Chronic Copper Overload Triggers Inflammation in Mesenteric PVAT Alongside Changes in Renin–Angiotensin System-Related Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Experimental Procedure
2.3. Adipocyte Isolation
2.4. Plasma Biochemical Analysis
2.5. Adipokine and Hormone Analyses
2.6. RNA Extraction and Quantitative Real-Time Polymerase
2.7. Lipolysis
2.8. Statistical Analysis
3. Results
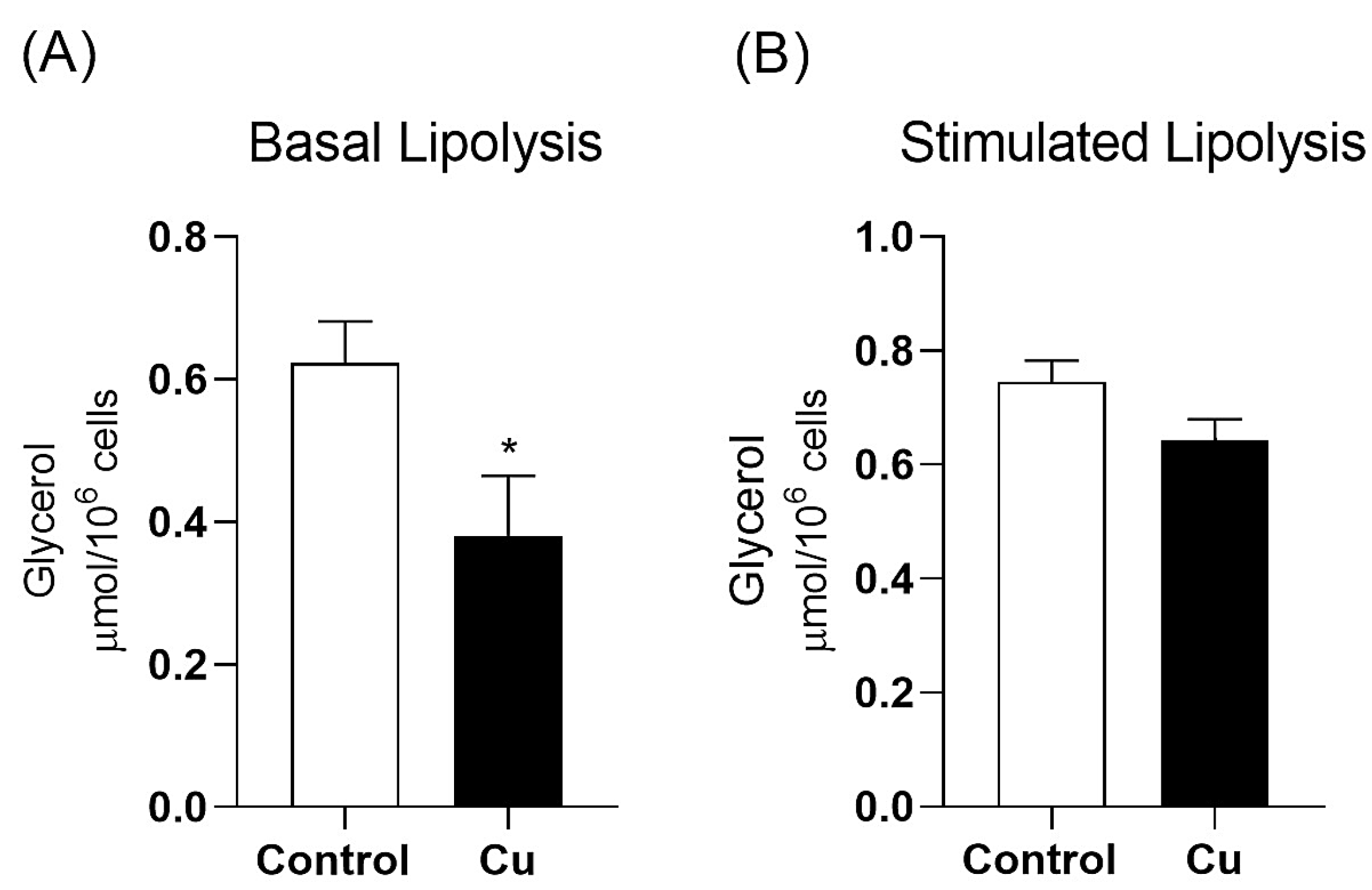
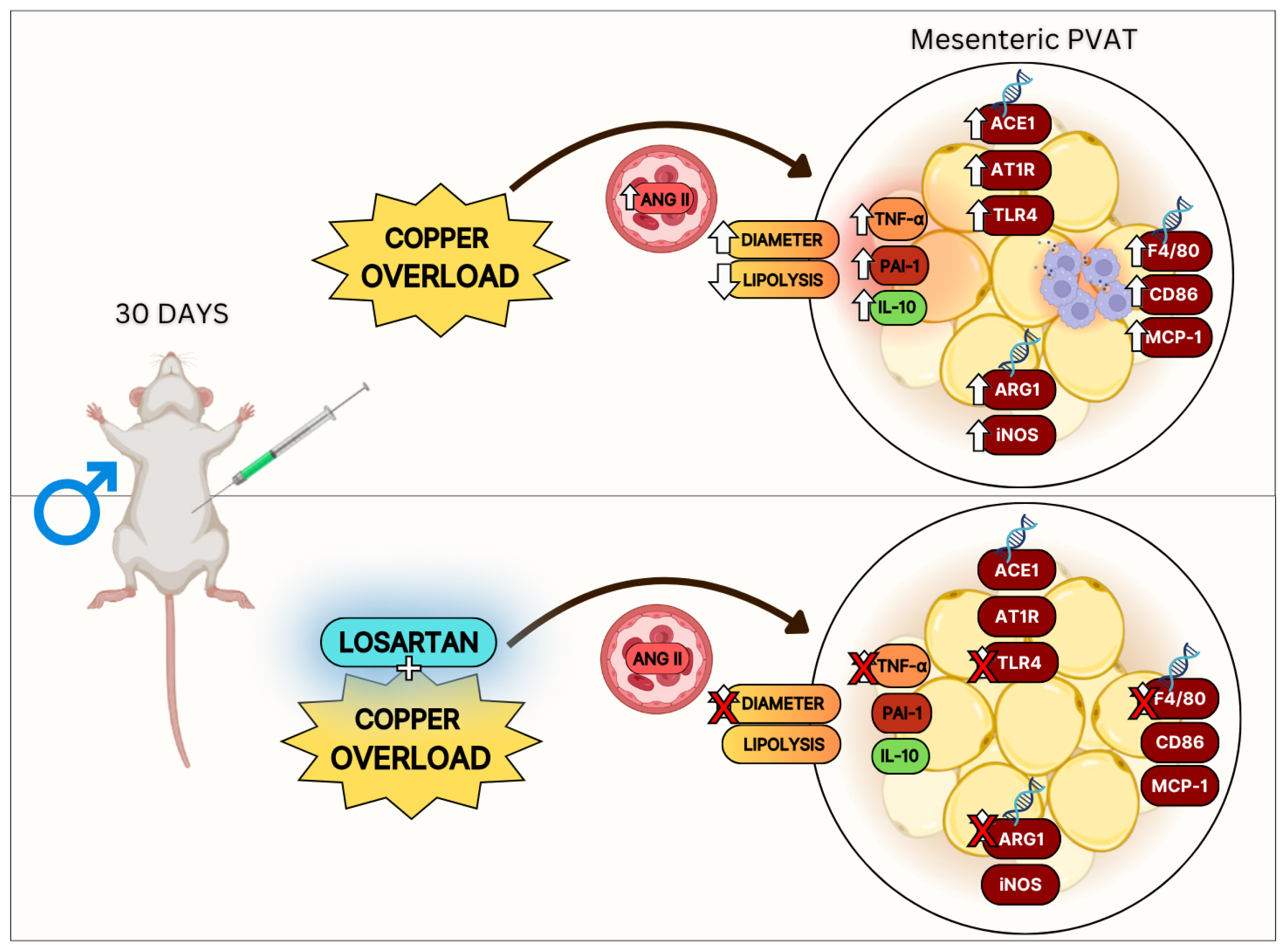
3.1. Chronic Copper Overload Increases Mesenteric PVAT Adipocyte Diameter and Decreases Basal Lipolysis Without Altering Total Adiposity
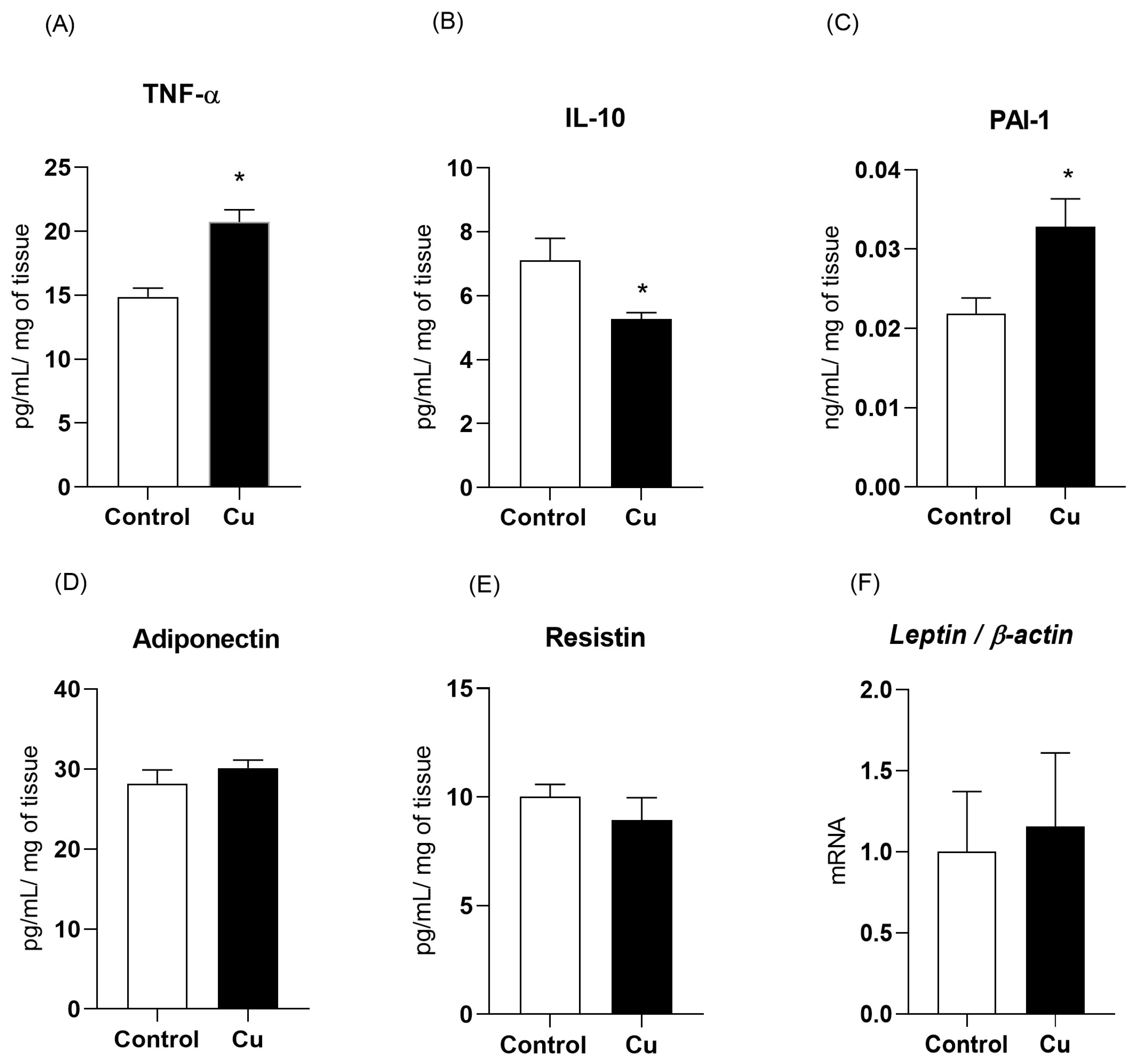
