The Impact of Cocoa Flavanols in Modulating Resting Cerebral Blood Flow During Prolonged Sitting in Healthy Young and Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants
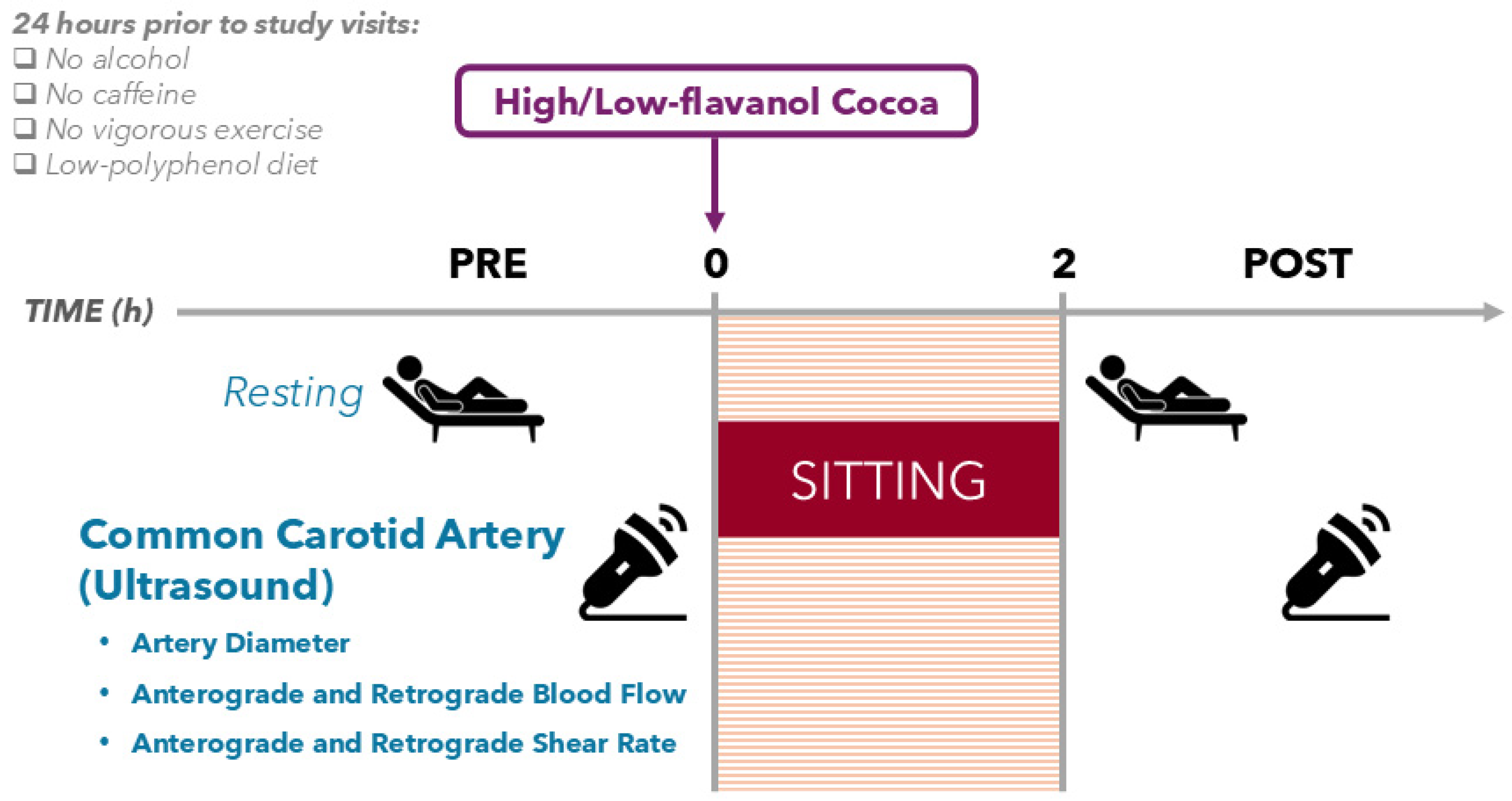
2.3. Study Design
2.4. Maximal Aerobic Capacity Test
2.5. High- and Low-Flavanol Interventions
2.6. Ultrasound Recording of the Common Carotid Artery
2.7. Blood Pressure and Heart Rate
2.8. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Habitual Physical Activity
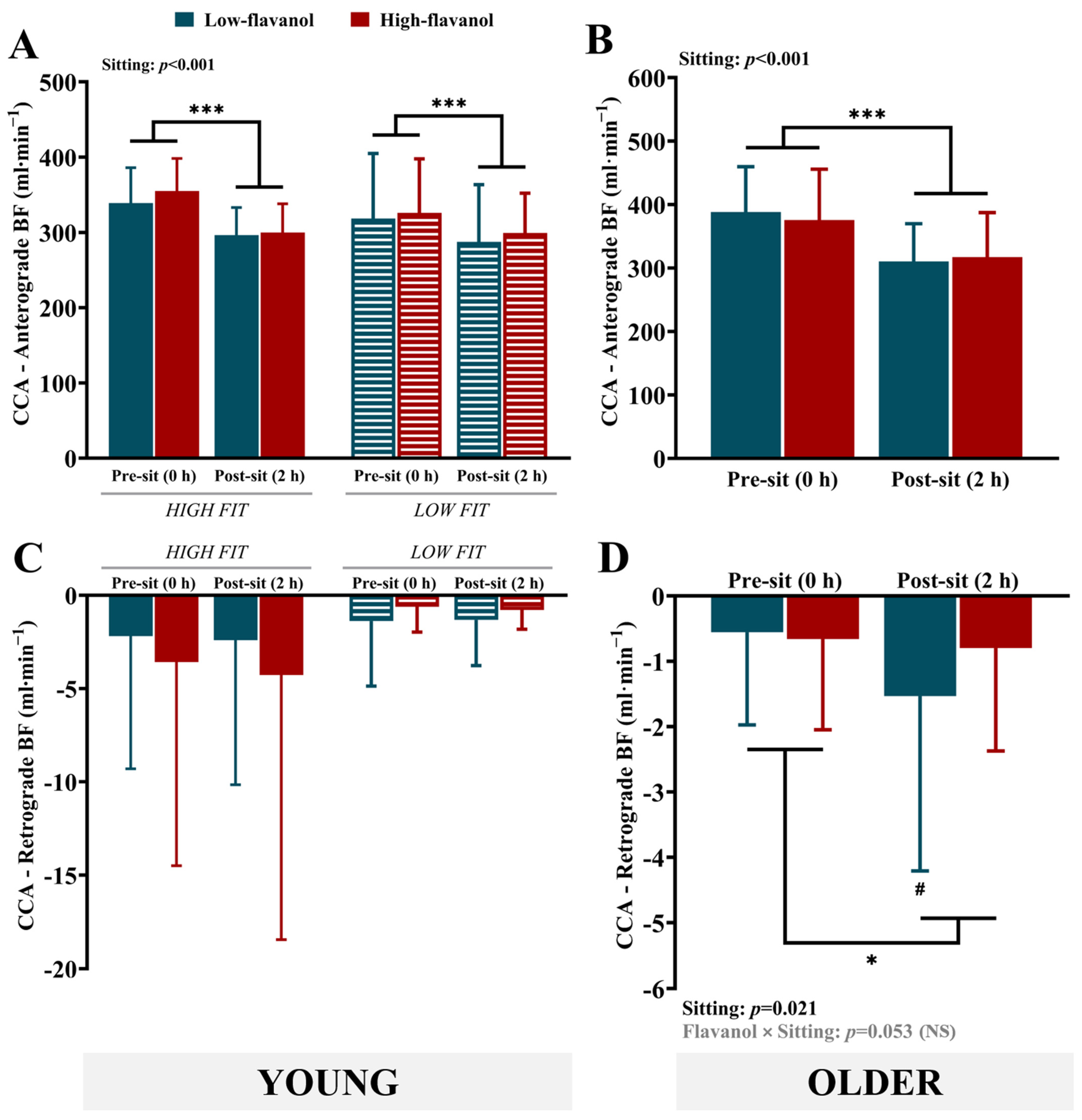
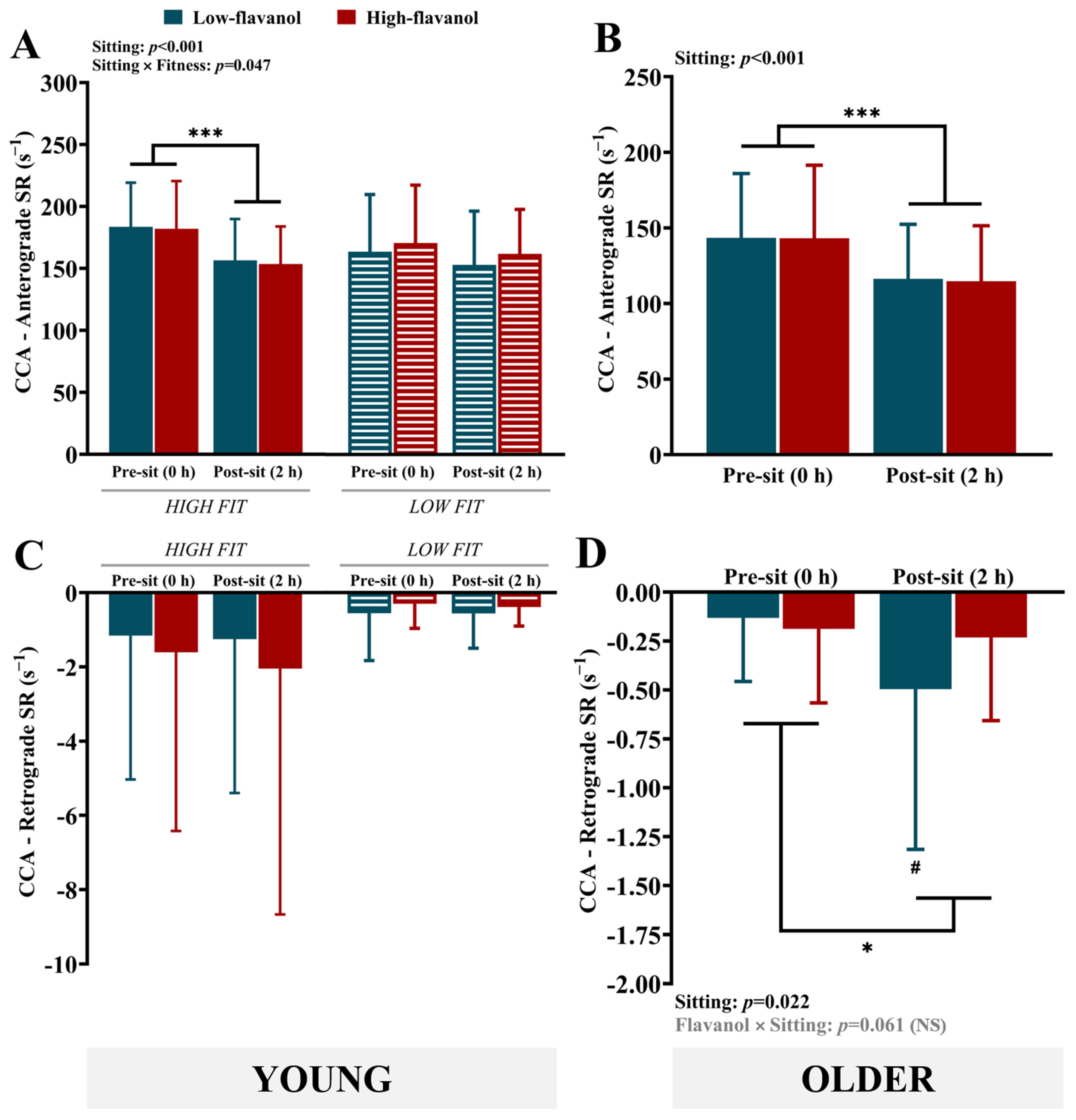
3.3. Common Carotid Artery (CCA) Vascular Measures
4. Discussion
4.1. Impact of Sitting on Carotid Blood Flow/Shear Rate: Role of Fitness and/or Aging
4.2. Impact of Flavanols on Carotid Blood Flow/Shear Rate During Sitting
5. Limitations
6. Conclusions
Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Cai, H.; Bai, W.; Su, Z.; Tang, Y.-L.; Ungvari, G.S.; Ng, C.H.; Zhang, Q.; Xiang, Y.-T. Global prevalence of mild cognitive impairment among older adults living in nursing homes: A meta-analysis and systematic review of epidemiological surveys. Transl. Psychiatry 2023, 13, 88. [Google Scholar] [CrossRef]
- Song, W.-x.; Wu, W.-w.; Zhao, Y.-y.; Xu, H.-l.; Chen, G.-c.; Jin, S.-y.; Chen, J.; Xian, S.-x.; Liang, J.-h. Evidence from a meta-analysis and systematic review reveals the global prevalence of mild cognitive impairment. Front. Aging Neurosci. 2023, 15, 1227112. [Google Scholar] [CrossRef]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement 2024, 20, 3708–3821. [CrossRef]
- Barrett, E.; Burns, A. Dementia Revealed: What Primary Care Needs to Know. A Primer for General Practice. NHS England. 2014. Available online: https://www.england.nhs.uk/wp-content/uploads/2014/09/dementia-revealed-toolkit.pdf (accessed on 1 October 2024).
