1. Introduction
Adipose tissue is recognized as an endocrine organ performing many physiologic roles, including functions in the storage of energy reserves, regulation of appetite, control of thermogenesis, and insulin sensitivity by secreting hormones (adipokines), cytokines, and lipids [
1]. Adipose tissue stores a large proportion of the body’s vitamin D (D represents D
2 or D
3). Also, it is an endocrine organ that can metabolize 25(OH)D
3 and has a vitamin D receptor permitting 1,25-dihydroxyvitamin D (1,25(OH)
2D
3) to regulate adipocyte maturation and cytokine production [
2]. It is also known that vitamin D is associated with the lipid droplet organelle inside of a mature adipocyte [
1]. However, with all of this knowledge, we have little understanding of the kinetics of vitamin D
3, 25(OH)D
3 and 1,25(OH)
2D
3 uptake in adipocytes [
3].
The fat-soluble vitamin D accumulates in adipose tissue [
4]. Early investigations using radiolabelled vitamin D
3 into vitamin D deficient rats revealed that a significant proportion, approximately 50%, was retained in adipose tissue as unmodified vitamin D
3 [
5]. Subsequent kinetic studies in vitamin D replete rats, following intracardiac administration of radiolabelled vitamin D
3, showed rapid accumulation of radioactivity in the liver and serum (notably, 70% in the liver within 20 min), whereas uptake in other tissues, such as the intestinal mucosa, kidney, bone, and muscle, occurred more gradually and eventually declined [
6]. In contrast, adipose tissue displayed a slow but steady accumulation of radioactivity that persisted, with no apparent clearance, and accounted for roughly 10% of the dose after one week. Notably, vitamin D metabolism was tissue specific; while polar metabolites dominated in the liver, serum, and mucosa after one week (80% of radioactivity), vitamin D
3 itself remained predominant in kidney, muscle, and adipose tissue (70%).
Findings from human studies supported these results. Postmortem and surgical samples confirmed vitamin D
3 accumulation in various fat depots [
7]. Using a high-sensitivity high-performance liquid chromatography (HPLC) method, Lawson et al. [
8] detected considerable concentrations of vitamin D
3 (50–100 ng/g) in perirenal, pericardial, cervical, and axillary fat. Of note, perirenal fat has been identified as a depot for brown adipose tissue (BAT) in humans [
9], suggesting that vitamin D
3 also accumulates in thermogenic fat. Furthermore, Lawson’s study indicated that stored vitamin D
3 is mobilized during periods of deficiency, with an estimated half-life of 12 days.
Beyond being sequestered in adipose tissue, vitamin D is also localized within intracellular lipid droplets of adipocytes [
10]. Studies comparing vitamin D metabolism in individuals with normal weight and those with higher body fat have shown significant differences in circulation dynamics [
11].
The mechanism by which vitamin D is stored and/or processed in adipose tissue remains the subject of significant interest and debate. Several hypotheses have been proposed to explain the diminished response to vitamin D supplementation. One suggests that vitamin D, being a fat-soluble vitamin, is sequestered in adipose tissue, leaving lower amounts available in circulation [
12]. Another posits volumetric dilution, whereby vitamin D is distributed across a larger adiposity mass, effectively reducing its circulating concentration [
13].
Little is known about how human adipocytes respond to being exposed to vitamin D3 and its major metabolites in a time-dependent manner. The goal of this study was to determine the kinetics for the uptake of vitamin D3, 25(OH)D3 and 1,25(OH)2D3 in cultured mature human adipocytes.
2. Materials and Methods
2.1. Cells
Frozen stocks of primary human white adipose stromal cells (ASCs) were obtained from the Boston University Adipose Biology and Nutrient Metabolism Core.
2.2. Differentiation of Human ASCs
Primary human ASCs (Passage 5) were seeded in 6-well plates (5000 to 15,000 cells/cm
2) and differentiated as previously described [
14]. Briefly, the preadipocytes were grown in growth medium containing alpha-MEM with 10% FBS and antibiotics until confluent. Differentiation was induced in serum-free media using an adipogenic cocktail that included insulin (Sigma-Aldrich, St. Louis, MO, USA; I0516-5 mL), dexamethasone (Sigma-Aldrich, D4902-100MG), IBMX (Sigma-Aldrich, I5879-100MG), and rosiglitazone (Sigma-Aldrich, R2408-10MG) for seven days. The medium was then changed to a lipogenic maintenance medium (NaHCO
3, d-biotin (Sigma-Aldrich, B4639-500MG), pantothenate (Sigma-Aldrich, P5155-100G) insulin, dexamethasone), for an additional seven days.