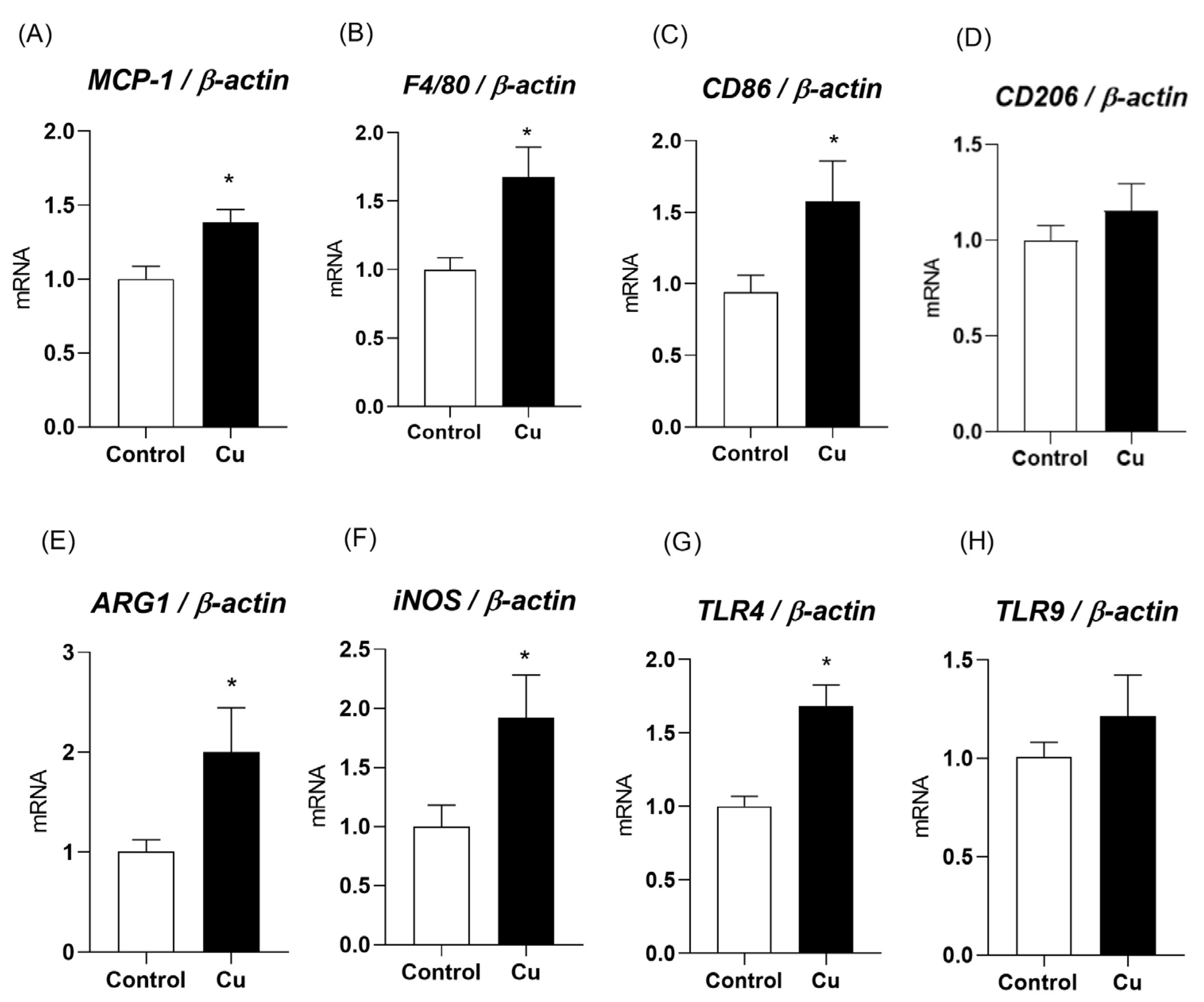
3.2. Chronic Copper Overload Impairs the Inflammatory and Vascular Regulatory Adipokines of Mesenteric PVAT
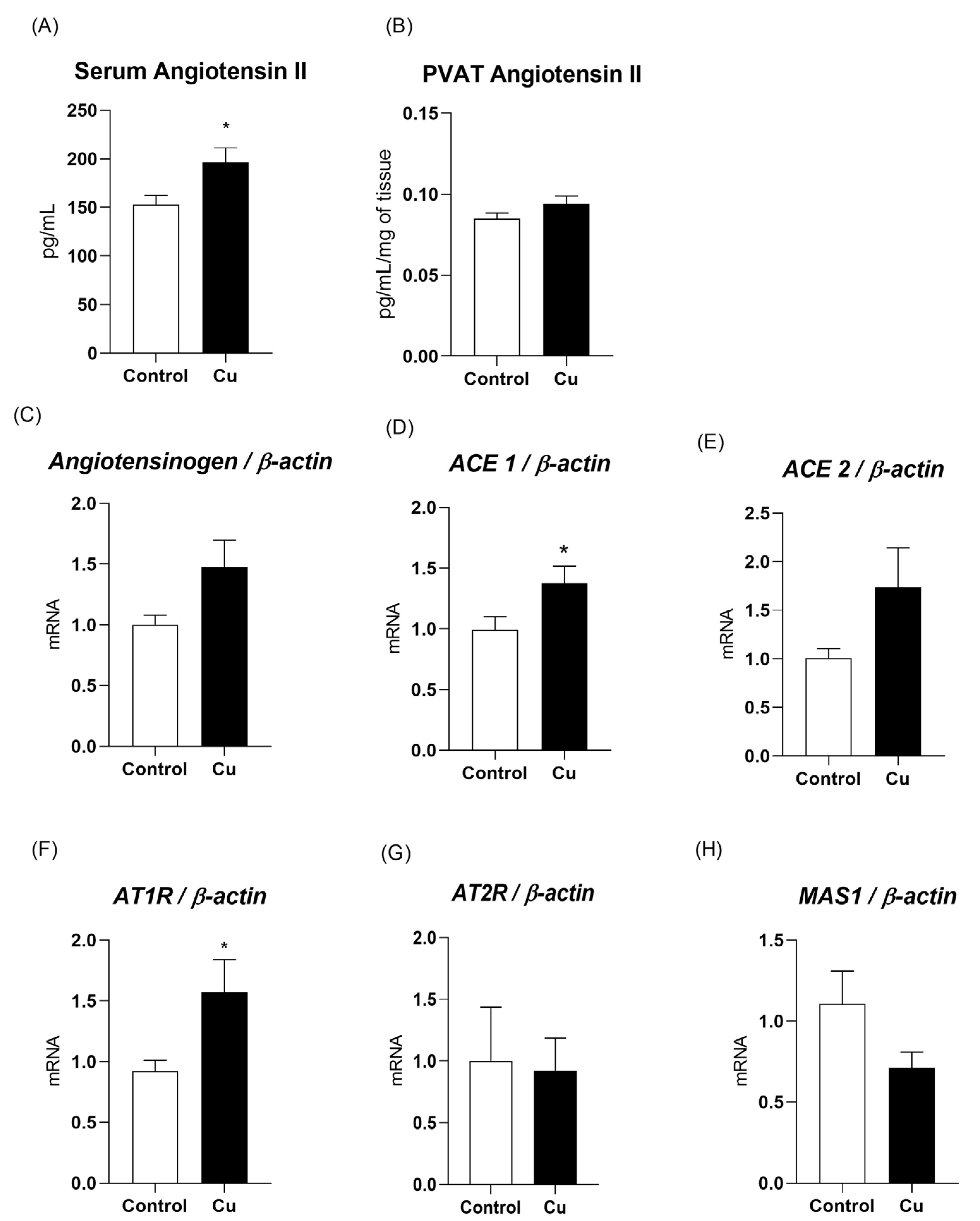
3.3. Chronic Copper Overload Promotes an Increase in Blood Angiotensin II Levels and Upregulation of Key Proteins in the Renin–Angiotensin System of Mesenteric PVAT
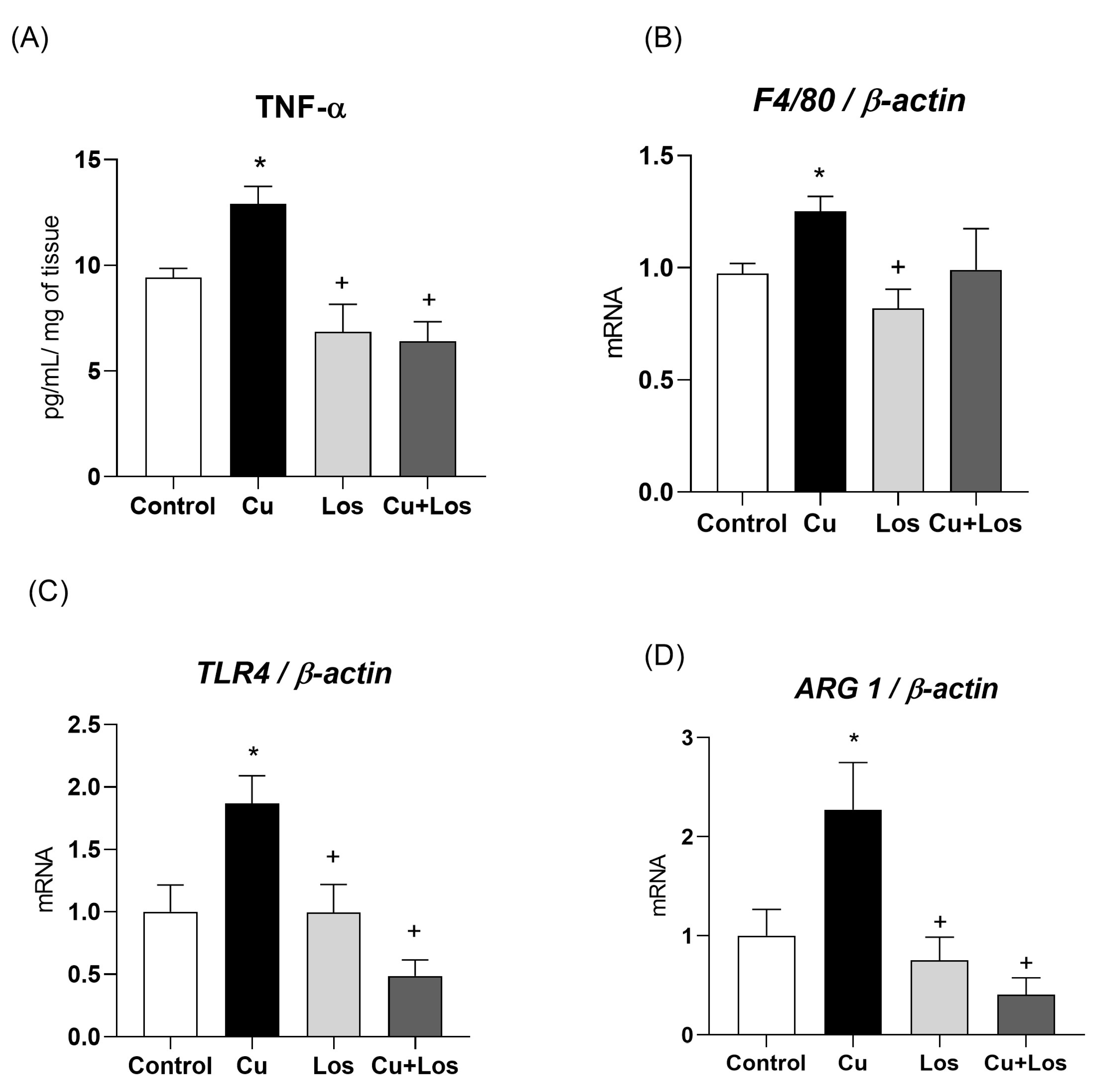
3.4. The Effects of Copper Overload on Inflammatory Markers in Mesenteric PVAT Are Partially Prevented by Losartan
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE1/2 | Angiotensin II type 1 receptor |
| ARG1 | Arginase-1 |
| AT1R | Angiotensin-converting enzyme 1/2 |
| CU | Copper |
| Los | Losartan |
| NO | Nitric oxide |
| PVAT | Perivascular adipose tissue |
| RAS | Reactive oxygen species |
| ROS | Renin–angiotensin system |
References
- Yang, L.; Chen, X.; Cheng, H.; Zhang, L. Dietary copper intake and risk of stroke in adults: A case-control study based on National Health and Nutrition Examination Survey 2013–2018. Nutrients 2022, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A.; Peroni, G. Copper as dietary supplement for bone metabolism: A review. Nutrients 2021, 13, 2246. [Google Scholar] [CrossRef] [PubMed]
- Eljazzar, S.; Abu-Hijleh, H.; Alkhatib, D.; Sokary, S.; Ismail, S.; Al-Jayyousi, G.F.; Tayyem, R. The role of copper intake in the development and management of type 2 diabetes: A systematic review. Nutrients 2023, 15, 1655. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Tsuji, J.; Garry, M.; McArdle, M.; Goodfellow, W.; Adams, W.; Menzie, C. Critical review of exposure and effects: Implications for setting regulatory health criteria for ingested copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef]
- World Health Organization. Copper in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Available online: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/copper.pdf (accessed on 9 March 2025).