- WHO. Dementia; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Patterson, C. World Alzheimer Report 2018; Alzheimer’s Disease International (ADI): London, UK, 2018. [Google Scholar]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular cognitive impairment and dementia: JACC scientific expert panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; García-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Iso-Markku, P.; Aaltonen, S.; Kujala, U.M.; Halme, H.-L.; Phipps, D.; Knittle, K.; Vuoksimaa, E.; Waller, K. Physical activity and cognitive decline among older adults: A systematic review and meta-analysis. JAMA Netw. Open 2024, 7, e2354285. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Aslan, D.H.; Sayre, M.K.; Bharadwaj, P.K.; Ally, M.; Maltagliati, S.; Lai, M.H.C.; Wilcox, R.R.; Klimentidis, Y.C.; Alexander, G.E. Sedentary behavior and incident dementia among older adults. JAMA 2023, 330, 934–940. [Google Scholar] [CrossRef]
- Arnoldy, L.; Gauci, S.; Young, L.M.; Marx, W.; Macpherson, H.; Pipingas, A.; Civier, O.; White, D.J. The association of dietary and nutrient patterns on neurocognitive decline: A systematic review of MRI and PET studies. Ageing Res. Rev. 2023, 87, 101892. [Google Scholar] [CrossRef]
- Morris, M.C. Nutrition and risk of dementia: Overview and methodological issues. Ann. N. Y. Acad. Sci. 2016, 1367, 31–37. [Google Scholar] [CrossRef]
- Kumareswaran, S. Detrimental impact of sedentary behaviour on health. Eur. J. Med. Health Sci. 2023, 5, 18–22. [Google Scholar] [CrossRef]
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too much sitting: The population health science of sedentary behavior. Exerc. Sport Sci. Rev. 2010, 38, 105–113. [Google Scholar] [CrossRef]
- Kongsvold, A.; Flaaten, M.; Logacjov, A.; Skarpsno, E.S.; Bach, K.; Nilsen, T.I.L.; Mork, P.J. Can the bias of self-reported sitting time be corrected? A statistical model validation study based on data from 23 993 adults in the Norwegian HUNT study. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Bennie, J.A.; Chau, J.Y.; van der Ploeg, H.P.; Stamatakis, E.; Do, A.; Bauman, A. The prevalence and correlates of sitting in European adults-a comparison of 32 Eurobarometer-participating countries. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.T.; Paterson, C.; Poles, J.; Higgins, S.; Stoner, L. The effect of sitting duration on peripheral blood pressure responses to prolonged sitting, with and without interruption: A systematic review and meta-analysis. Sports Med. 2024, 54, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.P.; Hewson, D.J.; Champion, R.B.; Sayegh, S.M. Sitting time and risk of cardiovascular disease and diabetes: A systematic review and meta-analysis. Am. J. Prev. Med. 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Gao, W.; Sanna, M.; Chen, Y.-H.; Tsai, M.-K.; Wen, C.-P. Occupational sitting time, leisure physical activity, and all-cause and cardiovascular disease mortality. JAMA Netw. Open 2024, 7, e2350680. [Google Scholar]
- Li, S.; Lear, S.A.; Rangarajan, S.; Hu, B.; Yin, L.; Bangdiwala, S.I.; Alhabib, K.F.; Rosengren, A.; Gupta, R.; Mony, P.K. Association of sitting time with mortality and cardiovascular events in high-income, middle-income, and low-income countries. JAMA Cardiol. 2022, 7, 796–807. [Google Scholar] [CrossRef]
- Liao, J.; Hu, M.; Imm, K.; Holmes, C.J.; Zhu, J.; Cao, C.; Yang, L. Association of daily sitting time and leisure-time physical activity with body fat among US adults. J. Sport Health Sci. 2024, 13, 195–203. [Google Scholar] [CrossRef]
- Cai, X.-y.; Qian, G.-p.; Wang, F.; Zhang, M.-y.; Da, Y.-j.; Liang, J.-h. Association between sedentary behavior and risk of cognitive decline or mild cognitive impairment among the elderly: A systematic review and meta-analysis. Front. Neurosci. 2023, 17, 1221990. [Google Scholar]
- van der Sluijs, K.M.; Bakker, E.A.; Kerstens, T.P.; Stens, N.A.; de Koning, I.A.; Thannhauser, J.; Malik, A.E.F.; Reesink, K.D.; Nabeel, P.M.; Raj, K.V. Association of Objectively Measured Sedentary Behavior With Arterial Stiffness: Findings From the Nijmegen Exercise Study. Scand. J. Med. Sci. Sports 2024, 34, e14757. [Google Scholar] [CrossRef]
- Huynh, Q.L.; Blizzard, C.L.; Sharman, J.E.; Magnussen, C.G.; Dwyer, T.; Venn, A.J. The cross-sectional association of sitting time with carotid artery stiffness in young adults. BMJ Open 2014, 4, e004384. [Google Scholar] [CrossRef]
- Ke, J.; Li, K.; Ke, T.; Zhong, X.; Zheng, Q.; Wang, Y.; Li, L.; Dai, Y.; Dong, Q.; Ji, B. Association of sedentary time and carotid atherosclerotic plaques in patients with type 2 diabetes. J. Diabetes 2022, 14, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Jin, W.; Lyu, P.-Y.; Liu, Y.; Li, R.; Hu, M.; Xiao, X.-J. Carotid atherosclerosis and cognitive impairment in nonstroke patients. Chin. Med. J. 2017, 130, 2375–2379. [Google Scholar] [PubMed]
- Khan, A.A.; Patel, J.; Desikan, S.; Chrencik, M.; Martinez-Delcid, J.; Caraballo, B.; Yokemick, J.; Gray, V.L.; Sorkin, J.D.; Cebral, J. Asymptomatic carotid artery stenosis is associated with cerebral hypoperfusion. J. Vasc. Surg. 2021, 73, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Tarumi, T.; Shah, F.; Tanaka, H.; Haley, A.P. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am. J. Hypertens. 2011, 24, 1108–1113. [Google Scholar] [CrossRef]
- Zeki Al Hazzouri, A.; Yaffe, K. Arterial stiffness and cognitive function in the elderly. J. Alzheimer’s Dis. 2014, 42, S503–S514. [Google Scholar] [CrossRef]
- Carter, S.E.; Draijer, R.; Holder, S.M.; Brown, L.; Thijssen, D.H.J.; Hopkins, N.D. Regular walking breaks prevent the decline in cerebral blood flow associated with prolonged sitting. J. Appl. Physiol. 2018, 125, 790–798. [Google Scholar] [CrossRef]
- Wheeler, M.J.; Dunstan, D.W.; Smith, B.; Smith, K.J.; Scheer, A.; Lewis, J.; Naylor, L.H.; Heinonen, I.; Ellis, K.A.; Cerin, E. Morning exercise mitigates the impact of prolonged sitting on cerebral blood flow in older adults. J. Appl. Physiol. 2019, 126, 1049–1055. [Google Scholar] [CrossRef]
