BMI-Specific Nutritional Education Priorities for Weight Management in Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Recruitment
2.3. Patient Survey Content
2.4. Data Handling
2.5. Quantitative Statistical Analyses
2.6. Qualitative Statistical Analyses
3. Results
3.1. Strategies for Weight Management and a Healthy Lifestyle
3.2. Vitamins, Minerals and Other Supplements
3.3. Foods and Nutrients That May Reduce Inflammation
3.4. Diets for Weight Loss
3.5. Resources and Additional Nutrition Topics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| BMI | Body Mass Index |
| HBMI | Higher Body Mass Index (dichotomized group) |
| LBMI | Lower Body Mass Index (dichotomized group) |
| OAAA | Osteoarthritis Action Alliance |
| OR | Odds Ratio |
| CBD | Cannabidiol |
| THC | Tetrahydrocannabinol |
References
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Goldin, R.H.; McAdam, L.; Louie, J.S.; Gold, R.; Bluestone, R. Clinical and radiological survey of the incidence of osteoarthrosis among obese patients. Ann. Rheum. Dis. 1976, 35, 349–353. [Google Scholar] [CrossRef]
- Messier, S.P.; Beavers, D.P.; Queen, K.; Mihalko, S.L.; Miller, G.D.; Losina, E.; Katz, J.N.; Loeser, R.F.; DeVita, P.; Hunter, D.J.; et al. Effect of Diet and Exercise on Knee Pain in Patients with Osteoarthritis and Overweight or Obesity: A Randomized Clinical Trial. JAMA 2022, 328, 2242–2251. [Google Scholar] [CrossRef]
- Messier, S.P.; Callahan, L.F.; Beavers, D.P.; Queen, K.; Mihalko, S.L.; Miller, G.D.; Losina, E.; Katz, J.N.; Loeser, R.F.; Quandt, S.A.; et al. Weight-loss and exercise for communities with arthritis in North Carolina (we-can): Design and rationale of a pragmatic, assessor-blinded, randomized controlled trial. BMC Musculoskelet. Disord. 2017, 18, 91. [Google Scholar] [CrossRef]
- Messier, S.P.; Loeser, R.F.; Miller, G.D.; Morgan, T.M.; Rejeski, W.J.; Sevick, M.A.; Ettinger, W.H., Jr.; Pahor, M.; Williamson, J.D. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: The Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004, 50, 1501–1510. [Google Scholar] [CrossRef]
- Messier, S.P.; Loeser, R.F.; Mitchell, M.N.; Valle, G.; Morgan, T.P.; Rejeski, W.J.; Ettinger, W.H. Exercise and weight loss in obese older adults with knee osteoarthritis: A preliminary study. J. Am. Geriatr. Soc. 2000, 48, 1062–1072. [Google Scholar] [CrossRef]
- Messier, S.P.; Mihalko, S.L.; Beavers, D.P.; Nicklas, B.J.; DeVita, P.; Carr, J.J.; Hunter, D.J.; Lyles, M.; Guermazi, A.; Bennell, K.L.; et al. Effect of High-Intensity Strength Training on Knee Pain and Knee Joint Compressive Forces Among Adults With Knee Osteoarthritis: The START Randomized Clinical Trial. JAMA 2021, 325, 646–657. [Google Scholar] [CrossRef]
