248–261. [Google Scholar] [CrossRef]
- Arabi, S.M.; Bahari, H.; Hamidipor, S.; Bahrami, L.S.; Feizy, Z.; Nematy, M.; Kesharwani, P.; Sahebkar, A. The effects of curcumin-containing supplements on inflammatory biomarkers in hemodialysis patients: A systematic review and meta-analysis. Phytother. Res. 2022, 36, 4361–4370. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.D.S.G.; Brum Da Costa, I.D.S.; De Vargas Reis, D.C.M.; Trugilho, L.; Chermut, T.R.; Esgalhado, M.; Cardozo, L.F.M.F.; Stenvinkel, P.; Shiels, P.G.; Mafra, D. Cinnamon: An Aromatic Condiment Applicable to Chronic Kidney Disease. Kidney Res. Clin. Pract. 2023, 42, 4–26. [Google Scholar] [CrossRef]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef]
- Moreira, L.D.S.G.; Fanton, S.; Cardozo, L.; Borges, N.A.; Combet, E.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. Pink Pressure: Beetroot (Beta vulgaris rubra) as a Possible Novel Medical Therapy for Chronic Kidney Disease. Nutr. Rev. 2022, 80, 1041–1061. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Anvarifard, P.; Ostadrahimi, A.; Ardalan, M.; Anbari, M.; Ghoreishi, Z. The effects of propolis on pro-oxidant-antioxidant balance, glycemic control, and quality of life in chronic kidney disease: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2023, 13, 9884. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji-Ping, L.; Ya-Fu, X.; Jia-Ying, P.; Yan, D.; Zhong-Yuan, G.; Ying-Bo, Y.; Zheng-Tao, W.; Xiao-Lu, J.; Li, Y. Plantaginis semen ameliorates diabetic kidney disease via targeting the sphingosine kinase 1/sphingosine-1-phosphate pathway. J. Ethnopharmacol. 2024, 331, 118221. [Google Scholar]
- Lesmana, R.; Tandean, S.; Christoper, A.; Suwantika, A.; Wathoni, N.; Abdulah, R.; Fearnley, J.; Bankova, V.; Zulhendri, F. Propolis as an autophagy modulator in relation to its roles in redox balance and inflammation regulation. Biomed. Pharmacother. 2024, 175, 116745. [Google Scholar] [CrossRef]
- Czajkowska, A.; Szponar, B. Krótkołańcuchowe kwasy tłuszczowe (SCFA) jako produkty metabolizmu bakterii jelitowych oraz ich znaczenie dla organizmu gospodarza. Postępy Hig. I Med. Doświadczalnej 2018, 72, 131–142. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Li, Y.; Qin, G.Q.; Liu, J. Short chain fatty acids for the risk of diabetic nephropathy in type 2 diabetes patients. Acta Diabetol. 2022, 59, 901–909. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Zhang, N. Protective effects of butyrate on renal ischemia-reperfusion injury in rats. Urol. Int. 2019, 102, 348–355. [Google Scholar] [CrossRef]
- Machado, R.A.; Constantino, L.d.S.; Tomasi, C.D.; Rojas, H.A.; Vuolo, F.S.; Vitto, M.F.; Cesconetto, P.A.; de Souza, C.T.; Ritter, C.; Dal-Pizzol, F. Sodium butyrate decreases the activation of NF-κB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Urol. Int. 2012, 27, 3136–3140. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, B.; Hong, X.; Zhang, X.; Kong, X. Histone deacetylase inhibitor, sodium butyrate, attenuates gentamicin-induced nephrotoxicity by increasing prohibitin protein expression in rats. Eur. J. Pharmacol. 2013, 707, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Felizardo, R.J.F.; de Almeida, D.C.; Pereira, R.L.; Watanabe, I.K.M.; Doimo, N.T.S.; Ribeiro, W.R.; Cenedeze, M.A.; Hiyane, M.I.; Amano, M.T.; Braga, T.T.; et al. Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic-and GPR109a-mediated mechanisms. FASEB J. 2019, 33, 11894–11908. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, Z.; Zhang, L.; Zhu, Y.; Deng, M.; Huang, C.; Liu, Y.; Zhu, Q.; Wang, L. Sodium butyrate ameliorates deoxycorticosterone acetate/salt-induced hypertension and renal damage by inhibiting the MR/SGK1 pathway. Hypertens. Res. 2021, 44, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, T.; He, Y.; Xie, Y.; Xu, Y.; Huang, W. The role and mechanism of butyrate in the prevention and treatment of diabetic kidney disease. Front. Microbiol. 