- Burnet, K.; Blackwell, J.; Kelsch, E.; Hanson, E.D.; Stone, K.; Fryer, S.; Credeur, D.; Palta, P.; Stoner, L. Cerebrovascular function response to prolonged sitting combined with a high-glycemic index meal: A double-blind, randomized cross-over trial. Psychophysiology 2021, 58, e13830. [Google Scholar] [CrossRef]
- Jones, R.; McArthur, D.; McCoy, S.M.; Stoner, L.; Credeur, D.P. Impact of a Brief Period of Uninterrupted Sitting on Cerebrovascular Hemodynamics: 524 Board# 7 May 29 1: 00 PM-3: 00 PM. Med. Sci. Sports Exerc. 2019, 51, 134. [Google Scholar]
- Jones, R.; McArthur, D.; McCoy, S.M.; Stoner, L.; Fryer, S.; Credeur, D.P. Impact of acute uninterrupted sitting on cerebrovascular hemodynamics. Int. J. Exerc. Sci. 2022, 15, 1156. [Google Scholar] [PubMed]
- Chandran, O.; Shruthi, P.; Sukumar, S.; Kadavigere, R.; Chakravarthy, K.; Rao, C.R.; Chandrasekaran, B. Effects of physical activity breaks during prolonged sitting on vascular and executive function—A randomised cross-over trial. J. Taibah Univ. Med. Sci. 2023, 18, 1065–1075. [Google Scholar] [CrossRef]
- Fryer, S.; Paterson, C.; Stoner, L.; Brown, M.A.; Faulkner, J.; Turner, L.A.; Aguirre-Betolaza, A.M.; Zieff, G.; Stone, K. Leg fidgeting improves executive function following prolonged sitting with a typical western meal: A randomized, controlled cross-over trial. Int. J. Environ. Res. Public Health 2022, 19, 1357. [Google Scholar] [CrossRef] [PubMed]
- Paramashiva, P.S.; Madhivanan, O.C.; Chandrasekaran, B.; Vaishali, K.; Sukumar, S.; Kadavigere, R. Alteration in central vascular and cognitive functions during simulated work conditions in males–a secondary analysis from a randomised controlled trial. F1000Research 2025, 11, 397. [Google Scholar]
- Carter, S.E.; Draijer, R.; Stewart, C.E.; Moss, A.D.; Thijssen, D.H.J.; Hopkins, N.D. Are acute sitting-induced changes in inflammation and cerebrovascular function related to impaired mood and cognition? Sport Sci. Health 2021, 17, 753–762. [Google Scholar] [CrossRef]
- Maasakkers, C.M.; Melis, R.J.F.; Kessels, R.P.C.; Gardiner, P.A.; Olde Rikkert, M.G.M.; Thijssen, D.H.J.; Claassen, J.A.H.R. The short-term effects of sedentary behaviour on cerebral hemodynamics and cognitive performance in older adults: A cross-over design on the potential impact of mental and/or physical activity. Alzheimer’s Res. Ther. 2020, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Guzman, V.A.; Gonzalez-Castellon, M.; Razlighi, Q.; Gu, Y.; Narkhede, A.; Janicki, S.; Ichise, M.; Stern, Y.; Manly, J.J. Cerebral autoregulation, beta amyloid, and white matter hyperintensities are interrelated. Neurosci. Lett. 2015, 592, 54–58. [Google Scholar] [CrossRef]
- Crippa, I.A.; Subirà, C.; Vincent, J.-L.; Fernandez, R.F.; Hernandez, S.C.; Cavicchi, F.Z.; Creteur, J.; Taccone, F.S. Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit. Care 2018, 22, 327. [Google Scholar] [CrossRef] [PubMed]
- Yew, B.; Nation, D.A.; Alzheimer’s Disease Neuroimaging, I. Cerebrovascular resistance: Effects on cognitive decline, cortical atrophy, and progression to dementia. Brain 2017, 140, 1987–2001. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, J.; Winkler, E.A.H.; Healy, G.N.; Dempsey, P.C.; Bellettiere, J.; Owen, N.; Dunstan, D.W. Descriptive epidemiology of interruptions to free-living sitting time in middle-age and older adults. Med. Sci. Sports Exerc. 2021, 53, 2503. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.-F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef]
- Yeh, T.-S.; Yuan, C.; Ascherio, A.; Rosner, B.A.; Willett, W.C.; Blacker, D. Long-term dietary flavonoid intake and subjective cognitive decline in US men and women. Neurology 2021, 97, e1041–e1056. [Google Scholar] [PubMed]
- Brickman, A.M.; Yeung, L.-K.; Alschuler, D.M.; Ottaviani, J.I.; Kuhnle, G.G.C.; Sloan, R.P.; Luttmann-Gibson, H.; Copeland, T.; Schroeter, H.; Sesso, H.D. Dietary flavanols restore hippocampal-dependent memory in older adults with lower diet quality and lower habitual flavanol consumption. Proc. Natl. Acad. Sci. USA 2023, 120, e2216932120. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.; Haddad, Z.; Deisling, S.; Ellinger, S. Effect of an (–)-Epicatechin Intake on Cardiometabolic Parameters—A Systematic Review of Randomized Controlled Trials. Nutrients 2022, 14, 4500. [Google Scholar] [CrossRef]
- Ebaditabar, M.; Djafarian, K.; Saeidifard, N.; Shab-Bidar, S. Effect of dark chocolate on flow-mediated dilatation: Systematic review, meta-analysis, and dose–response analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 36, 17–27. [Google Scholar] [CrossRef]
- Raman, G.; Avendano, E.E.; Chen, S.; Wang, J.; Matson, J.; Gayer, B.; Novotny, J.A.; Cassidy, A. Dietary intakes of flavan-3-ols and cardiometabolic health: Systematic review and meta-analysis of randomized trials and prospective cohort studies. Am. J. Clin. Nutr. 2019, 110, 1067–1078. [Google Scholar] [CrossRef]
- Decroix, L.; De Pauw, K.; Van Cutsem, J.; Pattyn, N.; Heyman, E.; Meeusen, R. Acute cocoa flavanols intake improves cerebral hemodynamics while maintaining brain activity and cognitive performance in moderate hypoxia. Psychopharmacology 2018, 235, 2597–2608. [Google Scholar] [CrossRef]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47, S215–S220. [Google Scholar] [CrossRef]
- Gratton, G.; Weaver, S.R.; Burley, C.V.; Low, K.A.; Maclin, E.L.; Johns, P.W.; Pham, Q.S.; Lucas, S.J.E.; Fabiani, M.; Rendeiro, C. Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults. Sci. Rep. 2020, 10, 19409. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Pal, D.; Moutsiana, C.; Field, D.T.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. Psychopharmacology 2015, 232, 3227–3234. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Eijsvogels, T.M.H. Coronary atherosclerosis in middle-aged athletes: Current insights, burning questions, and future perspectives. Clin. Cardiol. 2020, 43, 863–871. [Google Scholar] [CrossRef]
- Runacres, A.; Mackintosh, K.A.; McNarry, M.A. Health consequences of an elite sporting career: Long-term detriment or long-term gain? A meta-analysis of 165,000 former athletes. Sports Med. 2021, 51, 289–301. [Google Scholar] [CrossRef]