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Zhong, C.C.; Zhao, T.; Hogstrand, C.; Chen, F.; Song, C.C.; Luo, Z. Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J. Nutr. Biochem. 2022, 100, 108883. [Google Scholar] [CrossRef]
- Shan, J.; Geng, R.; Zhang, Y.; Wei, J.; Liu, J.; Bai, J. Identification of cuproptosis-related subtypes, establishment of a prognostic model and tumor immune landscape in endometrial carcinoma. Comput. Biol. Med. 2022, 149, 105988. [Google Scholar] [CrossRef]
- Filetti, F.M.; Vassallo, D.V.; Fioresi, M.; Simões, M.R. Reactive oxygen species impair the excitation-contraction coupling of papillary muscles after acute exposure to a high copper concentration. Toxicol. Vitr. 2018, 51, 106–113. [Google Scholar] [CrossRef]
- Nunes, K.Z.; Fioresi, M.; Marques, V.B.; Vassallo, D.V. Acute copper overload induces vascular dysfunction in aortic rings due to endothelial oxidative stress and increased nitric oxide production. J. Toxicol. Environ. Health Part A 2018, 81, 218–228. [Google Scholar] [CrossRef]
- Da Silva, J.A.D.; Filetti, F.M.; da Silva, N.P.; Gomes, K.N.; Graceli, J.B.; Lopes, A.B.; Vassallo, D.V.; Nunes, K.Z. Copper exposure at a daily dose twice the recommended in diabetic rats induces oxidative stress, vascular dysfunction and perivascular adipose tissue inflammation. Toxicol. Lett. 2025, 409, 97–108. [Google Scholar] [CrossRef]
- Toscano, C.M.; Filetti, F.M.; Almenara, C.C.P.; Fioresi, M.; Vassallo, D.V. Copper exposure for 30 days at a daily dose twice the recommended increases blood pressure and cardiac contractility. Life Sci. 2022, 300, 120579. [Google Scholar] [CrossRef] [PubMed]
- Victorio, J.A.; Davel, A.P. Perivascular adipose tissue oxidative stress on the pathophysiology of cardiometabolic diseases. Curr. Hypertens. Rev. 2020, 16, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.T.; Paula, S.M.; Lino, C.A.; Senger, N.; Couto, G.K.; Barreto-Chaves, M.L.d.M.; Mill, J.G.; Rossoni, L.V. Renin-angiotensin system overactivation in perivascular adipose tissue contributes to vascular dysfunction in heart failure. Clin. Sci. 2020, 134, 3195–3211. [Google Scholar] [CrossRef]
- Cheng, C.K.; Ding, H.; Jiang, M.; Yin, H.; Gollasch, M.; Huang, Y. Perivascular adipose tissue: Fine-tuner of vascular redox status and inflammation. Redox Biol. 2023, 62, 102683. [Google Scholar] [CrossRef]
- Grigoras, A.; Amalinei, C.; Balan, R.A.; Giusca, S.E.; Caruntu, I.D. Perivascular adipose tissue in cardiovascular diseases—An update. Anatol. J. Cardiol. 2019, 22, 219–231. [Google Scholar] [CrossRef]
- Saxton, S.N.; Clark, B.J.; Withers, S.B.; Eringa, E.C.; Heagerty, A.M. Mechanistic links between obesity, diabetes, and blood pressure: Role of perivascular adipose tissue. Physiol. Rev. 2019, 99, 1701–1763. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xia, N. The interplay between adipose tissue and vasculature: Role of oxidative stress in obesity. Front. Cardiovasc. Med. 2021, 8, 650214. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The role of obesity-induced perivascular adipose tissue (PVAT) dysfunction in vascular homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef]
- Victorio, J.A.; Barssotti, L.; Aprahamian, T.; Costa, R.G.; Mousovich-Neto, F.; Oliveira, H.C.F.; Mori, M.; Rossoni, L.V.; Davel, A.P. β-Adrenergic stimulation-induced PVAT dysfunction in male sex: A role for 11β-hydroxysteroid dehydrogenase-1. Endocrinology 2024, 165, bqae053. [Google Scholar] [CrossRef]
- Li, X.; Ma, Z.; Zhu, Y.Z. Regional heterogeneity of perivascular adipose tissue: Morphology, origin, and secretome. Front. Pharmacol. 2021, 12, 697720. [Google Scholar] [CrossRef]
- Wronska, A.; Kmiec, Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol. 2012, 205, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Perivascular adipose tissue oxidative stress in obesity. Antioxidants 2023, 12, 1595. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Carbone, F.; Tirandi, A.; Montecucco, F.; Liberale, L. Obesity phenotypes and cardiovascular risk: From pathophysiology to clinical management. Rev. Endocr. Metab. Disord. 2023, 24, 901–919. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Garcia-Barrio, M.T.; Chen, Y.E. Perivascular adipose tissue regulates vascular function by targeting vascular smooth muscle cells. Arter. Thromb. Vasc. Biol. 2020, 40, 1094–1109. [Google Scholar] [CrossRef]
- Dos Reis Costa, D.E.F.; Silveira, A.L.M.; Campos, G.P.; Nóbrega, N.R.C.; De Araújo, N.F.; de Figueiredo Borges, L.; dos Santos Aggum Capettini, L.; Ferreira, A.V.M.; Bonaventura, D. High-carbohydrate diet enhanced the anticontractile effect of perivascular adipose tissue through activation of renin-angiotensin system. Front. Physiol. 2020, 11, 628101. [Google Scholar] [CrossRef]
