Purple Potato Extract Suppresses Hypoxia-Induced Metabolic Reprogramming and Inhibits HIF-1α Signaling in Caco-2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Purple Potato Extract
2.2. Cell Culture
2.3. Immunoblotting
2.4. qRT-PCR Analysis
2.5. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis of Extracellular Lactate in Cell Culture Medium
2.6. Wound Healing Assay
2.7. Cell Proliferation Assay
2.8. Drug Resistance Assay
2.9. Statistical Analyses
3. Results
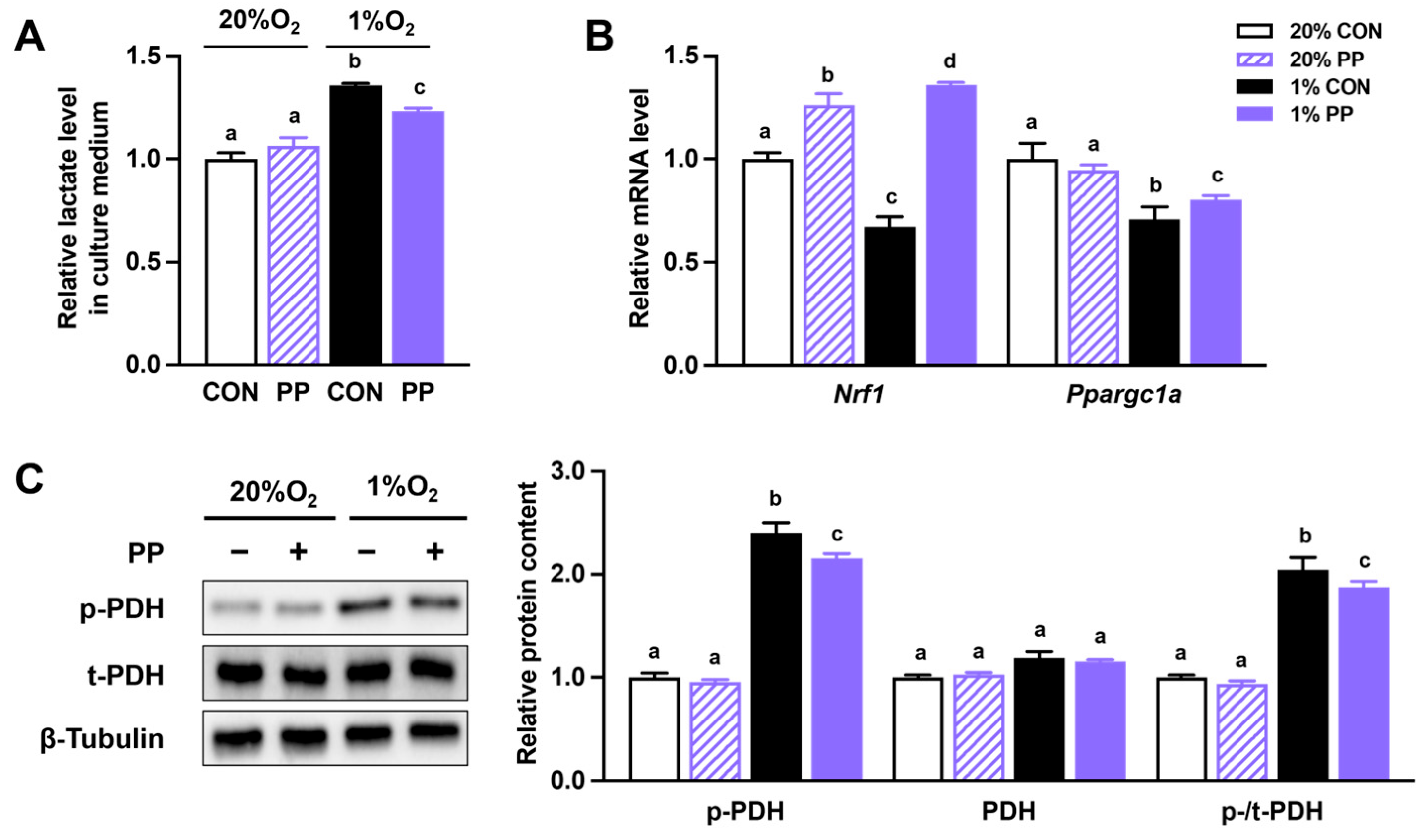
3.1. PP Extract Suppresses Glycolysis and Improves Oxidative Phosphorylation Under Hypoxia Stress
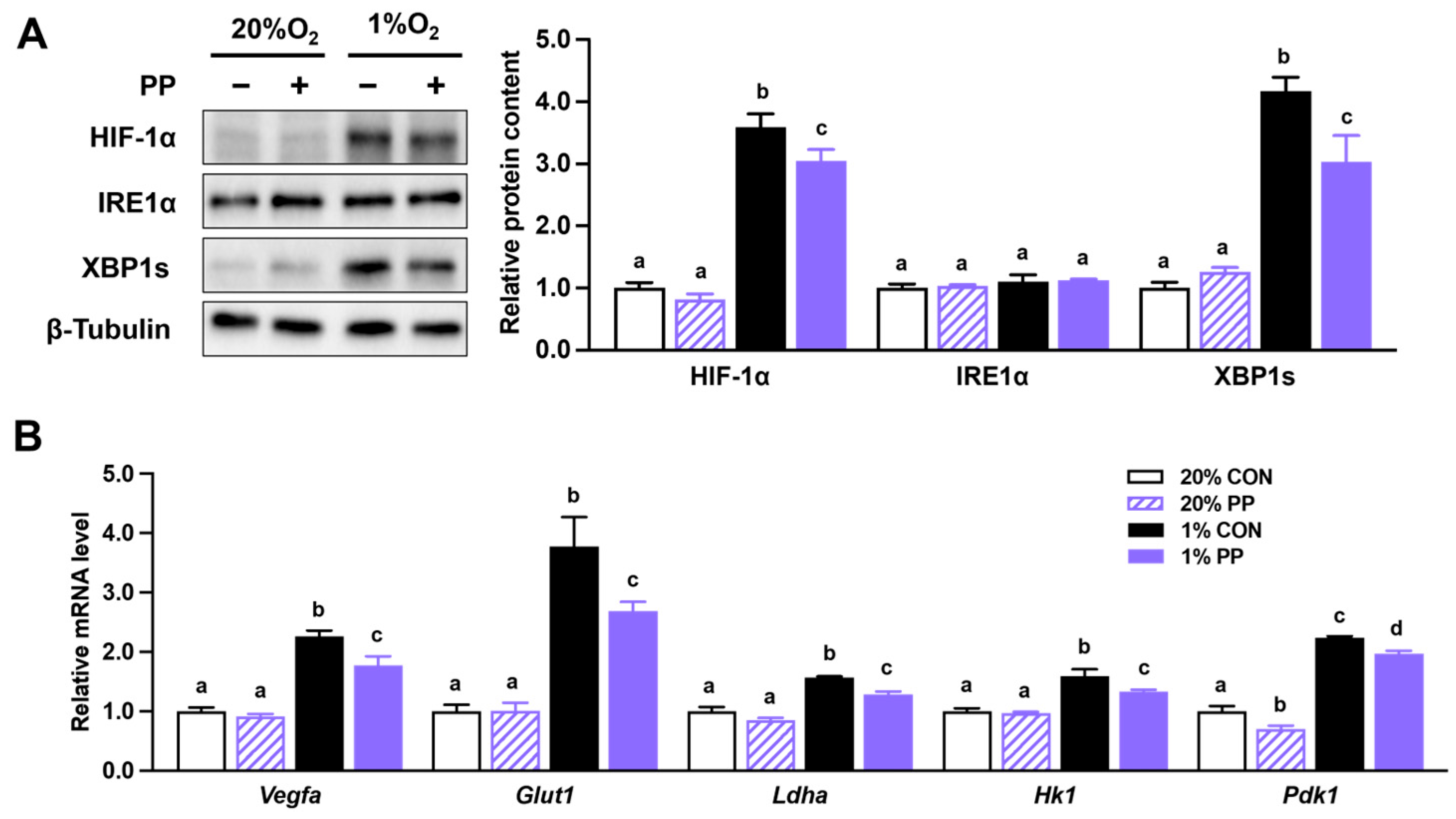
3.2. PP Extract Suppresses HIF-1α Accumulation and Downstream Gene Expression
3.3. PP Extract Suppresses Cell Proliferation and Migration Under Hypoxia Stress
3.4. PP Extract Suppresses Cell Drug Resistance and Stemness Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Tomlinson, I.P.M.; Novelli, M.R.; Bodmer, W.F. The mutation rate and cancer. Proc. Natl. Acad. Sci. USA 1996, 93, 14800–14803. [Google Scholar] [CrossRef] [PubMed]
- Brahimi-Horn, M.C.; Chiche, J.; Pouysségur, J. Hypoxia and cancer. J. Mol. Med. 2007, 85, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Robbins, P.A.; Ratcliffe, P.J. The human side of hypoxia-inducible factor. Br. J. Haematol. 2008, 141, 325–334. [Google Scholar] [CrossRef]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar]
- Haase, V.H. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int. 2017, 21, S110–S124. [Google Scholar] [CrossRef]
- Lisy, K.; Peet, D. Turn me on: Regulating HIF transcriptional activity. Cell Death Differ. 2008, 15, 642–649. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; Kriegsheim, A.v.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Peiris-Pages, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef]
- Simiantonaki, N.; Taxeidis, M.; Jayasinghe, C.; Kurzik-Dumke, U.; Kirkpatrick, C.J. Hypoxia-inducible factor I alpha expression increases during colorectal carcinogenesis and tumor progression. BMC Cancer 2008, 8, 320. [Google Scholar] [CrossRef]
- Chipurupalli, S.; Kannan, E.; Tergaonkar, V.; D’Andrea, R.; Robinson, N. Hypoxia Induced ER Stress Response as an Adaptive Mechanism in Cancer. Int. J. Mol. Sci. 2019, 20, 749. [Google Scholar] [CrossRef] [PubMed]