- Smith, E.C.; Pizzey, F.K.; Askew, C.D.; Mielke, G.I.; Ainslie, P.N.; Coombes, J.S.; Bailey, T.G. Effects of cardiorespiratory fitness and exercise training on cerebrovascular blood flow and reactivity: A systematic review with meta-analyses. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H59–H76. [Google Scholar] [CrossRef]
- Stacey, B.S.; Campbell, Z.; Bailey, D.M. Elevated cerebral perfusion and preserved cognition in elite Brazilian Jiu-Jitsu athletes: Evidence for neuroprotection. Scand. J. Med. Sci. Sports 2021, 31, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Stupin, M.; Stupin, A.; Rasic, L.; Cosic, A.; Kolar, L.; Seric, V.; Lenasi, H.; Izakovic, K.; Drenjancevic, I. Acute exhaustive rowing exercise reduces skin microvascular dilator function in young adult rowing athletes. Eur. J. Appl. Physiol. 2018, 118, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Štursová, P.; Budinska, X.; Nováková, Z.; Dobšák, P.; Babula, P. Sports activities and cardiovascular system change. Physiol. Res. 2023, 72, S429. [Google Scholar] [CrossRef]
- Sugawara, J.; Tomoto, T.; Repshas, J.; Zhang, R.; Tarumi, T. Middle-aged endurance athletes exhibit lower cerebrovascular impedance than sedentary peers. J. Appl. Physiol. 2020, 129, 335–342. [Google Scholar] [CrossRef]
- Ahmed, B.; Rahman, A.A.; Lee, S.; Malhotra, R. The implications of aging on vascular health. Int. J. Mol. Sci. 2024, 25, 11188. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Vyzas, C.; Mishra, K.; Graham, R.M.; Vatner, D.E. Vascular stiffness in aging and disease. Front. Physiol. 2021, 12, 762437. [Google Scholar] [CrossRef] [PubMed]
- Holder, S.M.; Dawson, E.A.; Brislane, Á.; Hisdal, J.; Green, D.J.; Thijssen, D.H.J. Fluctuation in shear rate, with unaltered mean shear rate, improves brachial artery flow-mediated dilation in healthy, young men. J. Appl. Physiol. 2019, 126, 1687–1693. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Dawson, E.A.; Tinken, T.M.; Cable, N.T.; Green, D.J. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 2009, 53, 986–992. [Google Scholar] [CrossRef]
- Tinken, T.M.; Thijssen, D.H.J.; Hopkins, N.; Black, M.A.; Dawson, E.A.; Minson, C.T.; Newcomer, S.C.; Laughlin, M.H.; Cable, N.T.; Green, D.J. Impact of shear rate modulation on vascular function in humans. Hypertension 2009, 54, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, T.H.A.; Green, D.J.; Hopman, M.T.E.; Thijssen, D.H.J. Acute impact of retrograde shear rate on brachial and superficial femoral artery flow-mediated dilation in humans. Physiol. Rep. 2014, 2, e00193. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar]
- Krause, D.N.; Duckles, S.P.; Pelligrino, D.A. Influence of sex steroid hormones on cerebrovascular function. J. Appl. Physiol. 2006, 101, 1252–1261. [Google Scholar] [CrossRef]
- Williams, J.S.; Dunford, E.C.; MacDonald, M.J. Impact of the menstrual cycle on peripheral vascular function in premenopausal women: Systematic review and meta-analysis. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1327–H1337. [Google Scholar] [CrossRef]
- Correale, M.; Leopizzi, A.; Mallardi, A.; Ranieri, A.; Suriano, M.P.; D’Alessandro, D.; Tricarico, L.; Mazzeo, P.; Tucci, S.; Pastore, G. Switch to direct anticoagulants and improved endothelial function in patients with chronic heart failure and atrial fibrillation. Thromb. Res. 2020, 195, 16–20. [Google Scholar] [CrossRef]
- Daniele, A.; Lucas, S.J.E.; Rendeiro, C. Cocoa flavanols rescue upper- and lower-limb endothelial function during sitting in high- and low-fit young healthy males. J. Physiol. 2025; submitted. [Google Scholar]
- Daniele, A.; Lucas, S.J.E.; Rendeiro, C. Cocoa flavanols protect endothelial function during prolonged sitting in healthy older adults. Food Funct. 2015; submitted. [Google Scholar]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.J.; Schroeter, H.; Crozier, A. Absorption, metabolism, distribution and excretion of (−)-epicatechin: A review of recent findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Ultrasound assessment of flow-mediated dilation. Hypertension 2010, 55, 1075–1085. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Thosar, S.S.; Bielko, S.L.; Mather, K.J.; Johnston, J.D.; Wallace, J.P. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med. Sci. Sports Exerc. 2015, 47, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef]
- Baynham, R.; van Zanten, J.J.C.S.V.; Rendeiro, C. Cocoa flavanols rescue stress-induced declines in endothelial function after a high-fat meal, but do not affect cerebral oxygenation during stress in young, healthy adults. Food Funct. 2024, 15, 11472–11490. [Google Scholar] [CrossRef]
- Heiss, C.; Kleinbongard, P.; Dejam, A.; Perré, S.; Schroeter, H.; Sies, H.; Kelm, M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2005, 46, 1276–1283. [Google Scholar] [CrossRef]
- Faridi, Z.; Njike, V.Y.; Dutta, S.; Ali, A.; Katz, D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008, 88, 58–63. [Google Scholar] [CrossRef]
- Sansone, R.; Ottaviani, J.I.; Rodriguez-Mateos, A.; Heinen, Y.; Noske, D.; Spencer, J.P.; Crozier, A.; Merx, M.W.; Kelm, M.; Schroeter, H. Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: Randomized, double-masked controlled studies. Am. J. Clin. Nutr. 2017, 105, 352–360. [Google Scholar] [CrossRef]
- Alsolmei, F.A.; Li, H.; Pereira, S.L.; Krishnan, P.; Johns, P.W.; Siddiqui, R.A. Polyphenol-enriched plum extract enhances myotubule formation and anabolism while attenuating colon cancer-induced cellular damage in C2C12 cells. Nutrients 2019, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J.; Leonczak, J.; Li, J.; Johnson, J.C.; Collins, T.; Kwik-Uribe, C.; Schmitz, H.H. Determination of flavanol and procyanidin (by degree of polymerization 1–10) content of chocolate, cocoa liquors, powder (s), and cocoa flavanol extracts by normal phase high-performance liquid chromatography: Collaborative study. J. AOAC Int. 2012, 95, 1153–1160. [Google Scholar] [CrossRef]
- Miller, K.B.; Hurst, W.J.; Payne, M.J.; Stuart, D.A.; Apgar, J.; Sweigart, D.S.; Ou, B. Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. J. Agric. Food Chem. 2008, 56, 8527–8533. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.N.; Lewis, N.C.S.; Hill, B.G.; Ainslie, P.N. Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R707–R720. [Google Scholar] [CrossRef]