2.3. Vitamin D3, 25(OH)D3, and 1,25(OH)D3 Incubation
Newly differentiated human adipocytes cultured in maintenance medium were treated with either 10−6 M vitamin D3, 10−6 M 1,25(OH)2D3, or a combination of 10−6 M vitamin D3, 10−6 M 25(OH)D3, and 10−6 M 1,25(OH)2D3 in the maintenance medium using three wells per condition. The combination of vitamin D3, 25(OH)D3, and 1,25(OH)2D3 10−6 M was incubated for 0, 1, 2, 6, 24, and 96 h. For vitamin D3 or 1,25(OH)2D3 alone, the incubation times were 1 h and 96 h. After incubation, the culture media was removed, and the cells were gently washed with 1 mL of PBS. To extract intracellular contents, 1 mL of methanol was added directly to the adherent cells, followed by an additional 1 mL of methanol, resulting in a total 2 mL of per well. Extracts from each of the three wells were collected separately each time. The methanol lysate was used for high-performance liquid chromatography (HPLC) analysis and triglyceride (TG) measurement analysis.
2.4. Triglyceride Quantification Using Glycerol-Based Enzymatic Assay
Cellular TG content was quantified using a modified fluorometric enzymatic assay based on the detection of glycerol. Samples (250 µL from the total of 2 mL) were first dried under a stream of nitrogen gas, and the lipid residue was resuspended in Cell Lysis Buffer (CLB, Cell Signaling Technology, Danvers, MA, USA, #9803). Following sonication to ensure complete lipid solubilization, samples were centrifuged to remove debris, and the resulting supernatants were serially diluted in CLB for downstream analysis.
A glycerol-based standard curve was prepared to allow for quantification of triglyceride equivalents. A 2.5 mg/mL stock solution of glycerol was serially diluted. All standards, diluted sample replicates, and non-triglyceride reagent controls were transferred to a black 96-well plate in technical duplicates. Each well received 25 µL of triglyceride reagent (Sigma-Aldrich, T2449), and plates were sealed with adhesive film and incubated at 37 °C for 1 h, with gentle agitation every 20 min to ensure homogeneity.
A glycine-based enzyme buffer was freshly prepared by dissolving 7.88 g of glycine (Sigma-Aldrich, G7126) in 300 mL of H2O (Milli-Q water), adjusting the pH to 9.1 with NaOH and adding 500 µL of 2 M MgCl2. The final volume was brought to 500 mL with mq H20, and 580 mg of ATP disodium salt (Sigma-Aldrich, A7599) was added to yield a final concentration of ~2.35 mM ATP. Ten-milliliter aliquots of this buffer were stored at −80 °C until use. For the assay, 50 µL of the enzyme buffer was added per well, followed by the addition of glycerol kinase (Sigma-Aldrich, G8121), glycerol-3-phosphate dehydrogenase (Sigma-Aldrich, G6501), and hydrazine hydrate (Sigma-Aldrich, 225819) to ensure complete conversion of glycerol to dihydroxyacetonephosphate and NADH in the presence of NAD+. After 20 min of incubation at room temperature, 3 µL of NAD+ (25 mg/mL; Sigma-Aldrich, N6522) was added to each well. Following an additional 30 min of incubation, fluorescence was measured at an excitation wavelength of 350 nm and emission at 466 nm. TG concentrations were determined based on a standard curve and expressed as triolein equivalents.
2.5. High-Performance Liquid Chromatography (HPLC) Procedure
The remaining 1.75 mL of methanol lysate was used for HPLC analysis. Cell-derived methanol extracts were dried under a stream of nitrogen and reconstituted in 1 mL of 5% isopropanol (IPA) in n-hexane. Samples were centrifuged to remove cellular debris, and the resulting supernatants were dried again under nitrogen. The final residues were reconstituted in 130 µL of 5% IPA/n-hexane for injection. All solvents used were of analytical HPLC grade.