- Messier, S.P.; Resnik, A.E.; Beavers, D.P.; Mihalko, S.L.; Miller, G.D.; Nicklas, B.J.; deVita, P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F.; et al. Intentional Weight Loss in Overweight and Obese Patients With Knee Osteoarthritis: Is More Better? Arthritis Care Res. 2018, 70, 1569–1575. [Google Scholar] [CrossRef]
- Allen, K.D.; Ambrose, K.R.; Booker, S.Q.; Buck, A.N.; Huffman, K.F. Non-Pharmacological Pain Management for Osteoarthritis: Review Update. Curr. Rheumatol. Rep. 2025, 27, 19. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Bartels, E.M.; Astrup, A.; Bliddal, H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2007, 66, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Chu, I.J.H.; Lim, A.Y.T.; Ng, C.L.W. Effects of meaningful weight loss beyond symptomatic relief in adults with knee osteoarthritis and obesity: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, C.L.; Hangaard, S.; Bartels, E.M.; Gudbergsen, H.; Christensen, R.; Bliddal, H.; Englund, M.; Conaghan, P.G.; Boesen, M. The effects of weight loss on imaging outcomes in osteoarthritis of the hip or knee in people who are overweight or obese: A systematic review. Osteoarthr. Cartil. 2020, 28, 10–21. [Google Scholar] [CrossRef]
- Felson, D.T.; Zhang, Y.; Anthony, J.M.; Naimark, A.; Anderson, J.J. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann. Intern. Med. 1992, 116, 535–539. [Google Scholar] [CrossRef]
- Gersing, A.S.; Schwaiger, B.J.; Nevitt, M.C.; Zarnowski, J.; Joseph, G.B.; Feuerriegel, G.; Jungmann, P.M.; Guimaraes, J.B.; Facchetti, L.; McCulloch, C.E.; et al. Weight loss regimen in obese and overweight individuals is associated with reduced cartilage degeneration: 96-month data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2019, 27, 863–870. [Google Scholar] [CrossRef]
- Gersing, A.S.; Solka, M.; Joseph, G.B.; Schwaiger, B.J.; Heilmeier, U.; Feuerriegel, G.; Nevitt, M.C.; McCulloch, C.E.; Link, T.M. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2016, 24, 1126–1134. [Google Scholar] [CrossRef]
- Shahid, A.; Thirumaran, A.J.; Christensen, R.; Venkatesha, V.; Henriksen, M.; Bowden, J.L.; Hunter, D.J. Comparison of weight loss interventions in overweight and obese adults with knee osteoarthritis: A systematic review and network meta-analysis of randomized trials. Osteoarthr. Cartil. 2025, 33, 518–529. [Google Scholar] [CrossRef]