2022, 13, 961536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cornall, L.M.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. The therapeutic potential of GPR43: A novel role in modulating metabolic health. Cell. Mol. Life Sci. 2013, 70, 4759–4770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, N.; Xu, L.; Shi, Y.; Zhuang, S. Podocyte autophagy: A potential therapeutic target to prevent the progression of diabetic nephropathy. J. Diabetes Res. 2017, 2017, 3560238. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.; et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef]
- Hida, M.; Aiba, Y.; Sawamura, S.; Suzuki, N.; Satoh, T.; Koga, Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996, 74, 349–355. [Google Scholar] [CrossRef]
- Taki, K.; Takayama, F.; Niwa, T. Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J. Ren. Nutr. 2005, 15, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Bodke, H.; Jogdand, S. Role of Probiotics in Human Health. Cureus 2022, 14, e31313. [Google Scholar] [CrossRef]
- Alatriste, P.V.M.; Urbina Arronte, R.; Gómez Espinosa, C.O.; Espinosa Cuevas, M.d.L. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr. Hosp. 2014, 29, 582–590. [Google Scholar]
- Firouzi, S.; Mohd-Yusof, B.-N.; Majid, H.-A.; Ismail, A.; Kamaruddin, N.-A. Effect of microbial cell preparation on renal profile and liver function among type 2 diabetics: A randomized controlled trial. BMC Complement. Altern. Med. 2015, 15, 433. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.A.B.; Soder, T.F.; Grokoski, K.C.; Benetti, F.; Mendes, R.H. Probiotics in the treatment of chronic kidney disease: A systematic review. J. Bras. Nefrol. 2018, 40, 278–286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbasi, B.; Ghiasvand, R.; Mirlohi, M. Kidney Function Improvement by Soy Milk Containing Lactobacillus plantarum A7 in Type 2 Diabetic Patients with Nephropathy: A Double-Blinded Randomized Controlled Trial. Iran. J. Kidney Dis. 2017, 11, 36–43. [Google Scholar] [PubMed]
- Miraghajani, M.; Zaghian, N.; Dehkohneh, A.; Mirlohi, M.; Ghiasvand, R. Probiotic Soy Milk Consumption and Renal Function Among Type 2 Diabetic Patients with Nephropathy: A Randomized Controlled Clinical Trial. Probiotics Antimicrob. Proteins 2019, 11, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Borges, N.A.; Carmo, F.L.; Stockler-Pinto, M.B.; de Brito, J.S.; Dolenga, C.J.; Ferreira, D.C.; Nakao, L.S.; Rosado, A.; Fouque, D.; Mafra, D. Probiotic Supplementation in Chronic Kidney Disease: A Double-blind, Randomized, Placebo-controlled Trial. J. Ren. Nutr. 2018, 28, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Pechenyak, B.; Vyas, U.; Ranganathan, P.; Weinberg, A.; Liang, P.; Mallappallil, M.C.; Norin, A.J.; Friedman, E.A.; Saggi, S.J. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. BioMed Res. Int. 2014, 2014, 568571. [Google Scholar] [CrossRef]
- Dai, Y.; Quan, J.; Xiong, L.; Luo, Y.; Yi, B. Probiotics improve renal function, glucose, lipids, inflammation and oxidative stress in diabetic kidney disease: A systematic review and meta-analysis. Ren. Fail. 2022, 44, 862–880. [Google Scholar] [CrossRef]