- Institute of Medicine Panel on Micronutrients. Dietary Reference intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US), National Academy of Sciences: Washington, DC, USA, 2001. [Google Scholar]
- Rodbell, M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 1964, 239, 375–380. [Google Scholar] [CrossRef]
- Bolsoni-Lopes, A.; Festuccia, W.T.; Farias, T.S.M.; Chimin, P.; Torres-Leal, F.L.; Derogis, P.B.M.; de Andrade, P.B.; Miyamoto, S.; Lima, F.B.; Curi, R.; et al. Palmitoleic acid (n-7) increases white adipocyte lipolysis and lipase content in a PPARα-dependent manner. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1093–E1102. [Google Scholar] [CrossRef]
- Bolsoni-Lopes, A.; Festuccia, W.T.; Chimin, P.; Farias, T.S.; Torres-Leal, F.L.; Cruz, M.M.; Andrade, P.B.; Hirabara, S.M.; Lima, F.B.; Alonso-Vale, M.I.C. Palmitoleic acid (n-7) increases white adipocytes GLUT4 content and glucose uptake in association with AMPK activation. Lipids Health Dis. 2014, 13, 199. [Google Scholar] [CrossRef]
- Brewer, G.J. Alzheimer’s disease causation by copper toxicity and treatment with zinc. Front. Aging. Neurosci. 2014, 6, 92. [Google Scholar] [CrossRef]
- Lamtai, M.; Zghari, O.; Ouakki, S.; Marmouzi, I.; Mesfioui, A.; El Hessni, A.; Ouichou, A. Chronic copper exposure leads to hippocampus oxidative stress and impaired learning and memory in male and female rats. Toxicol. Res. 2020, 36, 359–366. [Google Scholar] [CrossRef]
- Muñoz-Bravo, C.; Rodríguez-Moro, G.; Aparicio, V.; Pérez-Granados, A.M.; Delgado-Lista, J.; López-Miranda, J.; Tinahones, F.J.; Camargo, A. Serum copper levels and risk of major adverse cardiovascular events: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1217748. [Google Scholar] [CrossRef] [PubMed]
- Sarawi, W.S.; Alhusaini, A.M.; Fadda, L.M.; Alomar, H.A.; Albaker, A.B.; Aljrboa, A.S.; Alotaibi, A.M.; Hasan, I.H.; Mahmoud, A.M. Nano-curcumin prevents cardiac injury, oxidative stress and inflammation, and modulates TLR4/NF-κB and MAPK signaling in copper sulfate-intoxicated rats. Antioxidants 2021, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Sheline, C.T.; Choi, D.W. Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann. Neurol. 2004, 55, 645–653. [Google Scholar] [CrossRef]
- Rana, S.V. Perspectives in endocrine toxicity of heavy metals—A review. Biol. Trace Elem. Res. 2014, 160, 1–14. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, R.; Wang, Y.; Sun, H.; Liu, M.; Chen, Y. Obesity mediates the association between serum copper and inflammation: A cross-sectional and Mendelian randomization study. Biol. Trace Elem. Res. 2025, 203, 3009–3020. [Google Scholar] [CrossRef]
- Xu, S.; Lu, F.; Gao, J.; Yuan, Y. Inflammation-mediated metabolic regulation in adipose tissue. Obes. Rev. 2024, 25, e13724. [Google Scholar] [CrossRef]
- Azul, L.; Leandro, A.; Boroumand, P.; Klip, A.; Seiça, R.; Sena, C.M. Increased inflammation, oxidative stress and a reduction in antioxidant defense enzymes in perivascular adipose tissue contribute to vascular dysfunction in type 2 diabetes. Free Radic. Biol. Med. 2020, 146, 264–274. [Google Scholar] [CrossRef]
- Wong, Y.K.; Tse, H.F. Circulating biomarkers for cardiovascular disease risk prediction in patients with cardiovascular disease. Front. Cardiovasc. Med. 2021, 8, 713191. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Moraña-Fernández, S.; Anido-Varela, L.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Moscoso, I.; Gualillo, O.; González-Juanatey, J.R.; et al. Adipokines and inflammation: Focus on cardiovascular diseases. Int. J. Molec. Sci. 2020, 21, 7711. [Google Scholar] [CrossRef]
- Tamura, Y.; Kawao, N.; Yano, M.; Okada, K.; Matsuo, O.; Kaji, H. Plasminogen activator inhibitor-1 deficiency ameliorates insulin resistance and hyperlipidemia but not bone loss in obese female mice. Endocrinology 2014, 155, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Anavi, S.; Tirosh, O. iNOS as a metabolic enzyme under stress conditions. Free Radic. Biol. Med. 2020, 146, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K.; Kannikal, J.; Nath, N. Macrophage reprogramming and cancer therapeutics: Role of iNOS-derived NO. Cells 2021, 10, 3194. [Google Scholar] [CrossRef] [PubMed]
- Alghibiwi, H.K.; Alhusiani, A.M.; Sarawi, W.S.; Fadda, L.; Alomar, H.A.; Alsaab, J.S.; Hasan, I.H.; Alonazi, A.S.; Alrasheed, N.M.; Alhabardi, S. Coenzyme Q10 and its liposomal form prevent copper cardiotoxicity by attenuating oxidative stress, TLR-4/NF-κB signaling and necroptosis in rats. Cell. Mol. Biol. 2025, 71, 118–124. [Google Scholar] [CrossRef]