HPLC was performed on an Agilent 1100 series system equipped with a variable-wavelength UV detector. Separation was achieved using a Zorbax RX-SIL normal-phase column. The mobile phase consisted of a gradient from 5% to 25% IPA in n-hexane, with the flow rate gradually increasing from 0.6 mL/min to 1.0 mL/min. Analytes were detected at 265 nm with a minimum sensitivity threshold of 12.5 ng.
2.6. Vitamin D3 Efflux Experiment
Newly differentiated human adipocytes were incubated for 48 h with 10−6 M vitamin D3 and 100,000 CPM 3H-vitamin D3. Following incubation, cells were washed and preconditioned for 2 h in DMEM without insulin or dexamethasone. Subsequently, cells were treated for 2 h under basal conditions or 10 µM isopropanol-stimulated conditions. Samples of the media were collected and analyzed through scintillation counting. Data are presented as mean ± SEM. Statistical significance was assessed through an unpaired two-tailed Student’s t-test (p < 0.05).
2.7. Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM), unless otherwise specified. Technical replicates (n = 3 per condition) were used for each experimental time point. Statistical significance was determined either through one-way ANOVA with Dunnett’s post-test to compare all groups to the 1 h time point or an unpaired two-tailed Student’s t-test for comparisons between two groups. Linear regression analysis was applied to evaluate calibration curves for HPLC and fluorometric assays, with R2 values reported to assess fit quality. A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism (v9.0). In the figure, significance is indicated as follows: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3. Results
3.1. Morphological Assessment and Viability of Adipocytes Following Vitamin D3 Treatment
Prior to quantitative metabolite analyses, we assessed whether treatment with vitamin D3 or 1,25(OH)2D3 at 10−6 M altered adipocyte morphology or viability. Brightfield microscopy revealed no notable morphological changes in differentiated human adipocytes following treatment with either compound for up to 96 h. The cells maintained their characteristic rounded shape and lipid droplet-containing phenotype, comparable to untreated controls.
To evaluate cytotoxicity, cell death was assessed using trypan blue exclusion and LDH release assays at 1, 24, and 96 h post-treatment. Neither vitamin D3 nor 1,25(OH)2D3 induced a significant increase in cell death at any time point compared to vehicle controls. These findings suggest that treatment with vitamin D3 and 1,25(OH)2D3 at 10−6 M does not compromise adipocyte integrity or viability, thus validating the use of this concentration in subsequent metabolic uptake and retention analyses.
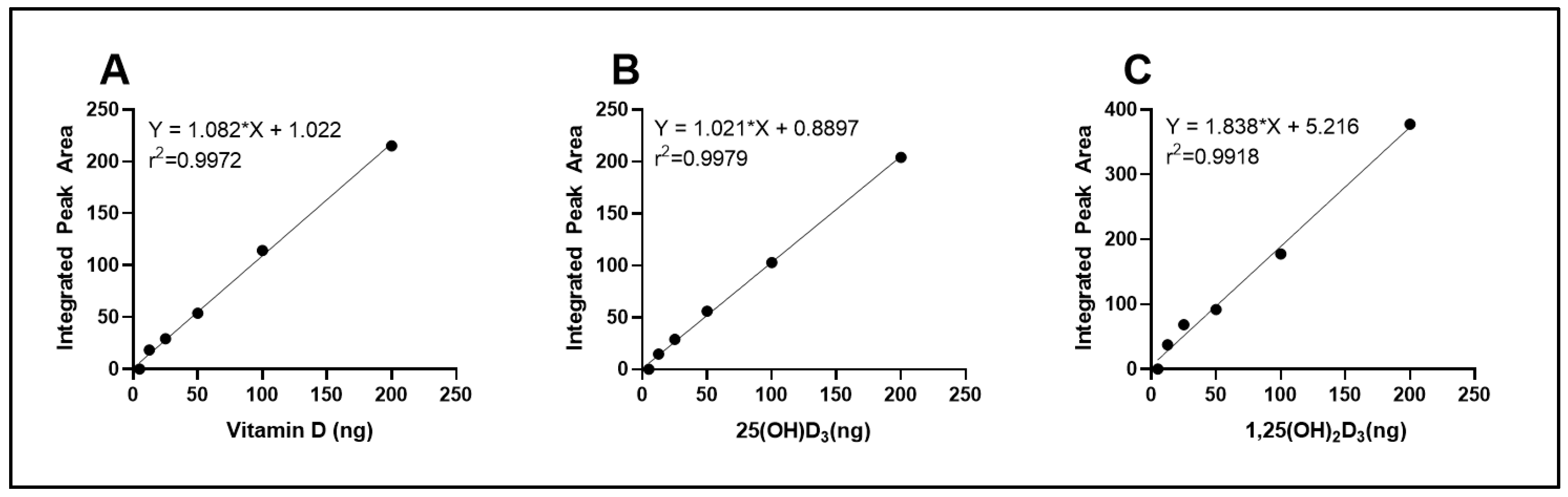
3.2. Quantitative Analysis of Vitamin D Metabolites Through HPLC
To quantify intracellular vitamin D
3 and its hydroxylated metabolites, a standard calibration curve was generated using increasing concentrations of vitamin D
3, 25(OH)D
3, and 1,25(OH)
2D
3 standards. The peak areas corresponding to each injected concentration were integrated and plotted against the known quantities, as shown in
Figure 1. The resulting calibration curve exhibited excellent linearity across the tested range (R
2 > 0.99), confirming the sensitivity and reliability of the method for quantification of cellular vitamin D
3, 25(OH)D
3 and 1,25(OH)
2D
3.