- Daniele, A.; Lucas, S.J.E.; Rendeiro, C. Variability of flow-mediated dilation across lower and upper limb conduit arteries. Eur. J. Appl. Physiol. 2024, 124, 3265–3278. [Google Scholar] [CrossRef]
- Hoiland, R.L.; Ainslie, P.N.; Wildfong, K.W.; Smith, K.J.; Bain, A.R.; Willie, C.K.; Foster, G.; Monteleone, B.; Day, T.A. Indomethacin-induced impairment of regional cerebrovascular reactivity: Implications for respiratory control. J. Physiol. 2015, 593, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Seidel, E.; Eicke, B.M.; Tettenborn, B.; Krummenauer, F. Reference values for vertebral artery flow volume by duplex sonography in young and elderly adults. Stroke 1999, 30, 2692–2696. [Google Scholar] [CrossRef]
- Nicolas, N.; de Tilly, A.; Roux, E. Blood shear stress during the cardiac cycle and endothelial cell orientation and polarity in the carotid artery of male and female mice. Front. Physiol. 2024, 15, 1386151. [Google Scholar] [CrossRef]
- Pyke, K.E.; Tschakovsky, M.E. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J. Physiol. 2005, 568, 357–369. [Google Scholar] [CrossRef]
- van den Munckhof, I.C.L.; Jones, H.; Hopman, M.T.E.; de Graaf, J.; Nyakayiru, J.; van Dijk, B.; Eijsvogels, T.M.H.; Thijssen, D.H.J. Relation between age and carotid artery intima-medial thickness: A systematic review. Clin. Cardiol. 2018, 41, 698–704. [Google Scholar] [CrossRef]
- Bauer, M.; Caviezel, S.; Teynor, A.; Erbel, R.; Mahabadi, A.A.; Schmidt-Trucksäss, A. Carotid intima-media thickness as a biomarker of subclinical atherosclerosis. Swiss Med. Wkly. 2012, 142, w13705. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Pencina, M.J.; Pencina, K.M.; O’Donnell, C.J.; Wolf, P.A.; D’Agostino, R.B., Sr. Carotid-wall intima–media thickness and cardiovascular events. N. Engl. J. Med. 2011, 365, 213–221. [Google Scholar] [CrossRef]
- Homma, S.; Hirose, N.; Ishida, H.; Ishii, T.; Araki, G. Carotid plaque and intima-media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke 2001, 32, 830–835. [Google Scholar] [CrossRef] [PubMed]
- de Weerd, M.; Greving, J.P.; de Jong, A.W.F.; Buskens, E.; Bots, M.L. Prevalence of asymptomatic carotid artery stenosis according to age and sex: Systematic review and metaregression analysis. Stroke 2009, 40, 1105–1113. [Google Scholar] [CrossRef]
- Miller, K.B.; Howery, A.J.; Rivera-Rivera, L.A.; Johnson, S.C.; Rowley, H.A.; Wieben, O.; Barnes, J.N. Age-related reductions in cerebrovascular reactivity using 4D flow MRI. Front. Aging Neurosci. 2019, 11, 281. [Google Scholar] [CrossRef]
- Mandalà, M.; Cipolla, M.J. Aging-related structural and functional changes in cerebral arteries: Caloric restriction (CR) intervention. J. Vasc. Med. Surg. 2021, 9, 1000002. [Google Scholar]
- Murman, D.L. The impact of age on cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Yamazaki, T.; Takano, D.; Maeda, T.; Fujimaki, Y.; Nakase, T.; Sato, Y. Cerebral circulation in aging. Ageing Res. Rev. 2016, 30, 49–60. [Google Scholar] [CrossRef]
- Bracko, O.; Cruz Hernández, J.C.; Park, L.; Nishimura, N.; Schaffer, C.B. Causes and consequences of baseline cerebral blood flow reductions in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2021, 41, 1501–1516. [Google Scholar] [CrossRef]
- Korte, N.; Nortley, R.; Attwell, D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020, 140, 793–810. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, Y.; Chen, R.; Liu, X.; Hu, Y.; Ma, Z.; Gao, L.; Jian, W.; Wang, L. Wall shear stress and its role in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1083547. [Google Scholar] [CrossRef] [PubMed]
- Tschiderer, L.; Seekircher, L.; Izzo, R.; Mancusi, C.; Manzi, M.V.; Baldassarre, D.; Amato, M.; Tremoli, E.; Veglia, F.; Tuomainen, T.P. Association of Intima-Media Thickness Measured at the Common Carotid Artery With Incident Carotid Plaque: Individual Participant Data Meta-Analysis of 20 Prospective Studies. J. Am. Heart Assoc. 2023, 12, e027657. [Google Scholar] [CrossRef]
- Pan, S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid. Redox Signal. 2009, 11, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.P.; Padilla, J.; Joyner, M.J. α-adrenergic vasoconstriction contributes to the age-related increase in conduit artery retrograde and oscillatory shear. Hypertension 2012, 60, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Murdock, M.E.; Duran, A.T.; Friel, C.P.; Serafini, M.A.; Ensari, I.; Cheung, Y.K.; Diaz, K.M. The Effects of Breaking Up Prolonged Sitting on Cognitive Performance in Middle-Aged and Older Adults: Dose Response Analysis of a Randomized Crossover Trial. Alzheimer’s Dement. 2023, 19, e083064. [Google Scholar] [CrossRef]
- Wanders, L.; Cuijpers, I.; Kessels, R.P.C.; van de Rest, O.; Hopman, M.T.E.; Thijssen, D.H.J. Impact of prolonged sitting and physical activity breaks on cognitive performance, perceivable benefits, and cardiometabolic health in overweight/obese adults: The role of meal composition. Clin. Nutr. 2021, 40, 2259–2269. [Google Scholar] [CrossRef]
- Wheeler, M.J.; Green, D.J.; Ellis, K.A.; Cerin, E.; Heinonen, I.; Naylor, L.H.; Larsen, R.; Wennberg, P.; Boraxbekk, C.-J.; Lewis, J. Distinct effects of acute exercise and breaks in sitting on working memory and executive function in older adults: A three-arm, randomised cross-over trial to evaluate the effects of exercise with and without breaks in sitting on cognition. Br. J. Sports Med. 2020, 54, 776–781. [Google Scholar] [CrossRef]
- Lang, J.J.; Prince, S.A.; Merucci, K.; Cadenas-Sanchez, C.; Chaput, J.-P.; Fraser, B.J.; Manyanga, T.; McGrath, R.; Ortega, F.B.; Singh, B. Cardiorespiratory fitness is a strong and consistent predictor of morbidity and mortality among adults: An overview of meta-analyses representing over 20.9 million observations from 199 unique cohort studies. Br. J. Sports Med. 2024, 58, 556–566. [Google Scholar] [CrossRef]
- Horiuchi, M.; Pomeroy, A.; Horiuchi, Y.; Stone, K.; Stoner, L. Effects of intermittent exercise during prolonged sitting on executive function, cerebrovascular, and psychological response: A randomized crossover trial. J. Appl. Physiol. 2023, 135, 1421–1430. [Google Scholar] [CrossRef]
