Camel Milk-Derived Extracellular Vesicles as a Functional Food Component Ameliorate Hypobaric Hypoxia-Induced Colonic Injury Through Microbiota–Metabolite Crosstalk
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics and Experimental Design
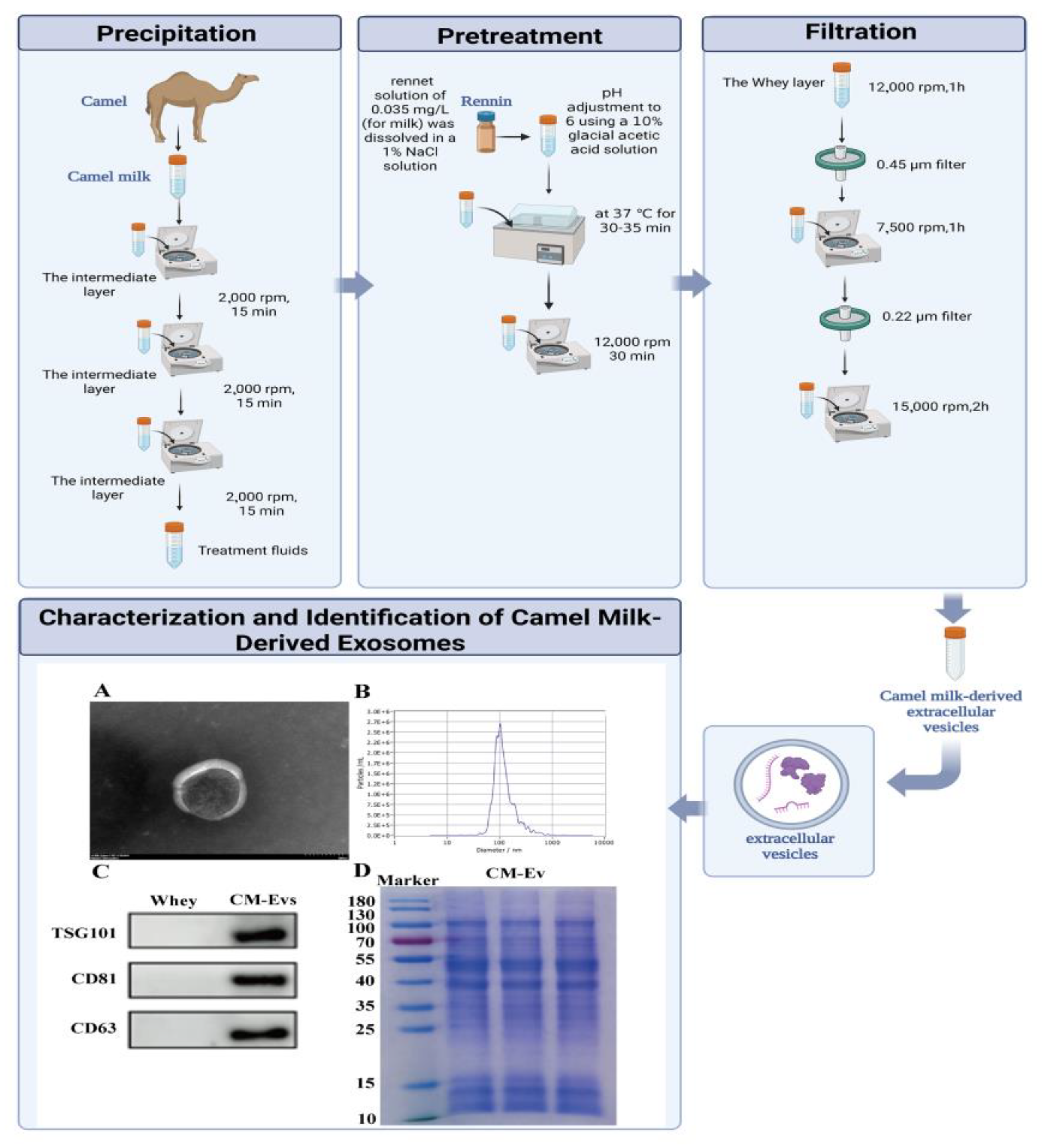
2.2. Optimized Protocol for Camel Milk Extracellular Vesicle (CM-EVs) Isolation
2.3. Comprehensive Characterization of CM-EVs
2.4. Hypobaric Hypoxia-Induced AMS Model and Disease Activity Index (DAI) Evaluation
2.5. Gut Microbiota Analysis via 16S rRNA Sequencing
2.6. Metabolomic Profiling and Pathway Analysis
2.7. Histopathological
2.8. Fecal Microbiota Transplantation Validation Experiment
2.9. Statistical Analysis
3. Results
3.1. Extraction and Characterization of CM-EVs
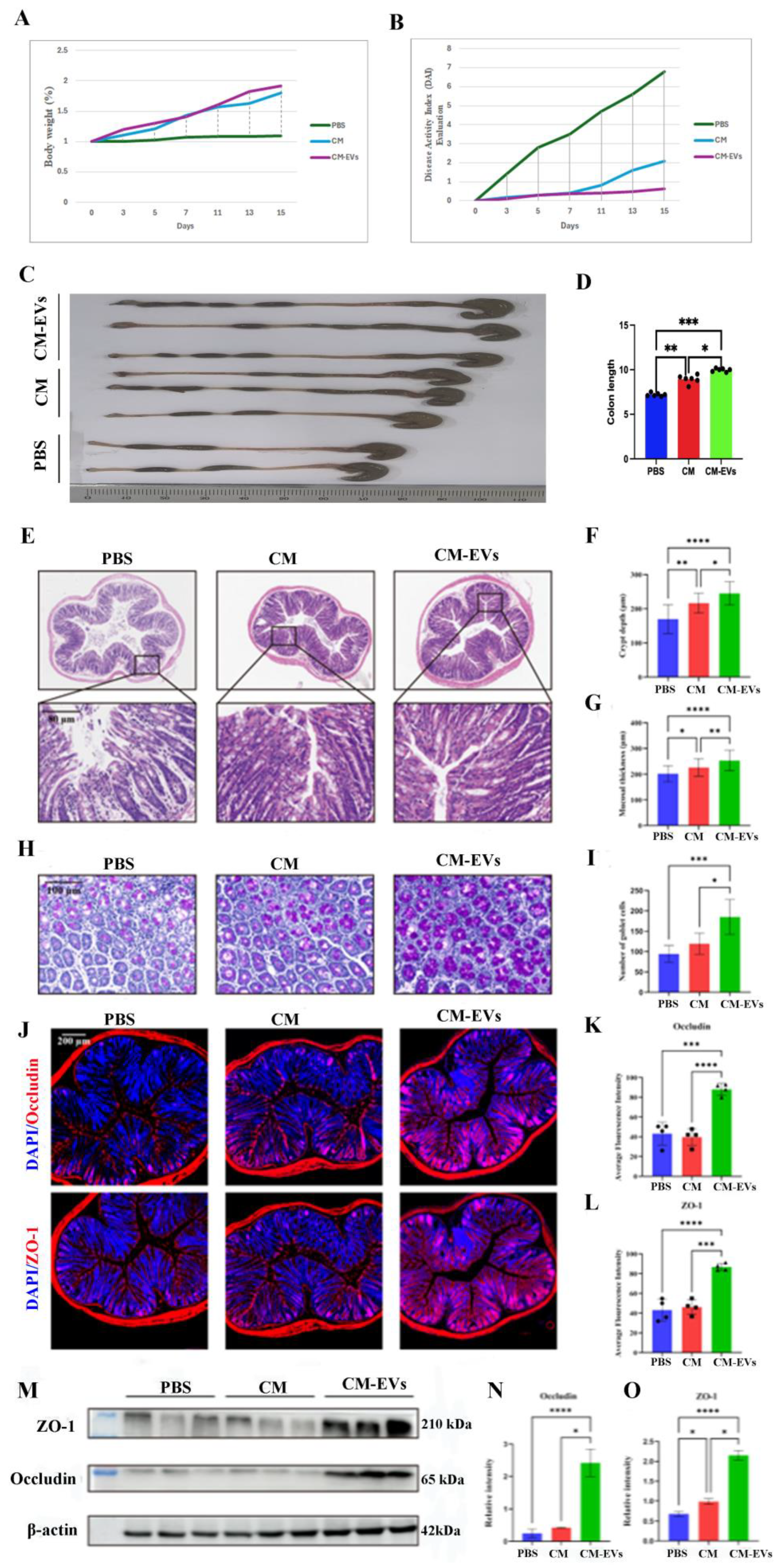
3.2. Hypobaric Hypoxia-Induced Colonic Injury in AMS Mice and DAI Evaluation
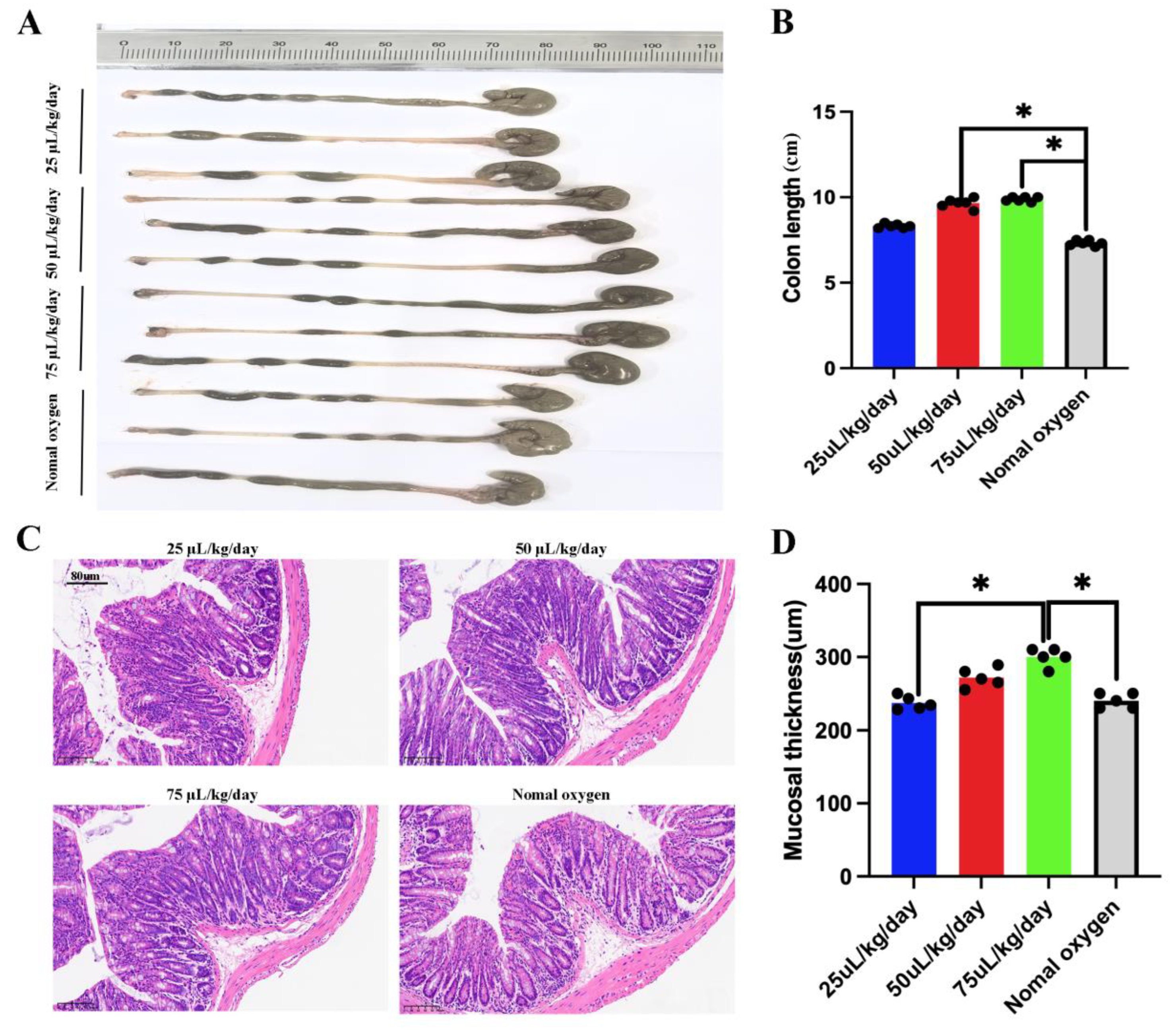
3.3. CM-EVs Attenuate Hypoxia-Induced Colonic Injury
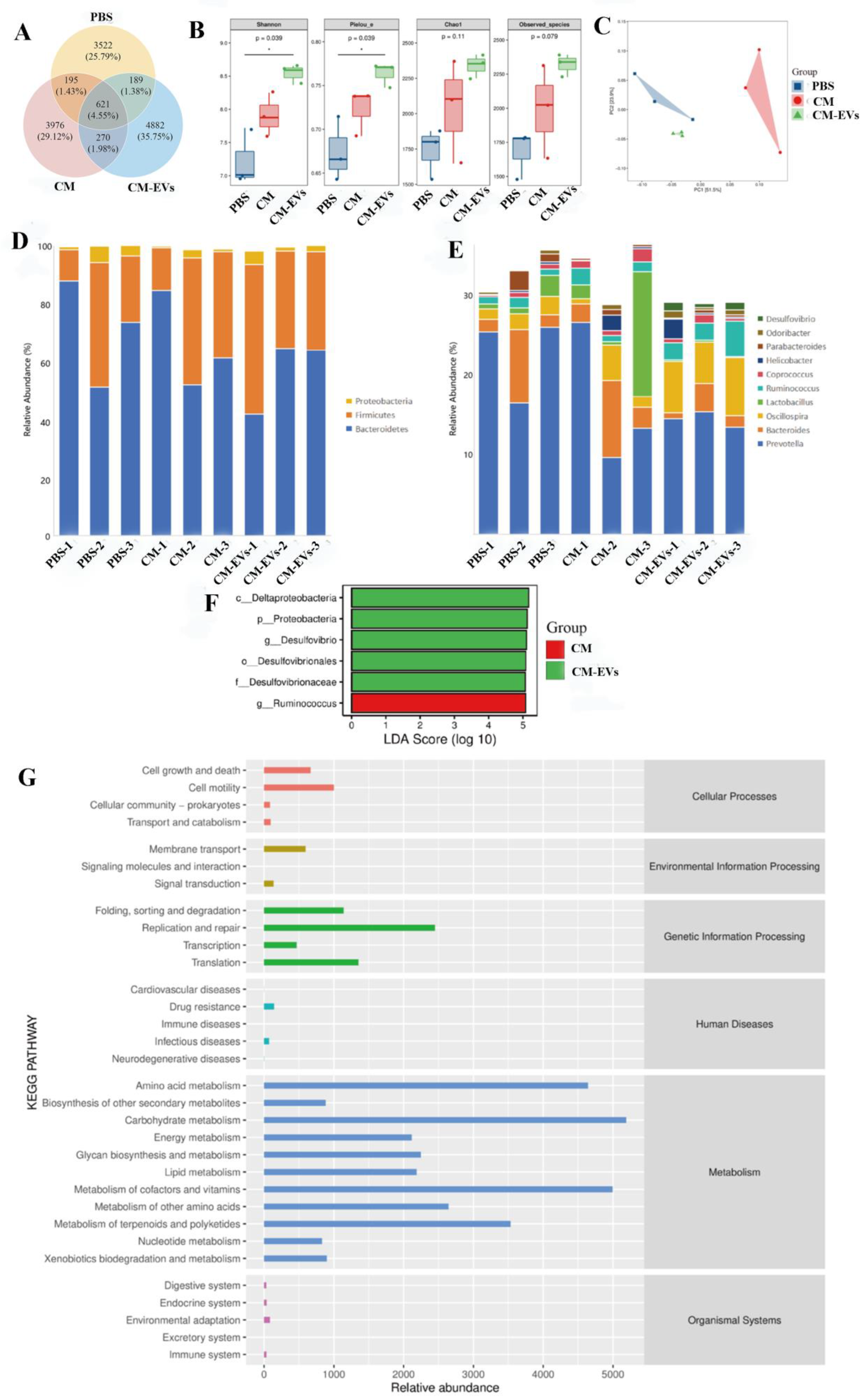
3.4. CM-EVs Modulate Gut Microbiota Composition
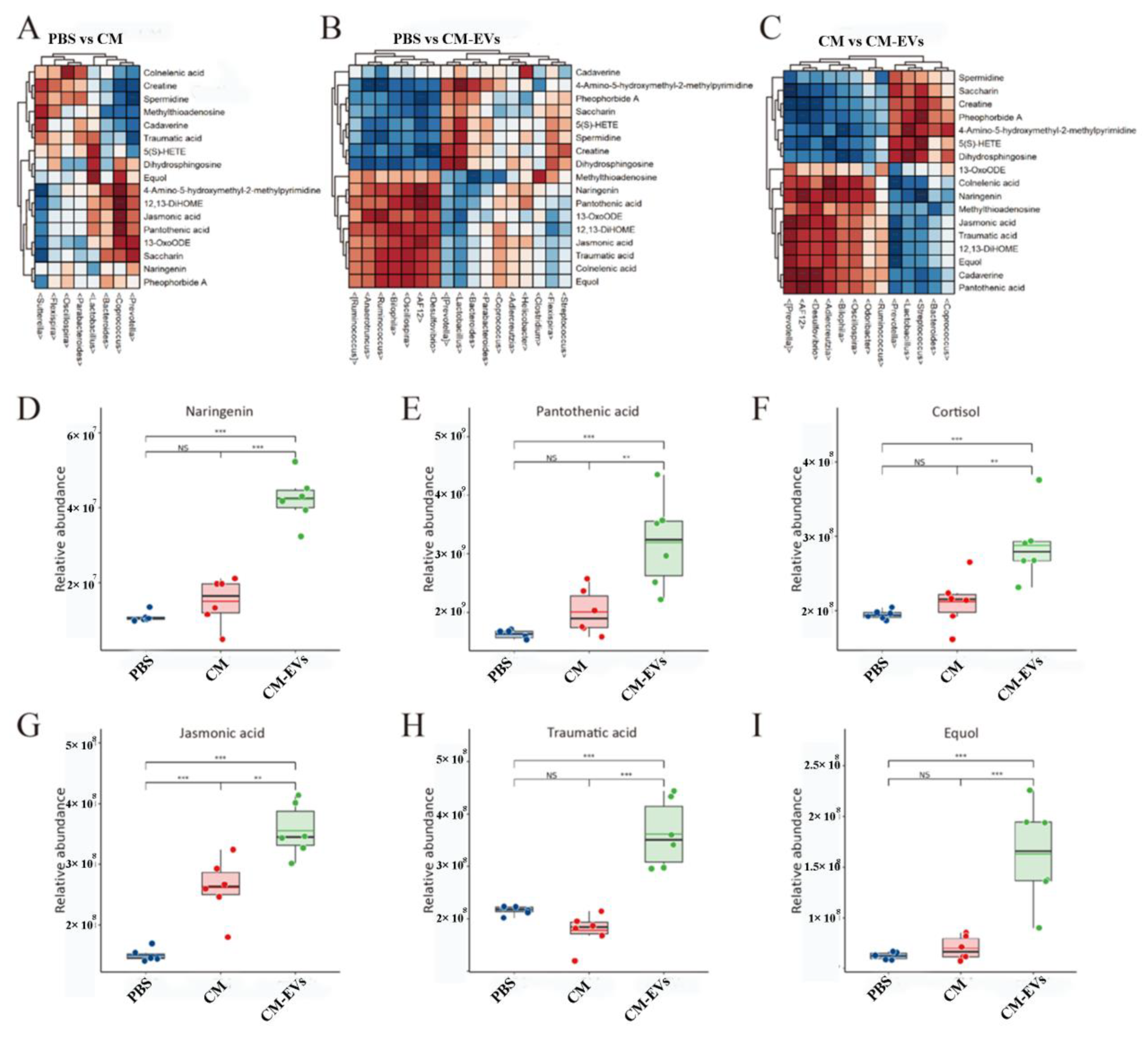
3.5. CM-EVs Regulate Fecal Metabolite Profiles
3.6. CM-EVs Suppress Pro-Inflammatory Cytokines
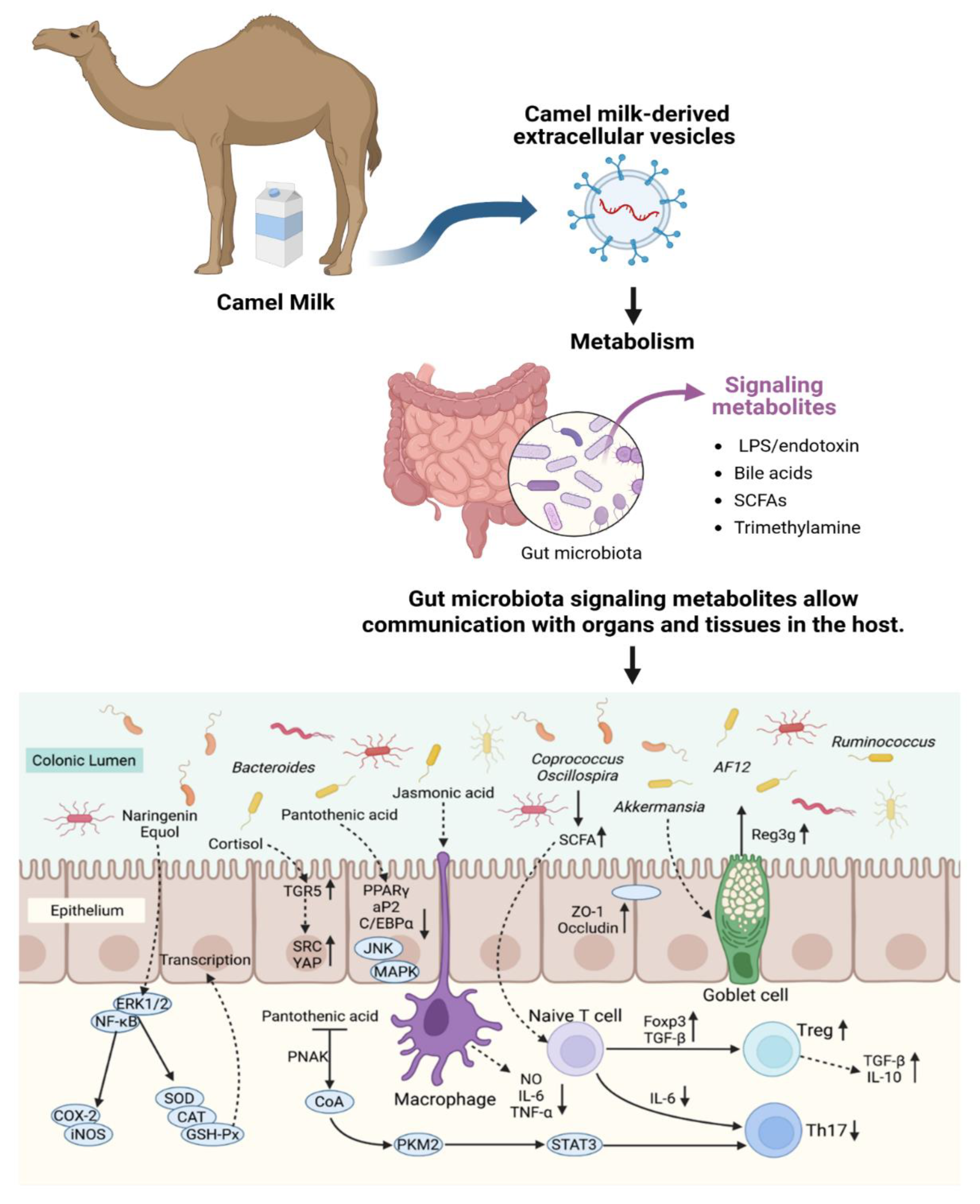
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMS | Acute Mountain Sickness |
| ANOVA | Analysis of Variance |
| ASV | Amplicon Sequence Variant |
| CM-EVs | Camel Milk-Derived Extracellular Vesicles |
| DAPI | 4’,6-Diamidino-2-Phenylindole |
| DNA | Deoxyribonucleic Acid |
| EVs | Extracellular Vesicles |
| ESI | Electrospray Ionization |
| FXR | Farnesoid X Receptor |
| H&E | Hematoxylin and Eosin Staining |
| IACUC | Institutional Animal Care and Use Committee |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| miRNA | MicroRNA |
| mRNA | Messenger RNA |
| MyD88 | Myeloid Differentiation Factor 88 |
| NF-κB | Nuclear Factor Kappa B |
| NTA | Nanoparticle Tracking Analysis |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PBS | Phosphate-Buffered Saline |