- Calvet, J.; Orellana, C.; Larrosa, M.; Navarro, N.; Chillaron, J.J.; Pedro-Botet, J.; Galisteo, C.; Garcia-Manrique, M.; Gratacos, J. High prevalence of cardiovascular co-morbidities in patients with symptomatic knee or hand osteoarthritis. Scand. J. Rheumatol. 2016, 45, 41–44. [Google Scholar] [CrossRef]
- Constantino de Campos, G.; Mundi, R.; Whittington, C.; Toutounji, M.J.; Ngai, W.; Sheehan, B. Osteoarthritis, mobility-related comorbidities and mortality: An overview of meta-analyses. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20981219. [Google Scholar] [CrossRef]
- Kivimäki, M.; Strandberg, T.; Pentti, J.; Nyberg, S.T.; Frank, P.; Jokela, M.; Ervasti, J.; Suominen, S.B.; Vahtera, J.; Sipilä, P.N.; et al. Body-mass index and risk of obesity-related complex multimorbidity: An observational multicohort study. Lancet Diabetes Endocrinol. 2022, 10, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Suri, P.; Morgenroth, D.C.; Hunter, D.J. Epidemiology of Osteoarthritis and Associated Comorbidities. PM&R 2012, 4, S10–S19. [Google Scholar]
- Zhao, T.; Winzenberg, T.; Aitken, D.; Graaff, B.d.; Ahmad, H.; Jones, G.; Palmer, A.J. The impact of comorbidities on health-related quality of life of people with osteoarthritis over 10 years. Rheumatology 2021, 61, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Anderson, J.J.; Naimark, A.; Walker, A.M.; Meenan, R.F. Obesity and knee osteoarthritis. The Framingham Study. Ann. Intern. Med. 1988, 109, 18–24. [Google Scholar] [CrossRef]
- Buck, A.N.; Shultz, S.P.; Huffman, K.F.; Vincent, H.K.; Batsis, J.A.; Newman, C.B.; Beresic, N.; Abbate, L.M.; Callahan, L.F. Mind the Gap: Exploring Nutritional Health Compared With Weight Management Interests of Individuals with Osteoarthritis. Curr. Dev. Nutr. 2022, 6, nzac084. [Google Scholar] [CrossRef]
- Goff, A.J.; De Oliveira Silva, D.; Merolli, M.; Bell, E.C.; Crossley, K.M.; Barton, C.J. Patient education improves pain and function in people with knee osteoarthritis with better effects when combined with exercise therapy: A systematic review. J. Physiother. 2021, 67, 177–189. [Google Scholar] [CrossRef]
- Faison, W.E.; Harrell, P.G.; Semel, D. Disparities across Diverse Populations in the Health and Treatment of Patients with Osteoarthritis. Healthcare 2021, 9, 1421. [Google Scholar] [CrossRef]
- Borkhoff, C.M.; Wieland, M.L.; Myasoedova, E.; Ahmad, Z.; Welch, V.; Hawker, G.A.; Li, L.C.; Buchbinder, R.; Ueffing, E.; Beaton, D.; et al. Reaching those most in need: A scoping review of interventions to improve health care quality for disadvantaged populations with osteoarthritis. Arthritis Care Res. 2011, 63, 39–52. [Google Scholar] [CrossRef]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The Association of Emotional Eating with Overweight/Obesity, Depression, Anxiety/Stress, and Dietary Patterns: A Review of the Current Clinical Evidence. Nutrients 2023, 15, 1173. [Google Scholar] [CrossRef]