- Vlachou, E.; Ntikoudi, A.; Govina, O.; Lavdaniti, M.; Kotsalas, N.; Tsartsalis, A.; Dimitriadis, G. Effects of Probiotics on Diabetic Nephropathy: A Systematic Review. Curr. Clin. Pharmacol. 2020, 15, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.G.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Asemi, Z.; Khorrami-Rad, A.; Alizadeh, S.A.; Shakeri, H.; Esmaillzadeh, A. Effects of Synbiotic Food Consumption on Metabolic Status of Diabetic Patients: A Double-Blind Randomized Cross-over Controlled Clinical Trial. Clin. Nutr. 2014, 33, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guida, B.; Germanò, R.; Trio, R.; Russo, D.; Memoli, B.; Grumetto, L.; Barbato, F.; Cataldi, M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.; Bammens, B.; De Moor, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008, 73, 1174–1180. [Google Scholar] [CrossRef]
- McFarlane, C.; Krishnasamy, R.; Stanton, T.; Savill, E.; Snelson, M.; Mihala, G.; Kelly, J.T.; Morrison, M.; Johnson, D.W.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology II (SYNERGY II): A Feasibility Randomized Controlled Trial. Nutrients 2021, 13, 4481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dehghani, H.; Heidari, F.; Mozaffari-Khosravi, H.; Nouri-Majelan, N.; Dehghani, A. Synbiotic Supplementations for Azotemia in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. Iran. J. Kidney Dis. 2016, 10, 351–357, Erratum in Iran. J. Kidney Dis. 2017, 11, 392. [Google Scholar] [PubMed]
- Firouzi, S.; Haghighatdoost, F. The effects of prebiotic, probiotic and synbiotic supplementation on blood parameters of renal function: A systematic review and meta-analysis of clinical trials. Nutrition 2018, 51–52, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Song, Y.; Sun, Y.; Li, C.; Du, H.; You, Q.; Cai, Y.; Lang, Y.; Shao, L. Association between Probiotic, Prebiotic, Synbiotics and Yogurt Supplements and diabetic kidney disease: The NHANES 2007–2016. J. Ren. Nutr. 2025, in press. [Google Scholar] [CrossRef]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, G.; Stasi, A.; Franzin, R.; Fiorentino, M.; Cimmarusti, M.T.; Deleonardis, A.; Palieri, R.; Pontrelli, P.; Gesualdo, L. Fecal Microbiota Transplantation in Reducing Uremic Toxins Accumulation in Kidney Diseases: Current Understanding and Future Perspectives. Toxins 2023, 15, 115. [Google Scholar] [CrossRef]
- Uchiyama, K.; Wakino, S.; Irie, J.; Miyamoto, J.; Matsui, A.; Tajima, T.; Itoh, T.; Oshima, Y.; Yoshifuji, A.; Kimura, I.; et al. Contribution of Uremic Dysbiosis to Insulin Resistance and Sarcopenia. Nephrol. Dial. Transplant. 2020, 35, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.M.C.; Simplício-Filho, A.; Sávio-Silva, C.; Oliveira, L.F.V.; Cruz, G.N.F.; Sousa, E.H.; Noronha, I.L.; Mangueira, C.L.P.; Quaglierini-Ribeiro, H.; Josefi-Rocha, G.R.; et al. Fecal Microbiota Transplant in a Pre-Clinical Model of Type 2 Diabetes Mellitus, Obesity and Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 3842. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Cui, W.; Guo, R.; Zhang, Y.; Wang, P.; Yu, W.; Zheng, X.; Wang, T.; Dong, Y.; Zhao, J.; et al. The harmful intestinal microbial community accumulates during DKD exacerbation and microbiome-metabolome combined validation in a mouse model. Front. Endocrinol. 2022, 13, 964389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Barba, C.; Soulage, C.O.; Caggiano, G.; Glorieux, G.; Fouque, D.; Koppe, L. Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD. Toxins 2020, 12, 741. [Google Scholar] [CrossRef]
- Arteaga-Muller, G.Y.; Flores-Treviño, S.; Bocanegra-Ibarias, P.; Robles-Espino, D.; Garza-González, E.; Fabela-Valdez, G.C.; Camacho-Ortiz, A. Changes in the Progression of Chronic Kidney Disease in Patients Undergoing Fecal Microbiota Transplantation. Nutrients 2024, 16, 1109. [Google Scholar] [CrossRef]