| PCA | Principal Component Analysis |
| PCoA | Principal Coordinate Analysis |
| qPCR | Quantitative Polymerase Chain Reaction |
| RNA | Ribonucleic Acid |
| SDS-PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| SCFA | Short-Chain Fatty Acids |
| SIMCA-P | Soft Independent Modeling of Class Analogy |
| SPF | Specific Pathogen-Free |
| TEM | Transmission Electron Microscopy |
| TGF-β | Transforming Growth Factor-Beta |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor-α |
| ZO-1 | Zonula Occludens-1 |
References
- Gatterer, H.; Villafuerte, F.C.; Ulrich, S.; Bhandari, S.S.; Keyes, L.E.; Burtscher, M. Altitude illnesses. Nat. Rev. Dis. Prim. 2024, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.-P.; Hermand, E.; Lhuissier, F.J. Cardiovascular physiology and pathophysiology at high altitude. Nat. Rev. Cardiol. 2024, 21, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Jury, N.J.; Guillen, E.G.; A Bremer, A. This is the moment: Advancing Food is Medicine through research. Am. J. Clin. Nutr. 2025, 121, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Y.; Gao, R.; Chen, J.; Chen, Y.; Li, M.; Gao, Y. Potential therapeutic effects of traditional Chinese medicine in acute mountain sickness: Pathogenesis, mechanisms and future directions. Front. Pharmacol. 2024, 15, 1393209. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Huey, S.L.; Shukla, A.P.; Kuriyan, R.; Finkelstein, J.L.; Mehta, S. Connecting precision nutrition with the Food is Medicine approach. Trends Endocrinol. Metab. 2024, 36, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Khalyfa, A.; Ericsson, A.C.; Puech, C.; McAdams, Z.; Bender, S.B.; Gozal, D. Gut microbiota mediate vascular dysfunction in a murine model of sleep apnoea: Effect of probiotics. Eur. Respir. J. 2023, 61, 2200002. [Google Scholar] [CrossRef] [PubMed]