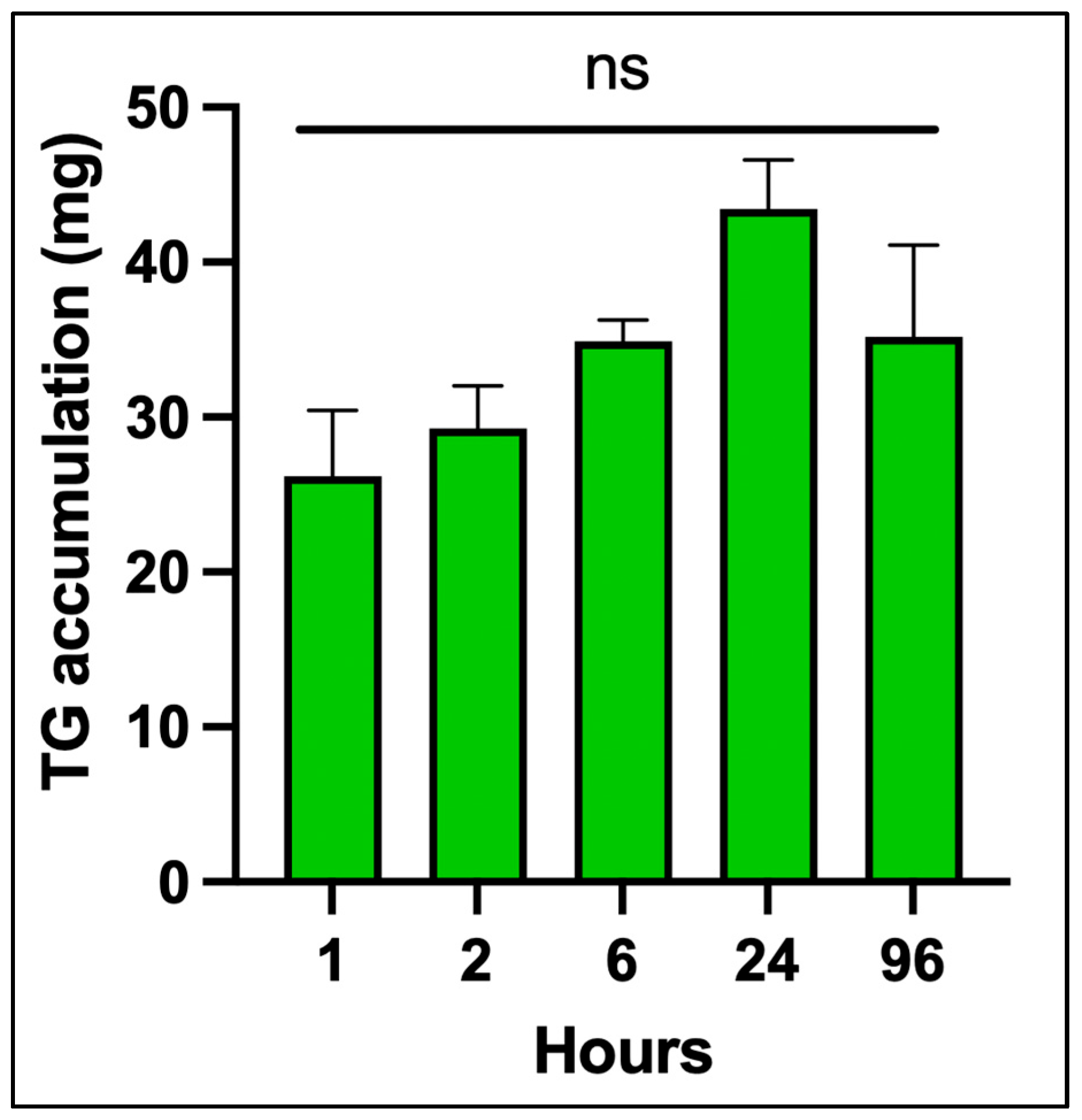
3.3. Fluorometric Quantification of Triglycerides
To quantify intracellular TG concentrations in differentiated human adipocytes, a fluorometric assay was employed using a glycerol standard curve. The standard curve was generated using serial dilutions of glycerol expressed in triolein equivalents, ranging from 0.01 to 0.6 mg/mL. Fluorescence intensity was measured and plotted against known concentrations, resulting in a non-linear regression curve with a strong fit (R2 > 0.99). The standard curve allowed for accurate interpolation of TG concentrations in experimental samples based on their fluorescence output. The use of this method provided sensitive and reliable quantification of cellular triglyceride content, which was subsequently used to normalize vitamin D3 and vitamin D3 metabolite concentrations.
Figure 2 shows the time course of TG accumulation in differentiated adipocytes from 0 to 96 h. There was no significant increase over time. This validated assay provided sensitive and reproducible quantification of intracellular triglycerides, which was essential for normalizing downstream measurements of vitamin D
3, 25(OH)D