- Baker, B.D.; Castelli, D.M. Prolonged sitting reduces cerebral oxygenation in physically active young adults. Front. Cogn. 2024, 3, 1370064. [Google Scholar] [CrossRef]
- Padilla, J.; Simmons, G.H.; Fadel, P.J.; Laughlin, M.H.; Joyner, M.J.; Casey, D.P. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: Role of nitric oxide. Hypertension 2011, 57, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Scholten, R.R.; Spaanderman, M.E.A.; Green, D.J.; Hopman, M.T.E.; Thijssen, D.H.J. Retrograde shear rate in formerly preeclamptic and healthy women before and after exercise training: Relationship with endothelial function. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H418–H425. [Google Scholar] [CrossRef]
- Cheng, N.; Bell, L.; Lamport, D.J.; Williams, C.M. Dietary flavonoids and human cognition: A meta-analysis. Mol. Nutr. Food Res. 2022, 66, 2100976. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.D.L.; Hollenberg, N.K. Aging and vascular responses to flavanol-rich cocoa. J. Hypertens. 2006, 24, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; Lucas, S.J.E.; Rendeiro, C. Detrimental effects of physical inactivity on peripheral and brain vasculature in humans: Insights into mechanisms, long-term health consequences and protective strategies. Front. Physiol. 2022, 13, 998380. [Google Scholar] [CrossRef]
- Moreno-Ulloa, A.; Mendez-Luna, D.; Beltran-Partida, E.; Castillo, C.; Guevara, G.; Ramirez-Sanchez, I.; Correa-Basurto, J.; Ceballos, G.; Villarreal, F. The effects of (−)-epicatechin on endothelial cells involve the G protein-coupled estrogen receptor (GPER). Pharmacol. Res. 2015, 100, 309–320. [Google Scholar] [CrossRef]
- Moreno-Ulloa, A.; Romero-Perez, D.; Villarreal, F.; Ceballos, G.; Ramirez-Sanchez, I. Cell membrane mediated (−)-epicatechin effects on upstream endothelial cell signaling: Evidence for a surface receptor. Bioorganic Med. Chem. Lett. 2014, 24, 2749–2752. [Google Scholar] [CrossRef]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D.L. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar]
- Bloomfield, P.M.; Fisher, J.P.; Shaw, D.M.; Gant, N. Cocoa flavanols protect cognitive function, cerebral oxygenation, and mental fatigue during severe hypoxia. J. Appl. Physiol. 2023, 135, 475–484. [Google Scholar] [CrossRef]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Tagougui, S.; Heyman, E.; Meeusen, R. Acute cocoa flavanol improves cerebral oxygenation without enhancing executive function at rest or after exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Marsh, C.E.; Carter, H.H.; Guelfi, K.J.; Smith, K.J.; Pike, K.E.; Naylor, L.H.; Green, D.J. Brachial and cerebrovascular functions are enhanced in postmenopausal women after ingestion of chocolate with a high concentration of cocoa. J. Nutr. 2017, 147, 1686–1692. [Google Scholar] [CrossRef]
- Carr, J.; Hoiland, R.L.; Caldwell, H.G.; Coombs, G.B.; Howe, C.A.; Tremblay, J.C.; Green, D.J.; Ainslie, P.N. Internal carotid and brachial artery shear-dependent vasodilator function in young healthy humans. J. Physiol. 2020, 598, 5333–5350. [Google Scholar] [CrossRef] [PubMed]
- Hoiland, R.L.; Caldwell, H.G.; Carr, J.M.J.R.; Howe, C.A.; Stacey, B.S.; Dawkins, T.; Wakeham, D.J.; Tremblay, J.C.; Tymko, M.M.; Patrician, A. Nitric oxide contributes to cerebrovascular shear-mediated dilatation but not steady-state cerebrovascular reactivity to carbon dioxide. J. Physiol. 2022, 600, 1385–1403. [Google Scholar] [CrossRef]
- Smith, K.J.; Hoiland, R.L.; Grove, R.; McKirdy, H.; Naylor, L.; Ainslie, P.N.; Green, D.J. Matched increases in cerebral artery shear stress, irrespective of stimulus, induce similar changes in extra-cranial arterial diameter in humans. J. Cereb. Blood Flow Metab. 2019, 39, 849–858. [Google Scholar] [CrossRef]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef] [PubMed]
- Ruediger, S.L.; Koep, J.L.; Keating, S.E.; Pizzey, F.K.; Coombes, J.S.; Bailey, T.G. Effect of menopause on cerebral artery blood flow velocity and cerebrovascular reactivity: Systematic review and meta-analysis. Maturitas 2021, 148, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef]
- Woolf, E.K.; Terwoord, J.D.; Litwin, N.S.; Vazquez, A.R.; Lee, S.Y.; Ghanem, N.; Michell, K.A.; Smith, B.T.; Grabos, L.E.; Ketelhut, N.B. Daily blueberry consumption for 12 weeks improves endothelial function in postmenopausal women with above-normal blood pressure through reductions in oxidative stress: A randomized controlled trial. Food Funct. 2023, 14, 2621–2641. [Google Scholar] [CrossRef]
- Monahan, K.D.; Feehan, R.P.; Kunselman, A.R.; Preston, A.G.; Miller, D.L.; Lott, M.E.J. Dose-dependent increases in flow-mediated dilation following acute cocoa ingestion in healthy older adults. J. Appl. Physiol. 2011, 111, 1568–1574. [Google Scholar] [CrossRef]
- Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G.G.C.; Spencer, J.P.E.; Schroeter, H.; Merx, M.W. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: A randomised, controlled, double-masked trial: The Flaviola Health Study. Br. J. Nutr. 2015, 114, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Manson, J.E.; Aragaki, A.K.; Rist, P.M.; Johnson, L.G.; Friedenberg, G.; Copeland, T.; Clar, A.; Mora, S.; Moorthy, M.V. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: The COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am. J. Clin. Nutr. 2022, 115, 1490–1500. [Google Scholar] [CrossRef]
- Thompson, A.S.; Jennings, A.; Bondonno, N.P.; Tresserra-Rimbau, A.; Parmenter, B.H.; Hill, C.; Perez-Cornago, A.; Kühn, T.; Cassidy, A. Higher habitual intakes of flavonoids and flavonoid-rich foods are associated with a lower incidence of type 2 diabetes in the UK Biobank cohort. Nutr. Diabetes 2024, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Spencer, J.P.E.; Schroeter, H.; Khaw, K.-T.; Kuhnle, G.G.C. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef]
- Rani, T.R.; Al Shibli, A.; Siraj, M.; Srimal, W.; Al Bakri, N.Z.S.; Radhika, T.S.L. ML-based approach to predict carotid arterial blood flow dynamics. Contemp. Math. 2024, 2858–2876. [Google Scholar] [CrossRef]