- Pral, L.P.; Fachi, J.L.; Corrêa, R.O.; Colonna, M.; Vinolo, M.A. Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 2021, 42, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; Parikh, K.; Heinrich, E.C. Hypoxia and Inflammation: Insights From High-Altitude Physiology. Front. Physiol. 2021, 12, 676782. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, M.; Sawicki, T.; Żulewska, J.; Staniewska, K.; Łobacz, A.; Przybyłowicz, K.E. The Role of Bovine Milk-Derived Exosomes in Human Health and Disease. Molecules 2024, 29, 5835. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.-J.; Wang, M.-H.; Ho, C.-T.; Pan, M.-H. Plant-Derived Extracellular Vesicles: A New Revolutionization of Modern Healthy Diets and Biomedical Applications. J. Agric. Food Chem. 2024, 72, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hu, Z.; Yan, X.; Lu, J.; Wang, C. Extracellular vesicles involved in growth regulation and metabolic modulation in Haematococcus pluvialis. Biotechnol. Biofuels Bioprod. 2024, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, e2403199. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Sluijter, J.P.G. Extracellular vesicles in cardiovascular homeostasis and disease: Potential role in diagnosis and therapy. Nat. Rev. Cardiol. 2025, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Z.H.; Wang, C.; Jin, Y. Extracellular Vesicle Transportation and Uptake by Recipient Cells: A Critical Process to Regulate Human Diseases. Processes 2021, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- French, K.C.; Antonyak, M.A.; Cerione, R.A. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin. Cell Dev. Biol. 2017, 67, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lorite, P.; Domínguez, J.N.; Palomeque, T.; Torres, M.I. Extracellular Vesicles: Advanced Tools for Disease Diagnosis, Monitoring, and Therapies. Int. J. Mol. Sci. 2024, 26, 189. [Google Scholar] [CrossRef] [PubMed]
- Visnovitz, T. Extracellular Vesicles: Biology and Therapeutic Applications. Int. J. Mol. Sci. 2024, 25, 13034. [Google Scholar] [CrossRef] [PubMed]