- Zhou, G.; Zeng, J.; Peng, L.; Wang, L.; Zheng, W.; Wu, D.; Yang, Y. Fecal microbiota transplantation for membranous nephropathy. CEN Case Rep. 2021, 10, 261–264. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, M.; Yang, X.; Wang, Y.; Li, R.; Sun, S. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: The first case reports. Ren. Fail. 2021, 43, 928–933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhi, W.; Yuan, X.; Song, W.; Jin, G.; Li, Y. Fecal Microbiota Transplantation May Represent a Good Approach for Patients with Focal Segmental Glomerulosclerosis: A Brief Report. J. Clin. Med. 2022, 11, 6700. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, L.; Zhang, Y.; Li, K.; Yang, J. The role and mechanism of the gut microbiota in the development and treatment of diabetic kidney disease. Front. Physiol. 2023, 14, 1166685. [Google Scholar] [CrossRef]
- Wu, S. Effect of Dietary Protein and Processing on Gut Microbiota—A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Lauriola, M. Food-Derived Uremic Toxins in Chronic Kidney Disease. Toxins 2023, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kikut, J.; Maciejewska, D.; Kulpa, D.; Celewicz, Z.; Ziętek, M. The Associations of SCFA with Anthropometric Parameters and Carbohydrate Metabolism in Pregnant Women. Int. J. Mol. Sci. 2020, 21, 9212. [Google Scholar] [CrossRef] [PubMed]
| Study | Probiotic Strain | Subjects and Duration of Supplementation | Results |
|---|---|---|---|
| Alatriste et al. [51] | Lactobacillus casei Shirota (16 × 109 CFU) | 30 patients with chronic kidney disease, stages 3 and 4, for 8 weeks | Lower ammonia levels in patients using probiotics. |
| Firouzi et al. 2015 [52] | Multi-strain preparation (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus lactis, Bifidobacterium bifidum, Bifidobacterium longum, and Bifidobacterium infantis) (6 × 1010 CFU) | 136 people with type 2 diabetes for 12 weeks | Probiotic supplementation reduced urea levels, especially among overweight or obese individuals. |
| Fagundes et al. [53] | Mix of L. acidophilus KB27, B. longum KB31, and S. thermophilus KB19 at a dose of 9 × 1010 CFU/day | 46 patients with chronic kidney disease, stages 3 and 4, for 6 months | Probiotic supplementation reduced blood urea nitrogen levels. Additionally, an improvement in well-being was observed among those using probiotics. |
| Borges et al. [56] | Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium longum, 90 billion CFU/day for 3 months | 46 hemodialysis patients | Probiotic supplementation did not reduce uremic toxins or inflammatory markers. |
| Natarajan et al. [57] | S. thermophilus KB 19, L. acidophilus KB 27, and B. longum KB 31 (90 billion CFU/day) | 22 people with chronic kidney disease, stage 5 | No statistically significant changes in uremic toxin levels were observed. |
| Study | Synbiotic | Subjects | Duration of Study | Result |
|---|---|---|---|---|
| Rossi et al. [62] 2016 | Prebiotic—inulin, fructooligosaccharides, and galactooligosaccharides; probiotic—Lactobacillus, Bifidobacteria, and Streptococcus. | 31 patients with chronic kidney disease | 6 weeks | Reduction in p-cresyl sulfate levels; no effect on indoxyl sulfate levels. Beneficial modification of gut microbiota. |
| Guida et al., 2014 [63] | Lactobacillus plantarum, 2 × 109 Lactobacillus casei subsp. rhamnosus, 2 × 109 Lactobacillus gasseri, 1 × 109 Bifidobacterium infantis, 1 × 109 Bifidobacterium longum, 1 × 109 Lactobacillus acidophilus, 1 × 109 Lactobacillus salivarius, 1 × 109 Lactobacillus sporogenes, and 5 × 109 Streptococcus thermophilus + prebiotic: inulin and 1.3 g of tapioca-resistant starch. | 30 patients with CKD, stages 3–4 | 4 weeks | Reduction in p-cresol levels. |
| McFarlane et al. 2021 [65] | The prebiotic was 20 g/day of high-resistant starch fiber supplement and the probiotic component provided 4.5 × 1011 colony-forming units (CFU)/day of nine different strains from three different genera (Bifidobacteria, Lactobacillus, and Streptococcus). | 68 patients with chronic kidney disease, stages 3–4 | 12 months | Modification of gut microbiota in patients using synbiotics—increase in Bifidobacterium and Blautia spp. Decrease in eGFR and increase in creatinine levels. |