- Rouschop, K.M.A.; van den Beucken, T.; Dubois, L.; Niessen, H.; Bussink, J.; Savelkouls, K.; Keulers, T.; Mujcic, H.; Landuyt, W.; Voncken, J.W.; et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Investig. 2010, 120, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Lee, K.; Tirasophon, W.; Shen, X.H.; Michalak, M.; Prywes, R.; Okada, T.; Yoshida, H.; Mori, K.; Kaufman, R.J. IREI-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Iliopoulos, D.; Zhang, Q.; Tang, Q.Z.; Greenblatt, M.B.; Hatziapostolou, M.; Lim, E.; Tam, W.L.; Ni, M.; Chen, Y.W.; et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1 alpha pathway. Nature 2014, 508, 103–107. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Keith, B.; Simon, M.C. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef]
- Krishnan, J.; Suter, M.; Windak, R.; Krebs, T.; Felley, A.; Montessuit, C.; Tokarska-Schlattner, M.; Aasum, E.; Bogdanova, A.; Perriard, E. Activation of a HIF1α-PPARγ axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009, 9, 512–524. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.; Husain, M. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci. Rep. 2018, 8, 8323. [Google Scholar] [CrossRef]
- Chen, J.; Xie, J.; Jiang, Z.; Wang, B.; Wang, Y.; Hu, X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 2011, 30, 4297–4306. [Google Scholar] [CrossRef]
- Sun, Q.; Iniguez, A.B.; Tian, Q.; Du, M.; Zhu, M.-J. PGC-1α in mediating mitochondrial biogenesis and intestinal epithelial differentiation promoted by purple potato extract. J. Funct. Foods 2022, 98, 105291. [Google Scholar] [CrossRef]
- Sun, Q.; Du, M.; Navarre, D.A.; Zhu, M.J. Effect of cooking methods on bioactivity of polyphenols in purple potatoes. Antioxidants 2021, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Hou, J.; Liu, C.; Shan, F.; Xiong, X.; Qin, A.; Chen, J.; Ren, W. The long non-coding RNA HOTAIRM1 suppresses cell progression via sponging endogenous miR-17-5p/B-cell translocation gene 3 (BTG3) axis in 5-fluorouracil resistant colorectal cancer cells. Biomed. Pharmacother. 2019, 117, 109171. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, L.; Hou, Z.; Liu, W.; Wang, H.; Zhou, T.; Li, Y.; Chen, S. LncRNA HAND2-AS1 inhibits 5-fluorouracil resistance by modulating miR-20a/PDCD4 axis in colorectal cancer. Cell. Signal. 2020, 66, 109483. [Google Scholar] [CrossRef]