3 and 1,25(OH)
2D
3.
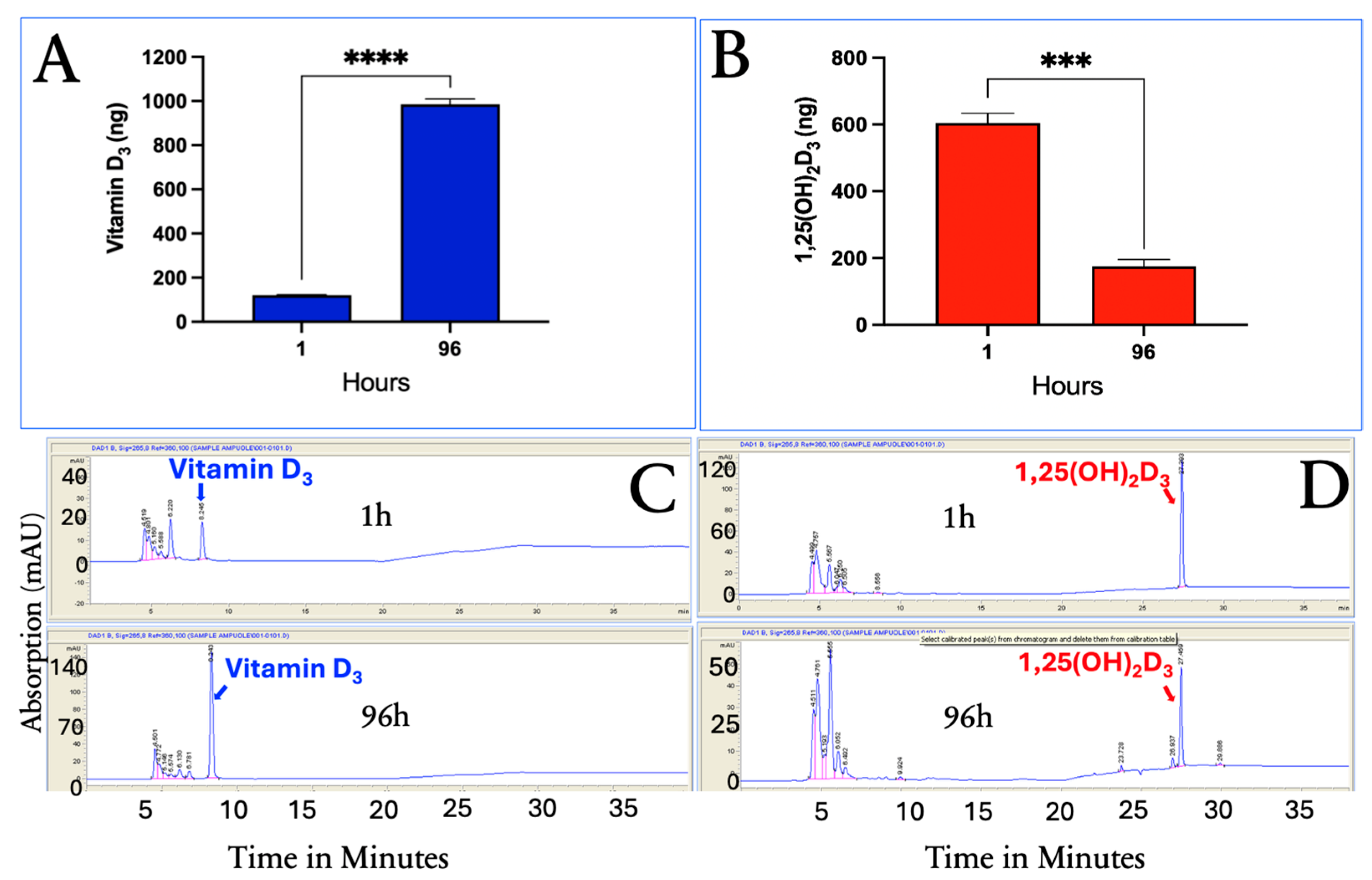
3.4. HPLC Analysis of Vitamin D3, 25(OH)D3, and 1,25(OH)2D3 in Methanol Extracts of Adipocytes
The HPLC system separated vitamin D
3, 25(OH)D
3, and 1,25(OH)
2D
3 (
Figure 3A). This system was used to quantitatively determine the presence of vitamin D
3, 25(OH)D
3, and 1,25(OH)
2D
3 in the methanol cellular extracts, as demonstrated in
Figure 3B,C, at 1 and 96 h.
3.5. Uptake of Vitamin D3, 25(OH)D3, and 1,25(OH)2D3 over Time in Cultured Human Differentiated Adipocytes
As shown in
Figure 4, vitamin D
3 concentrations increased over time, indicating continued cellular uptake and likely sequestration within lipid-rich compartments, such as lipid droplets. In contrast, 1,25(OH)
2D
3 reached its highest concentration 1 h after its addition and rapidly declined to undetectable levels by 96 h, suggesting rapid utilization, metabolism, or degradation. 25(OH)D
3 concentrations also peaked at 1 h and then quickly decreased over the first 24 h, followed by a slow decline through 96 h.