- Rosner, J.; Reddy, V.; Lui, F. Neuroanatomy, Circle of Willis; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Hasan, T.F.; Akinduro, O.O.; Haranhalli, N.; Tawk, R.G. Neurovascular Anatomy in Relation to Intracranial Neoplasms. In Comprehensive Overview of Modern Surgical Approaches to Intrinsic Brain Tumors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–51. [Google Scholar]
- Crowe-White, K.M.; Evans, L.W.; Kuhnle, G.G.C.; Milenkovic, D.; Stote, K.; Wallace, T.; Handu, D.; Senkus, K.E. Flavan-3-ols and Cardiometabolic Health: First Ever Dietary Bioactive Guideline. Adv. Nutr. 2022, 13, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, C.; Dong, H.; Saunders, C.; Harkness, L.; Blaze, M.; Hou, Y.; Belanger, R.L.; Altieri, V.; Nunez, M.A.; Jackson, K.G.; et al. Flavanone-rich citrus beverages counteract the transient decline in postprandial endothelial function in humans: A randomised, controlled, double-masked, cross-over intervention study. Br. J. Nutr. 2016, 116, 1999–2010. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P.E. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef]
| Low Flavanol | High Flavanol | |
|---|---|---|
| Total polyphenols * | 260.0 | 1246.8 |
| Total flavanols (mg) | 5.6 | 695.0 |
| Procyanidins (dimers-decamers; mg) | ND | 459.6 |
| (−)-Epicatechin (mg) | <6 | 150.0 |
| (−) and (+)-Catechin (mg) | <6 | 85.4 |
| Theobromine (mg) | 278.4 | 262.8 |
| Caffeine (mg) | 22.2 | 27.6 |
| Fat (g) | 1.3 | 1.7 |
| Carbohydrates (g) | 1.2 | 2.7 |
| Protein (g) | 2.7 | 2.7 |
| Fiber (g) | 4.0 | 1.8 |
| Energy (kcal) | 36.6 | 41.4 |
| Young | Older | ||
|---|---|---|---|
| High Fit | Low Fit | ||
| N | 20 | 20 | 20 |
| Anthropometric and Fitness | |||
| Age (yr.) | 22.2 ± 2.9 $ | 23.2 ± 4.1 $ | 72.4 ± 5.0 |
| Height (m) | 1.76 ± 0.06 ¢ | 1.79 ± 0.09 $ | 1.67 ± 0.10 |
| Weight (kg) | 70.4 ± 8.8 ** | 83.5 ± 11.9 ¢ | 68.7 ± 15.6 |
| BMI (kg∙m−2) | 22.7 ± 2.5 ** | 26.0 ± 2.3 | 24.4 ± 3.6 |
| VO2peak (mL∙kg−1∙min−1) | 56.4 ± 5.3 *** | 34.8 ± 4.6 | N/A |
| Common Carotid Artery | |||
| Artery diameter (mm) | 6.9 ± 0.3 $ | 6.9 ± 0.5 $ | 7.8 ± 0.8 |
| Anterograde blood flow (mL∙min−1) | 347.0 ± 41.3 | 322.2 ± 73.8 ¥ | 382.0 ± 66.9 |
| Retrograde blood flow (mL∙min−1) | −2.9 ± 9.0 | −1.0 ± 2.3 | −0.6 ± 1.3 |
| Anterograde shear rate (s−1) | 182.8 ± 34.3 ¢ | 167.0 ± 42.8 | 143.3 ± 43.9 |
| Retrograde shear rate (s−1) | −1.4 ± 4.3 | −0.4 ± 0.9 | −0.2 ± 0.3 |
| Blood Pressure and Heart Rate | |||
| Systolic BP (mm Hg) | 117.9 ± 9.1 ¢ | 121.3 ± 8.5 | 128.4 ± 12.6 |
| Diastolic BP (mm Hg) | 63.2 ± 5.4 **$ | 68.8 ± 4.5 ¢ | 74.5 ± 6.7 |
| Heart rate (bpm) | 50.9 ± 7.4 **¢ | 63.1 ± 9.6 | 61.5 ± 11.5 |
| Young | Older | ||
|---|---|---|---|
| Daily Physical Activity Pattern | High Fit | Low Fit | |
| Sedentary activity time (h) | 10.4 ± 1 | 10 ± 2.2 | 9.8 ± 1.5 |
| Light activity time (h) | 1.4 ± 0.4 ¥ | 1.2 ± 0.4 ¢ | 1.8 ± 0.6 |
| Moderate activity time (h) | 2.8 ± 0.5 | 2.6 ± 0.8 | 2.5 ± 1.1 |
| Vigorous activity time (h) | 0.6 ± 0.4 ***$ | 0.1 ± 0.1 | 0.0 ± 0.1 |
| Sedentary activity estimated MET∙min (mL∙kg−1∙min−1) | 765.2 ± 80.3 | 716.3 ± 158.4 | 747.1 ± 138.7 |
| Light activity estimated MET∙min (mL∙kg−1∙min−1) | 200.5 ± 52.7 ¥ | 176.5 ± 63.4 ¢ | 263.6 ± 95.3 |
| Moderate activity estimated MET∙min (mL∙kg−1∙min−1) | 685.1 ± 146.5 | 622.5 ± 205.2 | 533.7 ± 243.6 |
| Vigorous activity estimated MET∙min (mL∙kg−1∙min−1) | 336.0 ± 286.9 ***$ | 70.0 ± 75.2 | 25.2 ± 35.3 |
| Step count | 12,421.7 ± 2333.8 | 9896.5 ± 3663.6 | 10,651.8 ± 3883.6 |
| High Fit | Low Fit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low Flavanol | High Flavanol | Low Flavanol | High Flavanol | ||||||
| CCA | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Effect |
| Artery diameter (mm) | 6.8 ± 0.3 ** | 6.9 ± 0.3 ** | 7.0 ± 0.3 | 7.0 ± 0.4 | 6.9 ± 0.6 | 6.9 ± 0.6 | 6.9 ± 0.5 | 6.8 ± 0.6 | Flavanol × Fitness (p = 0.017) |
| Anterograde BF (mL∙min−1) | 339.0 ± 47.0 | 296.4 ± 36.6 | 354.9 ± 43.4 | 299.9 ± 38.0 | 318.5 ± 86.4 | 287.3 ± 76.1 | 326.0 ± 71.7 | 299.2 ± 52.9 | Sitting (p < 0.001) |
| Retrograde BF (mL∙min−1) | −2.2 ± 7.1 | −2.4 ± 7.7 | −3.6 ± 10.9 | −4.3 ± 14.2 | −1.4 ± 3.5 | −1.3 ± 2.4 | −0.6 ± 1.4 | −0.8 ± 1.0 | NS |
| Anterograde SR (s−1) | 183.6 ± 35.6 *** | 156.5 ± 33.4 | 182.0 ± 38.5 *** | 153.6 ± 30.3 | 163.5 ± 46.0 | 152.8 ± 43.4 | 170.6 ± 46.6 | 161.7 ± 35.8 | Sitting (p < 0.001); Sitting × Fitness (p = 0.047) |
| Retrograde SR (s−1) | −1.2 ± 3.9 | −1.3 ± 4.1 | −1.6 ± 4.8 | −2.0 ± 6.6 | −0.6 ± 1.3 | −0.6 ± 0.9 | −0.3 ± 0.7 | −0.4 ± 0.5 | NS |
| Low Flavanol | High Flavanol | ||||
|---|---|---|---|---|---|
| CCA | Pre | Post | Pre | Post | Effect |
| Artery diameter (mm) | 7.8 ± 0.8 | 7.8 ± 0.8 | 7.8 ± 0.8 | 7.9 ± 0.8 | NS |
| Anterograde BF (mL∙min−1) | 388.4 ± 71.3 | 310.6 ± 59.4 | 375.6 ± 80.2 | 317.2 ± 70.2 | Sitting (p < 0.001) |
| Retrograde BF (mL∙min−1) | −0.6 ± 1.4 | −1.5 ± 2.7 | −0.7 ± 1.4 | −0.8 ± 1.6 | Sitting (p = 0.021); Flavanol × Sitting (p = 0.053) (NS) |
| Anterograde SR (s−1) | 143.5 ± 42.5 | 116.3 ± 36.1 | 143.1 ± 48.3 | 114.7 ± 36.7 | Sitting (p < 0.001) |
| Retrograde SR (s−1) | −0.1 ± 0.3 | −0.5 ± 0.8 | −0.2 ± 0.4 | −0.2 ± 0.4 | Sitting (p = 0.022); Flavanol × Sitting (p = 0.061) (NS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniele, A.; Lucas, S.J.E.; Rendeiro, C. The Impact of Cocoa Flavanols in Modulating Resting Cerebral Blood Flow During Prolonged Sitting in Healthy Young and Older Adults. Nutrients 2025, 17, 2099. https://doi.org/10.3390/nu17132099
Daniele A, Lucas SJE, Rendeiro C. The Impact of Cocoa Flavanols in Modulating Resting Cerebral Blood Flow During Prolonged Sitting in Healthy Young and Older Adults. Nutrients. 2025; 17(13):2099. https://doi.org/10.3390/nu17132099
Chicago/Turabian StyleDaniele, Alessio, Samuel J. E. Lucas, and Catarina Rendeiro. 2025. "The Impact of Cocoa Flavanols in Modulating Resting Cerebral Blood Flow During Prolonged Sitting in Healthy Young and Older Adults" Nutrients 17, no. 13: 2099. https://doi.org/10.3390/nu17132099
APA StyleDaniele, A., Lucas, S. J. E., & Rendeiro, C. (2025). The Impact of Cocoa Flavanols in Modulating Resting Cerebral Blood Flow During Prolonged Sitting in Healthy Young and Older Adults. Nutrients, 17(13), 2099. https://doi.org/10.3390/nu17132099