| Dehghani et al. 2016 [66] | Prebiotic (fructooligosaccharide) + probiotic (seven strains across Lactobacillus, Bifidobacteria, and Streptococcus). | 66 patients with CKD (stages 3 and 4) | 6 weeks | Reduction in blood urea nitrogen levels after synbiotic use. No effect of synbiotic on creatinine levels. |
| Animal Studies | Donor | Recipient | Result |
|---|---|---|---|
| Uchiyama et al., 2020 [71] | Mice suffering from chronic kidney disease | Healthy, germ-free mice | Development of insulin resistance and sarcopenia in recipients. |
| Barba et al. [75] | Healthy mice | Mice with chronic kidney disease | Regulation of dysbiosis in mice with chronic kidney disease. Additionally, improvement in glucose tolerance. |
| Bastos et al. [72] | Healthy mice | Mice with chronic kidney disease | Reduction in albuminuria, prevention of weight gain, and lower expression of TNF-α in the ileum and colon. |
| Shang et al. [73] | Healthy mice | Mice with DKD | Restoration of normal gut microbiota can alleviate the course of DKD. |
| Human Studies | Donor | Recipient | Result |
| Wang et al., 2020 [74] | Patients with end-stage renal disease (223 patients) or healthy patients; control group (69 patients) | Rodents with adenine-induced chronic kidney disease | Gut microbiota of patients with chronic kidney disease induces higher production of uremic toxins and greater kidney fibrosis in mice. |
| Zhou et al., 2021 [77] | 14-year-old healthy male patient | Patient suffering from membranous nephropathy | Increase in serum albumin levels; decrease in creatinine and urea levels. |
| Zhao et al., 2021 [78] | Two healthy patients | Two patients suffering from IgA nephropathy | Lower protein excretion in 24 h urine collection; increase in blood albumin levels. |
| Zhi et al., 2022 [79] | Healthy patient | Patient suffering from focal segmental glomerulosclerosis | Reduction in urine protein levels; disease remission. |
| Arteaga-Muller et al., 2024 [76] | Healthy donors | Patients suffering from nephropathy secondary to diabetes and hypertension (13 in placebo group and 15 in FMT group); CKD in stages 2, 3, and 4 | A higher number of patients (53.8% in the placebo group) experienced CKD progression compared to the FMT group (13.3%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczuko, M.; Grudniewska, A.; Durma, A.; Małecki, R.; Filipczyńska, I.; Franek, E.; Kędzierska-Kapuza, K. Non-Pharmacological Interventions Aimed at Changing the Gut Microbiota for Preventing the Progression of Diabetic Kidney Disease. Nutrients 2025, 17, 2112. https://doi.org/10.3390/nu17132112
Szczuko M, Grudniewska A, Durma A, Małecki R, Filipczyńska I, Franek E, Kędzierska-Kapuza K. Non-Pharmacological Interventions Aimed at Changing the Gut Microbiota for Preventing the Progression of Diabetic Kidney Disease. Nutrients. 2025; 17(13):2112. https://doi.org/10.3390/nu17132112
Chicago/Turabian StyleSzczuko, Małgorzata, Anna Grudniewska, Anna Durma, Robert Małecki, Izabela Filipczyńska, Edward Franek, and Karolina Kędzierska-Kapuza. 2025. "Non-Pharmacological Interventions Aimed at Changing the Gut Microbiota for Preventing the Progression of Diabetic Kidney Disease" Nutrients 17, no. 13: 2112. https://doi.org/10.3390/nu17132112
APA StyleSzczuko, M., Grudniewska, A., Durma, A., Małecki, R., Filipczyńska, I., Franek, E., & Kędzierska-Kapuza, K. (2025). Non-Pharmacological Interventions Aimed at Changing the Gut Microbiota for Preventing the Progression of Diabetic Kidney Disease. Nutrients, 17(13), 2112. https://doi.org/10.3390/nu17132112