- Degerstedt, A.; Alinaghizadeh, H.; Thorstensson, C.A.; Olsson, C.B. High self-efficacy—A predictor of reduced pain and higher levels of physical activity among patients with osteoarthritis: An observational study. BMC Musculoskelet. Disord. 2020, 21, 380. [Google Scholar] [CrossRef]
- Lopez-Bravo, M.D.; Zamarron-Cassinello, M.D.; Touche, R.; Munoz-Plata, R.; Cuenca-Martinez, F.; Ramos-Toro, M. Psychological Factors Associated with Functional Disability in Patients with Hip and Knee Osteoarthritis. Behav. Med. 2021, 47, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.S.; Ammann, E.; Scamuffa, R.; Samuels, J.; Stokes, A.; Fegelman, E.; Hsiao, C.W. Association of body mass index and osteoarthritis with healthcare expenditures and utilization. Obes. Sci. Pract. 2020, 6, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, M.; Gullberg, B.; Merlo, J.; Lundqvist-Persson, C.; Råstam, L.; Lindblad, U. The validity of obesity based on self-reported weight and height: Implications for population studies. Obesity 2007, 15, 197–208. [Google Scholar] [CrossRef]
- Green, J.; Browne, J. Principles of Social Research; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Kamberelis, G.; Dimitriadis, G. Focus Groups: Strategic Articulations of Pedagogy, Politics, and Inquiry. In The Sage handbook of Qualitative Research, 3rd ed.; Sage Publications Ltd.: Thousand Oaks, CA, USA, 2005; pp. 887–907. [Google Scholar]
- Gökçe Kutsal, Y.; Eyigör, S.; Karahan, S.; Günaydın, R.; İrdesel, J.; Sarıdoğan, M.; Borman, P.; Sarı, A.; Ordu Gökkaya, K.; Binay Safer, V. Incorporating patient preferences into osteoarthritis treatment. Arch. Rheumatol. 2021, 36, 577–586. [Google Scholar] [CrossRef]
- Betancourt-Nunez, A.; Torres-Castillo, N.; Martinez-Lopez, E.; De Loera-Rodriguez, C.O.; Duran-Barajas, E.; Marquez-Sandoval, F.; Bernal-Orozco, M.F.; Garaulet, M.; Vizmanos, B. Emotional Eating and Dietary Patterns: Reflecting Food Choices in People with and without Abdominal Obesity. Nutrients 2022, 14, 1371. [Google Scholar] [CrossRef]
- Mathieu, J. What should you know about mindful and intuitive eating? J. Am. Diet. Assoc. 2009, 109, 1982–1987. [Google Scholar] [CrossRef]
- Castellini, G.; Graffigna, G. “Food is more than just a source of nutrients”: A qualitative phenomenological study on Food Involvement. Appetite 2022, 178, 106179. [Google Scholar] [CrossRef]