These results indicate that while adipocytes gradually accumulate vitamin D3, the cells rapidly acquire the highest intracellular concentrations of 25(OH)D3 within the first hour. Both hydroxylated metabolites declined rapidly within the first 6 h, with 1,25(OH)2D3 becoming undetectable by 96 h, while 25(OH)D3 stabilized and gradually decreased over the remaining incubation period.
These distinct kinetics suggest that adipocytes serve not only as passive storage sites for vitamin D3 but also as dynamic regulators of vitamin D metabolism, reflecting the varying solubility, receptor interactions, and metabolic fates of these compounds.
To determine whether vitamin D
3 is metabolized to either 25(OH)D
3 or 1,25(OH)
2D
3, in the differentiated human adipocytes, adipocytes were incubated with vitamin D
3 or 1,25(OH)
2D
3 alone for 1 and 96 h. The results indicated that neither 25(OH)D
3 nor 1,25(OH)
2D
3 were produced from vitamin D
3 treatment, and no vitamin D esters were detected (
Figure 5C). The percentage changes in the intracellular uptake of vitamin D
3 and 1,25(OH)
2D
3 at 1 h and 96 h were similar to what was observed when all three vitamin D species were incubated together. Vitamin D
3 showed a 91.8% increase in accumulation at 96 h compared to a 71.0% decrease in 1,25(OH)
2D
3 concentrations.