- Oshchepkova, A.; Zenkova, M.; Vlassov, V. Extracellular Vesicles for Therapeutic Nucleic Acid Delivery: Loading Strategies and Challenges. Int. J. Mol. Sci. 2023, 24, 7287. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; El-Hofey, S.M.; Shaban, A.M.; Orif, S.E.; Uyanıkgil, Y.; El-Magd, M.A. Camel milk extracellular vesicles/exosomes: A fascinating frontier in isolation and therapeutic potential. Food Funct. 2025, 16, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Mohammed-Geba, K.; Tawfic, A.A.; El-Magd, M.A. Camel milk exosomes modulate cyclophosphamide-induced oxidative stress and immuno-toxicity in rats. Food Funct. 2019, 10, 7523–7532. [Google Scholar] [CrossRef] [PubMed]
- Karbasi, S.; Erfanian, N.; Dehghan, H.; Zarban, A.; Namaei, M.H.; Hanafi-Bojd, M.Y.; Nasseri, S. Assessment of the Anti-cancer Effects of Camel Milk Exosomes (CMEXOs) on Murine Colorectal Cancer Cell Line (CT-26). Iran. J. Allergy Asthma Immunol. 2024, 23, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.A.M.; Sun, D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018 [Issued 6 February 2018 Effective from 1 September 2018]. Anim. Model. Exp. Med. 2020, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Huang, Y.; Qiao, W.; Zhang, X.; Wang, Y.; Zhang, M.; Fu, J.; Zhao, J.; Chen, L. Advances in the isolation and characterization of milk-derived extracellular vesicles and their functions. Front. Nutr. 2024, 11, 1512939. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Sanwlani, R.; Pathan, M.; Anand, S.; Johnston, E.L.; Ang, C.-S.; Kaparakis-Liaskos, M.; Mathivanan, S. Isolation and Characterization of Cow-, Buffalo-, Sheep- and Goat-Milk-Derived Extracellular Vesicles. Cells 2023, 12, 2491. [Google Scholar] [CrossRef] [PubMed]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1440132. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ouyang, X.; Mu, Y.; Chen, Y. Human breast milk-derived exosomes and their positive role on neonatal intestinal health. Pediatr. Res. 2025, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gündeş, Ş.E.; Boyacioğlu, S.Ö.; Abbak, M.; Bulca, S.; Boyacioğlu, O. Whey proteins from camel’s milk have higher in vitro wound-healing effect than whey proteins from cow’s milk. J. Sci. Food Agric. 2025, 105, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Almehdar, H.A.; El-Baky, N.A.; Alhaider, A.A.; Almuhaideb, S.A.; Albiheyri, R.S.; Uversky, V.N.; Redwan, E.M. Synergistic Killing of Pathogenic Escherichia coli Using Camel Lactoferrin from Different Saudi Camel Clans and Various Antibiotics. Protein J. 2019, 38, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.B.; Anwar, I.; Redwan, E.M.; Palakkott, A.; Ashraf, A.; Kizhakkayil, J.; Iratni, R.; Maqsood, S.; Ayoub, M.A. Camel and bovine milk lactoferrins activate insulin receptor and its related AKT and ERK1/2 pathways. J. Dairy Sci. 2022, 105, 1848–1861. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Redha, A.A.; Nirmal, N.P.; Maqsood, S. In vitro antidiabetic and antihypercholesterolemic activities of camel milk protein hydrolysates derived upon simulated gastrointestinal digestion of milk from different camel breeds. J. Dairy Sci. 2023, 106, 3098–3108. [Google Scholar] [CrossRef] [PubMed]
- Benmeziane-Derradji, F. Evaluation of camel milk: Gross composition—A scientific overview. Trop. Anim. Health Prod. 2021, 53, 308. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Li, W.; Wang, S.; Zheng, J.; Xu, G.; Li, G.; Shen, X.; Yang, J. Gut microbiota imbalance mediates intestinal barrier damage in high-altitude exposed mice. FEBS J. 2022, 289, 4850–4868. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Amevor, F.K.; Zhao, X.; Mou, C.; Pang, J.; Peng, X.; Liu, A.; Lan, X.; Liu, L. Potential therapeutic effects of milk-derived exosomes on intestinal diseases. J. Nanobiotechnol. 2023, 21, 496. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tian, W.; Yang, H.; Hu, H.; Zheng, J.; Yao, X.; Hu, B.; Liu, H. Shen-Ling-Bai-Zhu-San alleviates the imbalance of intestinal homeostasis in dextran sodium sulfate-induced colitis mice by regulating gut microbiota and inhibiting the NLRP3 inflammasome activation. J. Ethnopharmacol. 2024, 319, 117136. [Google Scholar] [CrossRef] [PubMed]
- Gonza, I.; Goya-Jorge, E.; Douny, C.; Boutaleb, S.; Taminiau, B.; Daube, G.; Scippo, M.; Louis, E.; Delcenserie, V. Food additives impair gut microbiota from healthy individuals and IBD patients in a colonic in vitro fermentation model. Food Res. Int. 2024, 182, 114157. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Paparo, L.; Nocerino, R.; Della Gatta, G.; Carucci, L.; Russo, R.; Pasolli, E.; Ercolini, D.; Canani, R.B. Specific gut microbiome signatures and the associated pro-inflamatory functions are linked to pediatric allergy and acquisition of immune tolerance. Nat. Commun. 2021, 12, 5958. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Yuan, W.-Q.; Wu, S.-M.; Yang, Y.-H.; Cui, D.-J. Novel mechanisms of intestinal flora regulation in high-altitude hypoxia. Heliyon 2024, 10, e38220. [Google Scholar] [CrossRef] [PubMed]
- Basak, C.; Chakraborty, R. Effect of Hypoxia on the Gut Microflora of a Facultative Air-Breathing Loach Lepidocephalichthys guntea. Curr. Microbiol. 2024, 81, 406. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liao, Y.; Chai, S.; Yang, Y.; Ga, Q.; Ge, R.; Wang, S.; Liu, S. Modification of Intestinal Flora Can Improve Host Metabolism and Alleviate the Damage Caused by Chronic Hypoxia. Curr. Issues Mol. Biol. 2024, 46, 12733–12745. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Sleiman, M.B.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Jarocka-Karpowicz, I.; Markowska, A. Therapeutic Potential of Jasmonic Acid and Its Derivatives. Int. J. Mol. Sci. 2021, 22, 8437. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Pankiewicz, W.; Czerpak, R. Traumatic Acid Reduces Oxidative Stress and Enhances Collagen Biosynthesis in Cultured Human Skin Fibroblasts. Lipids 2016, 51, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wen, Z.; Gao, Y.; Xiao, F.; Yan, J.; Wang, X.; Meng, T. Pantothenic Acid Alleviates Fat Deposition and Inflammation by Suppressing the JNK/P38 MAPK Signaling Pathway. J. Med. Food 2024, 27, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, W.; Zhou, T.; Liu, Q.; Han, C.; Huang, Z.; Chen, S.; Mei, Q.; Zhang, C.; Zhang, K.; et al. Vitamin B5 rewires Th17 cell metabolism via impeding PKM2 nuclear translocation. Cell Rep. 2022, 41, 111741. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wen, H.; Zhou, H.; Zhang, D.; Lan, D.; Liu, S.; Li, C.; Dai, X.; Song, T.; Wang, X.; et al. Naringenin: A flavanone with anti-inflammatory and anti-infective properties. Biomed. Pharmacother. 2023, 164, 114990. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Shi, F.; Zhang, X.; Zhang, Y.; Bi, K.; Chen, X.; Li, L.; Diao, H. Phospholipid metabolites of the gut microbiota promote hypoxia-induced intestinal injury via CD1d-dependent γδ T cells. Gut Microbes 2022, 14, 2096994. [Google Scholar] [CrossRef] [PubMed]
- Yung, C.; Zhang, Y.; Kuhn, M.; Armstrong, R.J.; Olyaei, A.; Aloia, M.; Scottoline, B.; Andres, S.F. Neonatal enteroids absorb extracellular vesicles from human milk-fed infant digestive fluid. J. Extracell. Vesicles 2024, 13, e12422. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Ding, Y.; Yang, J.; Zheng, Z.; Lu, J.; Luo, H.; Wang, J.; Lin, F.; Chen, J.; Li, Q.; et al. Milk exosomes from gestational diabetes mellitus parturients demonstrate weaker ability to promote intestinal development in offspring. Mol. Nutr. Food Res. 2025, 69, 1. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, S.; Pietrucci, D.; Milanesi, M.; Capomaccio, S.; Pascucci, L.; Evangelista, C.; Basiricò, L.; Bernabucci, U.; Chillemi, G.; Cappelli, K. Comparison of colostrum and milk extracellular vesicles small RNA cargo in water buffalo. Sci. Rep. 2024, 14, 17991. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, S.; Liu, Q.; Huang, C.; Hao, H.; Tan, M.S.; Yu, X.; Lou, C.K.L.; Huang, R.; Zhang, Z.; et al. Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis. Sci. Adv. 2023, 9, eade5041. [Google Scholar] [CrossRef] [PubMed]
- Munir, J.; Sadri, M.; Zempleni, J. Tsg101 knockout in the mammary gland leads to a decrease in small extracellular vesicles in milk from C57BL/6J dams and contributes to leakiness of the gut mucosa and reduced postnatal weight gain in suckling pups. J. Nutr. Biochem. 2025, 135, 109782. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Stremmel, W.; Weiskirchen, R.; John, S.M.; Schmitz, G. Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Er, D.; Wang, Y.-H.; Zhai, B.-T.; Ge, R. Camel Milk-Derived Extracellular Vesicles as a Functional Food Component Ameliorate Hypobaric Hypoxia-Induced Colonic Injury Through Microbiota–Metabolite Crosstalk. Nutrients 2025, 17, 2431. https://doi.org/10.3390/nu17152431
Yang H, Er D, Wang Y-H, Zhai B-T, Ge R. Camel Milk-Derived Extracellular Vesicles as a Functional Food Component Ameliorate Hypobaric Hypoxia-Induced Colonic Injury Through Microbiota–Metabolite Crosstalk. Nutrients. 2025; 17(15):2431. https://doi.org/10.3390/nu17152431
Chicago/Turabian StyleYang, Hui, Demtu Er, Yu-Huan Wang, Bin-Tao Zhai, and Rili Ge. 2025. "Camel Milk-Derived Extracellular Vesicles as a Functional Food Component Ameliorate Hypobaric Hypoxia-Induced Colonic Injury Through Microbiota–Metabolite Crosstalk" Nutrients 17, no. 15: 2431. https://doi.org/10.3390/nu17152431
APA StyleYang, H., Er, D., Wang, Y.-H., Zhai, B.-T., & Ge, R. (2025). Camel Milk-Derived Extracellular Vesicles as a Functional Food Component Ameliorate Hypobaric Hypoxia-Induced Colonic Injury Through Microbiota–Metabolite Crosstalk. Nutrients, 17(15), 2431. https://doi.org/10.3390/nu17152431