- Fuentes Artiles, R.; Staub, K.; Aldakak, L.; Eppenberger, P.; Rühli, F.; Bender, N. Mindful eating and common diet programs lower body weight similarly: Systematic review and meta-analysis. Obes. Rev. 2019, 20, 1619–1627. [Google Scholar] [CrossRef]
- Spinelli, S.; Monteleone, E. Food Preferences and Obesity. Endocrinol. Metab. 2021, 36, 209–219. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Yu, X.; Pu, H.; Voss, M. Overview of anti-inflammatory diets and their promising effects on non-communicable diseases. Br. J. Nutr. 2024, 132, 898–918. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia—What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Bassin, S.R.; De Carvalho, J.F.; Gulati, M. A Review of Plant-Based Diets for Obesity Management. Endocr. Pract. 2024, 30, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.N.; Vincent, H.K.; Newman, C.B.; Batsis, J.A.; Abbate, L.M.; Huffman, K.F.; Bodley, J.; Vos, N.; Callahan, L.F.; Shultz, S.P. Evidence-Based Dietary Practices to Improve Osteoarthritis Symptoms: An Umbrella Review. Nutrients 2023, 15, 3050. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Overton, C.; Nelson, A.E.; Neogi, T. Osteoarthritis Treatment Guidelines from Six Professional Societies: Similarities and Differences. Rheum. Dis. Clin. North. Am. 2022, 48, 637–657. [Google Scholar] [CrossRef]
- Butler, R.; McClinchy, J.; Morreale-Parker, C.; Marsh, W.; Rennie, K.L. BMI calculation in older people: The effect of using direct and surrogate measures of height in a community-based setting. Clin. Nutr. ESPEN 2017, 22, 112–115. [Google Scholar] [CrossRef]
- Drieskens, S.; Demarest, S.; Bel, S.; De Ridder, K.; Tafforeau, J. Correction of self-reported BMI based on objective measurements: A Belgian experience. Arch. Public Health 2018, 76, 10. [Google Scholar] [CrossRef]


| Entire Study Sample | BMI: ≥30 kg/m2 | BMI: <30 kg/m2 | p-Value | |
|---|---|---|---|---|
| n (%) | 296 (100%) | 172 (58.10%) | 124 (41.89%) | |
| Age | 60.30 ± 10.80 | 58.93 ± 10.55 | 62.24 ± 10.91 | 0.06 |
| BMI | 33.19 ± 8.41 | 38.67 ± 6.59 | 25.59 ± 3.00 | <0.001 |
| Sex | 0.35 | |||
| Male, n (%) | 47 (15.88%) | 25 (14.53%) | 22 (17.74%) | |
| Female, n (%) | 249 (84.12%) | 147 (85.47%) | 102 (82.26%) | |
| Race | 0.07 | |||
| Caucasian, n (%) | 253 (85.47%) | 139 (80.81%) | 114 (91.94%) | |
| Other Races, n (%) | 43 (14.53%) | 33 (19.19%) | 10 (8.06%) | |
| US Region | ||||
| Atlantic | 112 (37.84%) | 61 (35.47%) | 51 (41.13%) | |
| Southern | 63 (21.28% | 37 (21.51%) | 26 (20.97%) | |
| Central | 66 (22.30%) | 44 (25.58%) | 22 (17.74%) | |
| Pacific | 49 (16.55%) | 28 (16.28%) | 21 (16.94%) |