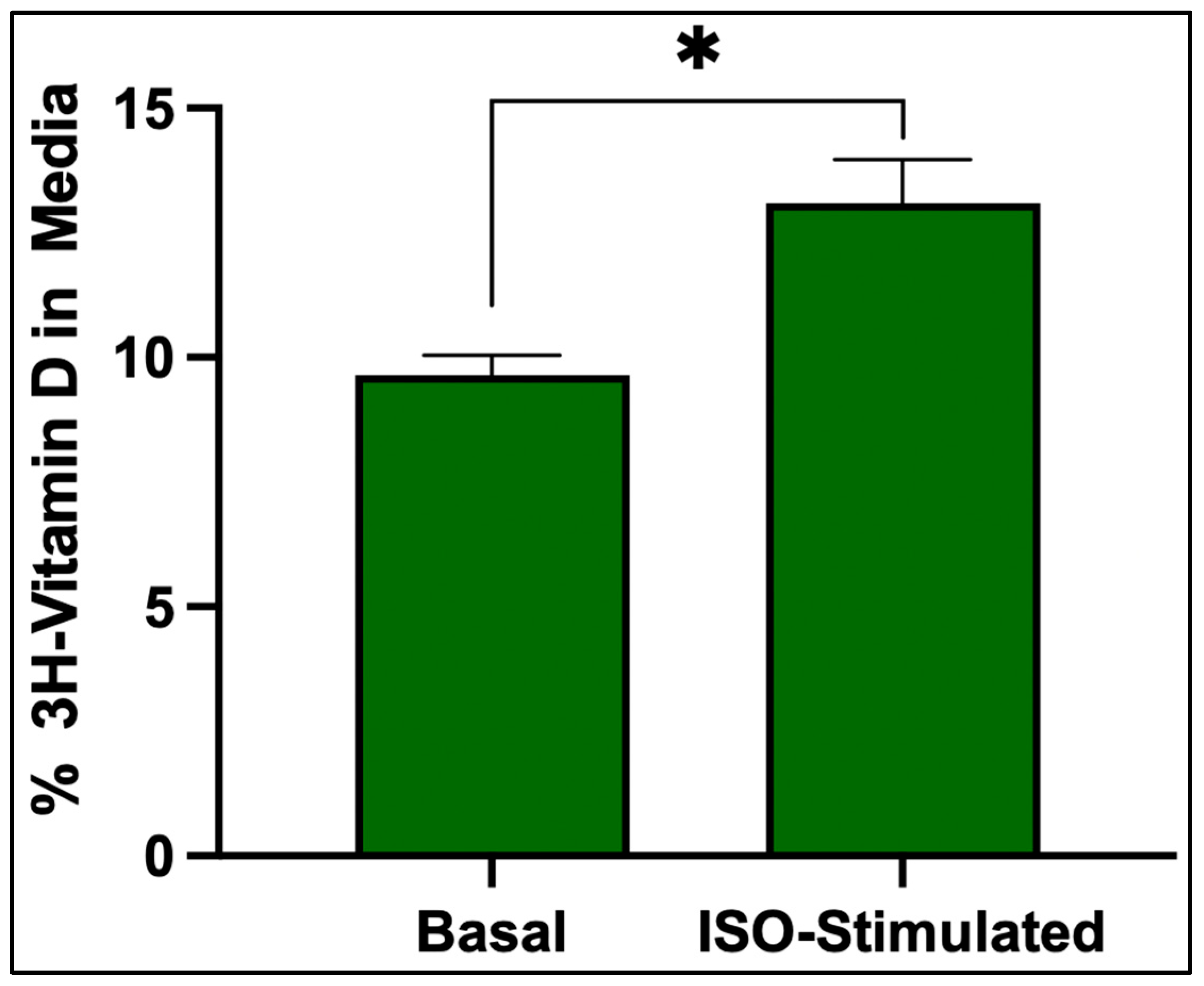
3.6. Vitamin D3 Release
Isoproterenol stimulation significantly increased the release of
3H-vitamin D
3 from adipocytes that were treated with isoproterenol compared to the basal condition, indicating that lipolysis increases the release of vitamin D
3 from adipocytes (
Figure 6).
4. Discussion
Our findings indicate that differentiated human adipocytes exhibit distinct intracellular handling of vitamin D3 and two of its hydroxylated metabolites, which may contribute to—but does not fully explain—variations in systemic vitamin D bioavailability. Further mechanistic studies, including gene expression and in vivo validation, are required to clarify these relationships.