- Liu, P.; Huang, G.; Cao, Z.; Xie, Q.; Wei, T.; Huang, C.; Li, Q.; Sun, M.; Shen, W.; Gao, P. Haematopoietic TLR4 deletion attenuates perivascular brown adipose tissue inflammation in atherosclerotic mice. Biochim. Biophys. Acta. Mol. Cell. Biol. Lipids 2017, 1862, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, inflammation, Toll-like receptor 4 and fatty acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Tsan, M.F.; Gao, B. Endogenous ligands of Toll-like receptors. J. Leukoc. Biol. 2004, 76, 514–519. [Google Scholar] [CrossRef]
- Jeon, M.J.; Leem, J.; Ko, M.S.; Jang, J.E.; Park, H.-S.; Kim, H.S.; Kim, M.; Yoo, H.J.; Lee, C.-H.; Park, I.-S.; et al. Mitochondrial dysfunction and activation of iNOS are responsible for the palmitate-induced decrease in adiponectin synthesis in 3T3L1 adipocytes. Exp. Mol. Med. 2012, 44, 562–570. [Google Scholar] [CrossRef]
- Kapur, S.; Picard, F.; Perreault, M.; Deshaies, Y.; Marette, A. Nitric oxide: A new player in the modulation of energy metabolism. Int. J. Obes. Relat. Metab. Disord. 2000, 24, S36–S40. [Google Scholar] [CrossRef]
- Lin, J.-R.; Ding, L.-L.; Xu, L.; Huang, J.; Zhang, Z.-B.; Chen, X.-H.; Cheng, Y.-W.; Ruan, C.-C.; Gao, P.-J. Brown adipocyte ADRB3 mediates cardioprotection via suppressing exosomal iNOS. Circ. Res. 2022, 131, 133–147. [Google Scholar] [CrossRef]
- Kalezic, A.; Korac, A.; Korac, B.; Jankovic, A. l-Arginine induces white adipose tissue browning—A new pharmaceutical alternative to cold. Pharmaceutics 2022, 14, 1368. [Google Scholar] [CrossRef]
- Arishe, O.; McKenzie, J.; Priviero, F.; Ebeigbe, A.B.; Webb, R.C. L-arginase induces vascular dysfunction in old spontaneously hypertensive rats. J. Afr. Assoc. Physiol. Sci. 2019, 7, 119–127. [Google Scholar] [CrossRef]
- Shatanawi, A.; Romero, M.J.; Iddings, J.A.; Chandra, S.; Umapathy, N.S.; Verin, A.D.; Caldwell, R.W. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. Am. J. Physiol. Cell. Physiol. 2011, 300, C1181–C1192. [Google Scholar] [CrossRef]
- Johnson, F.K.; Peyton, K.J.; Liu, X.-M.; Azam, M.A.; Shebib, A.R.; Johnson, R.A.; Durante, W. Arginase promotes endothelial dysfunction and hypertension in obese rats. Obesesity 2015, 23, 383–390. [Google Scholar] [CrossRef]
- Shemyakin, A.; Kövamees, O.; Rafnsson, A.; Böhm, F.; Svenarud, P.; Settergren, M.; Jung, C.; Pernow, J. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation 2012, 126, 2943–2950. [Google Scholar] [CrossRef]
- Bhatta, A.; Yao, L.; Xu, Z.; Toque, H.A.; Chen, J.; Atawia, R.T.; Fouda, A.Y.; Bagi, Z.; Lucas, R.; Caldwell, R.B.; et al. Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovasc. Res. 2017, 113, 1664–1676. [Google Scholar] [CrossRef]
- Tengbom, J.; Cederström, S.; Verouhis, D.; Böhm, F.; Eriksson, P.; Folkersen, L.; Gabrielsen, A.; Jernberg, T.; Lundman, P.; Persson, J.; et al. Arginase 1 is upregulated at admission in patients with ST-elevation myocardial infarction. J. Intern. Med. 2021, 290, 1061–1070. [Google Scholar] [CrossRef]
- Yao, J.; Wu, D.; Qiu, Y. Adipose tissue macrophage in obesity-associated metabolic diseases. Front. Immunol. 2022, 13, 977485. [Google Scholar] [CrossRef]
- Jacks, R.D.; Lumeng, C.N. Macrophage and T cell networks in adipose tissue. Nat. Rev. Endocrinol. 2024, 20, 50–61. [Google Scholar] [CrossRef]
- Pahlavani, M.; Kalupahana, N.S.; Ramalingam, L.; Moustaid-Moussa, N. Regulation and functions of the renin-angiotensin system in white and brown adipose tissue. Compr. Physiol. 2017, 7, 1137–1150. [Google Scholar] [CrossRef]
- Hillock-Watling, C.; Gotlieb, A.I. The pathobiology of perivascular adipose tissue (PVAT), the fourth layer of the blood vessel wall. Cardiovasc. Pathol. 2022, 61, 107459. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Biancardi, V.C.; Stranahan, A.M.; Krause, E.G.; de Kloet, A.D.; Stern, J.E. Cross talk between AT1 receptors and Toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H404–H415. [Google Scholar] [CrossRef]
- Nunes, K.P.; de Oliveira, A.A.; Mowry, F.E.; Biancardi, V.C. Targeting toll-like receptor 4 signalling pathways: Can therapeutics pay the toll for hypertension? Br. J. Pharmacol. 2019, 176, 1864–1879. [Google Scholar] [CrossRef]
- Mowry, F.E.; Peaden, S.C.; Stern, J.E.; Biancardi, V.C. TLR4 and AT1R mediate blood-brain barrier disruption, neuroinflammation, and autonomic dysfunction in spontaneously hypertensive rats. Pharmacol. Res. 2021, 174, 105877. [Google Scholar] [CrossRef]
- Chen, X.-S.; Wang, S.-H.; Liu, C.-Y.; Gao, Y.-L.; Meng, X.-L.; Wei, W.; Shou, S.-T.; Liu, Y.-C.; Chai, Y.-F. Losartan attenuates sepsis-induced cardiomyopathy by regulating macrophage polarization via TLR4-mediated NF-κB and MAPK signaling. Pharmacol. Res. 2022, 185, 106473. [Google Scholar] [CrossRef]