| Survey Topic and Question | Logistic Models with Dichotomous BMI Predictor a | Logistic Models Continuous BMI Predictor b | ||
|---|---|---|---|---|
| 1. Weight Management and Healthy Lifestyle Topics | Unadjusted | Adjusted c | Unadjusted | Adjusted c |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
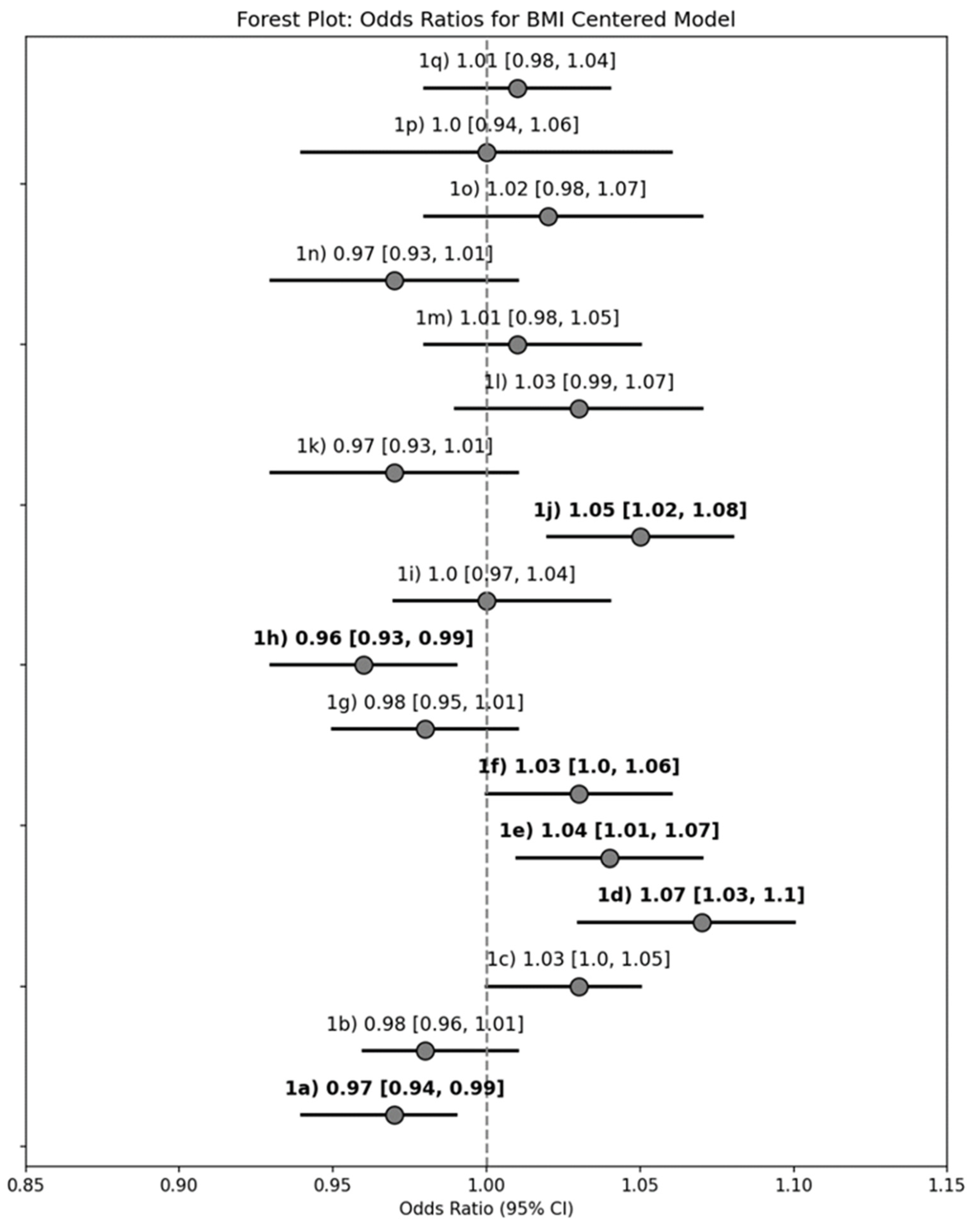
| 1a. Foods that make OA symptoms worse | 0.69 (0.42, 1.13) | 0.66 (0.39, 1.13) | 0.97 (0.94, 0.99) | 0.96 (0.92, 0.99) |
| 1b. Foods to help reduce inflammation | 0.66 (0.41, 1.05) | 0.73 (0.44, 1.22) | 0.98 (0.96, 1.01) | 0.98 (0.96, 1.01) |
| 1c. Healthy weight loss for older adults | 1.74 (1.08, 2.80) | 2.64 (1.52, 4.58) | 1.03 (1.00, 1.05) | 1.05 (1.02, 1.08) |
| 1d. Ways to control emotional eating | 2.72 (1.59, 4.68) | 2.39 (1.35, 4.23) | 1.07 (1.03, 1.10) | 1.06 (1.03, 1.10) |
| 1e. Strategies to feel full | 1.47 (0.89, 2.42) | 1.62 (0.94, 2.78) | 1.04 (1.01, 1.07) | 1.04 (1.01, 1.08) |
| 1f. Information on affordable food options | 1.47 (0.89, 2.42) | 1.42 (0.84, 2.42) | 1.03 (1.00, 1.06) | 1.03 (1.00, 1.06) |
| 1g. Tips for healthy snacking | 0.78 (0.47, 1.28) | 0.83 (0.49, 1.42) | 0.98 (0.95, 1.01) | 0.98 (0.95, 1.01) |
| 1h. General info on vitamins and minerals | 0.54 (0.32, 0.91) | 0.54 (0.30, 0.94) | 0.96 (0.93, 0.99) | 0.96 (0.92, 0.99) |
| 1i. Awareness of food triggers | 1.06 (0.63, 1.81) | 0.99 (0.55, 1.76) | 1.00 (0.97, 1.04) | 1.00 (0.96, 1.03) |
| 1j. Special diet considerations | 1.70 (0.97, 2.98) | 1.95 (1.05, 3.62) | 1.05 (1.02, 1.08) | 1.06 (1.02, 1.10) |
| 1k. Mindful eating techniques | 0.64 (0.35, 1.16) | 0.52 (0.27, 1.02) | 0.97 (0.93, 1.01) | 0.96 (0.92, 1.00) |
| 1l. Ways to monitor/control caloric intake | 1.60 (0.82, 3.11) | 1.66 (0.80, 3.44) | 1.03 (0.99, 1.07) | 1.03 (0.99, 1.07) |
| 1m. Benefits to fasting/intermittent fasting | 1.67 (0.89, 3.13) | 1.40 (0.71, 2.76) | 1.01 (0.98, 1.05) | 1.00 (0.96, 1.04) |
| 1n. Impacts of organic vs. regular food | 0.54 (0.28, 1.06) | 0.60 (0.29, 1.21) | 0.97 (0.93, 1.01) | 0.97 (0.93, 1.02) |
| 1o. Ways to control portion size | 1.25 (0.60, 2.59) | 1.33 (0.58, 3.02) | 1.02 (0.98, 1.07) | 1.05 (1.00, 1.10) |
| 1p. Food labels to guide dietary decisions | 1.47 (0.49, 4.41) | 1.34 (0.43, 4.18) | 1.00 (0.94, 1.06) | 0.99 (0.93, 1.05) |
| 2. Vitamins, Minerals, and Supplements | Unadjusted | Adjusted c | Unadjusted | Adjusted c |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| 2a. Glucosamine | 1.53 (0.94, 2.49) | 1.43 (0.86, 2.36) | 1.02 (0.99, 1.05) | 1.01 (0.98, 1.04) |
| 2b. Vitamin D | 0.84 (0.52, 1.36) | 0.83 (0.50, 1.36) | 0.98 (0.96, 1.01) | 0.98 (0.95, 1.01) |