The gradual accumulation and prolonged retention of vitamin D3 within adipocytes suggest that it is stored within lipid-rich compartments, likely lipid droplets, potentially limiting its availability for systemic metabolism and use. In contrast, 25(OH)D3 and 1,25(OH)2D3 showed rapid uptake and clearance, indicating that they are not stored but rather utilized in adipocytes. This selective accumulation and retention of vitamin D3 supports the hypothesis that adipose tissue acts as a long-term storage site for vitamin D3 and not for 25(OH)D3 or 1,25(OH)2D3. Furthermore, we observed that treatment of the adipocytes with isoproterenol induces lipolysis, increasing the percentage of 3H vitamin D3 in the media when compared to the adipocytes treated with control media.
This selective retention aligns with previous studies suggesting that the capacity of adipocytes to sequester vitamin D
3 leads to lower circulating vitamin D concentrations, impacting overall vitamin D status in obese patients [
15,
16]. Our recent study [
17] supports that vitamin D
3 is sequestered in adipose tissue, likely within lipid droplets, reducing its availability for metabolism and utilization.
It is important to highlight that vitamin D
3 and its metabolites play essential roles in regulating calcium ion concentrations, which are critical for bone mineralization, neuromuscular function, and cellular signaling [
4,
11]. By promoting intestinal calcium absorption and renal calcium reabsorption, 1,25(OH)
2D
3 maintains serum calcium within a narrow physiological range. The sequestration of vitamin D
3 in adipocytes observed in this study may reduce its bioavailability, potentially impairing these calcium-dependent processes. We do not fully understand why vitamin D
3 is sequestered in adipocytes. However, it has been speculated that storage of precious vitamin D
3 during the summer was critically important for the evolution and survival of humans as they left their ancestral home in equatorial Africa. Exposure of hunter–gatherers to sunlight during the summer provided them with their vitamin D requirement. The unutilized fat-soluble vitamin was deposited in adipose tissue with the intent of it being released in the winter in far northern and southern latitudes when no vitamin D could be produced. Due to lack of an energy source of food during the winter, the adipocyte’s lipid droplets provided an excellent source of energy while at the same time releasing vitamin D
3 to prevent rickets in children and metabolic bone disease in adults.
These dynamics underscore the importance of adipocyte handling of vitamin D3 in predicting physiological outcomes and may inform future strategies for optimizing vitamin D supplementation and therapeutic use.
In addition to their role as potential reservoirs for vitamin D
3, adipocytes express the vitamin D receptor (VDR) and possess the enzymatic machinery required for the local metabolism of vitamin D metabolites [
2]. Notably, 1,25(OH)
2D
3 binds to VDR, inducing the expression of CYP24A1, the enzyme responsible for 24-hydroxylation, which converts both 25(OH)D
3 and 1,25(OH)
2D
3 into inactive, water-soluble metabolites destined for excretion [
18]. This rapid induction of CYP24A1 likely explains the sharp decline observed for 1,25(OH)
2D
3 and the slower decline in 25(OH)D
3 in our study.
These patterns may be further influenced by inflammatory mediators present in obese adipose tissue potentially exacerbating vitamin D sequestration. Chronic low-grade inflammation associated with obesity alters adipocyte function and may impair vitamin D mobilization from lipid stores. The interplay between adipocyte inflammation and vitamin D metabolism may contribute to the reduced circulating 25(OH)D concentration observed in obesity, highlighting the importance of considering both metabolic and inflammatory pathways in understanding vitamin D deficiency in this population.
4.1. Future Directions
Further investigation is warranted to directly assess the expression of vitamin-D-related genes in adipocytes. Specifically, quantitative PCR or immunofluorescence studies assessing VDR and CYP24A1 expression, with and without exposure to vitamin D3, 25(OH)D3, or 1,25(OH)2D3, could clarify the regulatory mechanisms underlying local vitamin D metabolism. In addition, examining the uptake and retention of vitamin D metabolites in other relevant cell types, such as bone-marrow-derived mesenchymal stem cells (BMMSCs), would provide insight into tissue-specific responses and functional implications, including the potential for vitamin D to influence osteogenesis via calcium handling.
4.2. Strengths and Limitations
A strength of this study is the use of direct quantification via HPLC to track intracellular concentrations of vitamin D3, 25(OH)D3, and 1,25(OH)2D3 over time in human adipocytes. However, a limitation is that some experiments were conducted using differentiation batches with variable efficiency, which may have affected lipid content and vitamin D handling. Future studies should explore intracellular localization of vitamin D metabolites and the specific transporters or binding proteins involved.