- Dengler, V.L.; Galbraith, M.D.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Fan, F.; Wang, R.; Ye, X.; Xia, L.; Boulbes, D.; Ellis, L.M. Intracrine VEGF signalling mediates colorectal cancer cell migration and invasion. Br. J. Cancer 2017, 117, 848–855. [Google Scholar] [CrossRef]
- Park, J.B. Flavonoids are potential inhibitors of glucose uptake in U937 cells. Biochem. Biophys. Res. Commun. 1999, 260, 568–574. [Google Scholar] [CrossRef]
- Sánchez-Quintero, M.J.; Rodríguez-Díaz, C.; Rodríguez-González, F.J.; Fernández-Castañer, A.; García-Fuentes, E.; López-Gómez, C. Role of mitochondria in inflammatory bowel diseases: A systematic review. Int. J. Mol. Sci. 2023, 24, 17124. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, X.; Gheinani, P.T.; Guan, X.; Sharma, S.; Zhou, Y.; Jin, C.; Yang, Z.; Naren, A.P.; Yin, J. Epithelial SMYD5 exaggerates IBD by down-regulating mitochondrial functions via post-translational control of PGC-1α stability. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 375–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- LaGory, E.L.; Wu, C.; Taniguchi, C.M.; Ding, C.-K.C.; Chi, J.-T.; von Eyben, R.; Scott, D.A.; Richardson, A.D.; Giaccia, A.J. Suppression of PGC-1α is critical for reprogramming oxidative metabolism in renal cell carcinoma. Cell Rep. 2015, 12, 116–127. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Ajani, J.A.; Song, S. Drug resistance and Cancer stem cells. Cell Commun. Signal. 2021, 19, 19. [Google Scholar] [CrossRef]
- Shin, A.E.; Giancotti, F.G.; Rustgi, A.K. Metastatic colorectal cancer: Mechanisms and emerging therapeutics. Trends Pharmacol. Sci. 2023, 44, 222–236. [Google Scholar] [CrossRef]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updates 2011, 14, 191–201. [Google Scholar] [CrossRef]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism: Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, G.; Tang, X.; Chen, J.L.-Y.; Wu, J.; Ding, C.-K.C.; Keenan, M.M.; Sangokoya, C.; Kung, H.-N.; Ilkayeva, O.; Boros, L.G. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab. 2013, 1, 23. [Google Scholar] [CrossRef]
- Dong, Q.; Zhou, C.; Ren, H.; Zhang, Z.; Cheng, F.; Xiong, Z.; Chen, C.; Yang, J.; Gao, J.; Zhang, Y. Lactate-induced MRP1 expression contributes to metabolism-based etoposide resistance in non-small cell lung cancer cells. Cell Commun. Signal. 2020, 18, 167. [Google Scholar] [CrossRef]
- Koltai, T. The complex relationship between multiple drug resistance and the tumor pH gradient: A review. Cancer Drug Resist. 2022, 5, 277. [Google Scholar] [CrossRef]