- Sun, J.; Luo, J.; Ruan, Y.; Xiu, L.; Fang, B.; Zhang, H.; Wang, M.; Chen, H. Free fatty acids activate renin-angiotensin system in 3T3-L1 adipocytes through nuclear factor-kappa B pathway. J. Diabetes. Res. 2016, 2016, 1587594. [Google Scholar] [CrossRef]
- Alexandre-Santos, B.; Magliano, D.C.; Giori, I.G.; Medeiros, G.R.d.O.; Vieira, C.P.; Conte-Junior, C.A.; da Nobrega, A.C.L.; Frantz, E.D.C. Renin-angiotensin system modulation through enalapril and/or exercise training improves visceral adiposity in obese mice. Life Sci. 2022, 291, 120269. [Google Scholar] [CrossRef]
- De Batista, P.R.; Palacios, R.; Martín, A.; Hernanz, R.; Médici, C.T.; Silva, M.A.S.C.; Rossi, E.M.; Aguado, A.; Vassallo, D.V.; Salaices, M.; et al. Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLoS ONE 2014, 9, e104020. [Google Scholar] [CrossRef]
| Gene | 5′ Primer (5′–3′) | 3′ Primer (5′–3′) |
|---|---|---|
| ACE1 | CCACGTCCCGGAAATACGAA | CCTGCATCAGAGTAGCCGTT |
| ACE2 | GCCCAAAAGATGAACGAGGC | GACGCTTGATGGTCGCATTC |
| Angiotensinogen | CATCTTCACCCCCTCGAGTA | TGCTTCTGTGTGTCCTTTAGC |
| Arg1 | GGTGGAGACCACAGTATGGC | GCAGATTCCCAGAGCTGGTT |
| AT1R | GGATTCGTGGCTTGAGTCCT | CGAAATCCACTTGACCTGGTG |
| AT2R | TGCTCTGACCTGGATGGGTA | AGCTGTTGGTGAATCCCAGG |
| CD206 | GGAGGGTGCGGTACACTAAC | GTAGCCGGGATTTCGTCTGA |
| CD86 | AAGACATGTGTAACCTGCACCA | AAGCTTGCCTCTTCACAGGA |
| F4/80 | GGACAAAGACTTAACGGTGTGA | TGCTGGGCAGAAAACCTTGT |
| B-actin | ACACCCGCCACCAGTTCG | CCCACGATGGAGGGGAAGAC |
| Leptin | ATTTCACACACGCAGTCGGT | CCAGGGTCTGGTCCATCTTG |
| MAS1 | GGAAGACCAGCCCACAGTTAC | ATCACAGGAAGAGAGCCTCG |
| MCP-1 | TGTCTCAGCCAGATGCAGTT | CAGCCGACTCATTGGGATCA |
| NOS 2/iNOS | GGTGAGGGGACTGGACTTTT | TTCTCCGTGGGGCTTGTAGT |
| TLR4 | TCTGAGCTTCAACCCCCTGA | TTGTCTCAATTTCACACCTGGA |
| TLR9 | CCATTTTCCATCATGGTTCTCTG | GCCATGAGGCTTCAGTTCAC |
| Control | Copper | |
|---|---|---|
| Body weight gain (g) | 89 ± 7.9 | 97 ± 9.8 |
| Feed efficiency (g/g) | 0.14 ± 0.007 | 0.15 ± 0.009 |
| Food intake (g/day) | 29.3 ± 0.5 | 27.1 ± 0.4 * |
| Blood glucose (mg/dL) | 104 ± 6.1 | 105 ± 4.7 |
| Total cholesterol (mg/dL) | 75 ± 3 | 81 ± 5.7 |
| Triglyceride (mg/dL) | 122 ± 12.7 | 126 ± 13.2 |
| Inguinal WAT (mg/g BW) | 12.1 ± 0.4 | 11.8 ± 0.8 |
| Visceral WAT (mg/g BW) | 8.6 ± 0.6 | 8.8 ± 1.1 |
| Mesenteric Adipocyte diameter (µm) | 48.3 ± 2 | 58.9 ± 3.2 * |
| Control | Copper | Losartan | Copper + Losartan | |
|---|---|---|---|---|
| Body weight gain (g) | 110 ± 9.6 | 121 ± 8.5 | 108 ± 3.3 | 104 ± 4.3 |
| Feed efficiency (g/g) | 0.11 ± 0.006 | 0.10 ± 0.007 | 0.10 ± 0.007 | 0.11 ± 0.01 |
| Food intake (g/day) | 29.8 ± 1.2 | 28 ± 1.1 * | 28.7 ± 0.8 | 28.8 ± 0.9 |
| Blood glucose (mg/dL) | 103 ± 1.7 | 108 ± 6.6 | 102 ± 7.4 | 100 ± 5 |
| Total cholesterol (mg/dL) | 99 ± 3.8 | 98 ± 4.9 | 87 ± 5.4 | 101 ± 5.7 |
| Triglyceride (mg/dL) | 90 ± 9.1 | 112 ± 21 | 88 ± 10 | 99 ± 24 |
| Inguinal WAT (mg/g BW) | 11.3 ± 0.5 | 11.9 ± 0.7 | 10.6 ± 0.8 | 10.5 ± 0.9 |
| Visceral WAT (mg/g BW) | 8.1 ± 1.1 | 8.2 ± 0.9 | 8.5 ± 0.7 | 8.4 ± 0.4 |
| Mesenteric adipocyte diameter (µm) | 41.3 ± 0.5 | 49.9 ± 0.7 * | 41.0 ± 0.8 | 44.2 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mawandji, N.B.d.S.; Lisboa, N.A.d.S.; Gomes, K.N.; Vieira, J.M.; Simão, J.d.J.; Alonso-Vale, M.I.; Nunes, K.Z.; Vassallo, D.V.; Bolsoni-Lopes, A. Chronic Copper Overload Triggers Inflammation in Mesenteric PVAT Alongside Changes in Renin–Angiotensin System-Related Pathways. Nutrients 2025, 17, 2082. https://doi.org/10.3390/nu17132082
Mawandji NBdS, Lisboa NAdS, Gomes KN, Vieira JM, Simão JdJ, Alonso-Vale MI, Nunes KZ, Vassallo DV, Bolsoni-Lopes A. Chronic Copper Overload Triggers Inflammation in Mesenteric PVAT Alongside Changes in Renin–Angiotensin System-Related Pathways. Nutrients. 2025; 17(13):2082. https://doi.org/10.3390/nu17132082
Chicago/Turabian StyleMawandji, Nina Bruna de Souza, Nayara Ariel da Silva Lisboa, Karoline Neumann Gomes, Júlia Martins Vieira, Jussara de Jesus Simão, Maria Isabel Alonso-Vale, Karolini Zuqui Nunes, Dalton Valentim Vassallo, and Andressa Bolsoni-Lopes. 2025. "Chronic Copper Overload Triggers Inflammation in Mesenteric PVAT Alongside Changes in Renin–Angiotensin System-Related Pathways" Nutrients 17, no. 13: 2082. https://doi.org/10.3390/nu17132082
APA StyleMawandji, N. B. d. S., Lisboa, N. A. d. S., Gomes, K. N., Vieira, J. M., Simão, J. d. J., Alonso-Vale, M. I., Nunes, K. Z., Vassallo, D. V., & Bolsoni-Lopes, A. (2025). Chronic Copper Overload Triggers Inflammation in Mesenteric PVAT Alongside Changes in Renin–Angiotensin System-Related Pathways. Nutrients, 17(13), 2082. https://doi.org/10.3390/nu17132082