- Kim, H.; Jang, H.; Kim, T.W.; Kang, B.-H.; Lee, S.E.; Jeon, Y.K.; Chung, D.H.; Choi, J.; Shin, J.; Cho, E.-J. Core pluripotency factors directly regulate metabolism in embryonic stem cell to maintain pluripotency. Stem Cells 2015, 33, 2699–2711. [Google Scholar] [CrossRef]
- Loh, J.-J.; Ma, S. Hallmarks of cancer stemness. Cell Stem Cell 2024, 31, 617–639. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Jeong, H.K.; Lee, J.Y.; Yang, J.; Lee, E.J.; Kim, S.Y.; Youn, S.W.; Lee, J.; Kim, W.J.; Kim, K.W. Hypoxic priming of mESCs accelerates vascular-lineage differentiation through HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol. Med. 2012, 4, 924–938. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, P.; Alcaina, Y.; Tapia, N.; Sabour, D.; Arauzo-Bravo, M.J.; Sainz de la Maza, D.; Berra, E.; O’Mara, A.N.; Nistal, M.; Ortega, S. Hypoxia induces pluripotency in primordial germ cells by HIF1α stabilization and Oct4 deregulation. Antioxid. Redox Signal. 2015, 22, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wu, M.; Yang, H.; Diao, R.; Zeng, H. Hypoxia promotes conversion to a stem cell phenotype in prostate cancer cells by activating HIF-1α/Notch1 signaling pathway. Clin. Transl. Oncol. 2023, 25, 2138–2152. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Q.; Sun, Q.; Iniguez, A.B.; Li, X.; Du, M.; Zhu, M.-J. Purple Potato Extract Suppresses Hypoxia-Induced Metabolic Reprogramming and Inhibits HIF-1α Signaling in Caco-2 Cells. Nutrients 2025, 17, 2079. https://doi.org/10.3390/nu17132079
Cui Q, Sun Q, Iniguez AB, Li X, Du M, Zhu M-J. Purple Potato Extract Suppresses Hypoxia-Induced Metabolic Reprogramming and Inhibits HIF-1α Signaling in Caco-2 Cells. Nutrients. 2025; 17(13):2079. https://doi.org/10.3390/nu17132079
Chicago/Turabian StyleCui, Qiaorong, Qi Sun, Alejandro Bravo Iniguez, Xinrui Li, Min Du, and Mei-Jun Zhu. 2025. "Purple Potato Extract Suppresses Hypoxia-Induced Metabolic Reprogramming and Inhibits HIF-1α Signaling in Caco-2 Cells" Nutrients 17, no. 13: 2079. https://doi.org/10.3390/nu17132079
APA StyleCui, Q., Sun, Q., Iniguez, A. B., Li, X., Du, M., & Zhu, M.-J. (2025). Purple Potato Extract Suppresses Hypoxia-Induced Metabolic Reprogramming and Inhibits HIF-1α Signaling in Caco-2 Cells. Nutrients, 17(13), 2079. https://doi.org/10.3390/nu17132079