| 2c. Omega 3 Fatty Acids | 0.88 (0.55, 1.44) | 0.92 (0.56, 1.52) | 0.98 (0.96, 1.01) | 0.99 (0.96, 1.02) |
| 2d. Calcium | 0.87 (0.52, 1.42) | 0.87 (0.52, 1.45) | 0.99 (0.96, 1.02) | 0.99 (0.96, 1.02) |
| 2e. Chondroitin | 1.22 (0.74, 2.02) | 1.29 (0.77, 2.18) | 1.03 (1.00, 1.06) | 1.03 (1.00, 1.06) |
| 2f. Amino Acids | 0.71 (0.41, 1.21) | 0.70 (0.40, 1.22) | 1.00 (0.97, 1.03) | 1.00 (0.97, 1.03) |
| 2g. Dietary Fiber | 0.94 (0.53, 1.65) | 0.98 (0.54, 1.75) | 1.01 (0.98, 1.04) | 1.01 (0.98, 1.05) |
| 3. Foods and Nutrients that may Reduce Inflammation | Unadjusted | Adjusted c | Unadjusted | Adjusted c |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| 3a. Spices and Herbs | 0.77 (0.45, 1.29) | 0.90 (0.52, 1.54) | 1.00 (0.97, 1.03) | 1.01 (0.98, 1.04) |
| 3b. Fruits and Vegetables | 1.27 (0.78, 2.08) | 1.28 (0.77, 2.12) | 1.00 (0.98, 1.03) | 1.01 (0.98, 1.04) |
| 3c. Nuts | 1.38 (0.84, 2.26) | 1.39 (0.84, 2.32) | 1.02 (0.99, 1.05) | 1.03 (1.00, 1.06) |
| 3d. Olive Oil | 1.08 (0.64, 1.80) | 1.15 (0.68, 1.94) | 1.00 (0.97, 1.03) | 1.00 (0.97, 1.03) |
| 3e. Green Tea | 2.01 (1.17, 3.47) | 1.71 (1.00, 3.00) | 1.04 (1.01, 1.08) | 1.03 (1.00, 1.06) |
| 3f. Fish Oil | 0.62 (0.35, 1.08) | 0.65 (0.37, 1.16) | 0.96 (0.93, 0.99) | 0.96 (0.92, 1.00) |
| 3g. Plant-Based Alternatives for Meat | 0.41 (0.24, 0.72) | 0.38 (0.21, 0.68) | 0.96 (0.93, 1.00) | 0.95 (0.91, 0.98) |
| 4. Diets for Weight Loss | Unadjusted | Adjusted c | Unadjusted | Adjusted c |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| 4a. Mediterranean Diet | 0.76 (0.47, 1.24) | 0.82 (0.49, 1.36) | 0.99 (0.96, 1.01) | 0.99 (0.96, 1.02) |
| 4b. Low Carbohydrate Diet | 1.92 (1.17, 3.15) | 1.92 (1.15, 3.19) | 1.04 (1.01, 1.07) | 1.04 (1.01, 1.08) |
| 4c. Fasting and Intermittent Fasting | 1.03 (0.63, 1.68) | 0.90 (0.54, 1.51) | 1.00 (0.97, 1.03) | 0.99 (0.97, 1.03) |
| 4d. High Protein Diet | 1.03 (0.63, 1.70) | 1.03 (0.61, 1.73) | 0.99 (0.97, 1.02) | 0.99 (0.96, 1.02) |
| 4e. DASH Diet | 1.42 (0.82, 2.44) | 1.61 (0.91, 2.87) | 1.02 (0.99, 1.06) | 1.03 (1.00, 1.07) |
| 4f. Ketogenic Diet | 2.30 (1.29, 4.09) | 2.05 (1.13, 3.70) | 1.07 (1.03, 1.10) | 1.06 (1.02, 1.10) |
| 4g. Vegetarian or Vegan Diets | 0.38 (0.21, 0.69) | 0.40 (0.22, 0.74) | 0.94 (0.90, 0.98) | 0.94 (0.90, 0.98) |
| Survey Topic and Question | LBMI | HBMI | p-Value | ||
|---|---|---|---|---|---|
| 1. Weight Management and Healthy Lifestyle Topics | n = “No” | n = “Yes” | n = “No” | n = “Yes” | |
| 1a. Foods that make OA symptoms worse | 38 | 86 | 67 | 105 | 0.177 |
| 1b. Foods to help reduce inflammation | 45 | 79 | 80 | 92 | 0.102 |
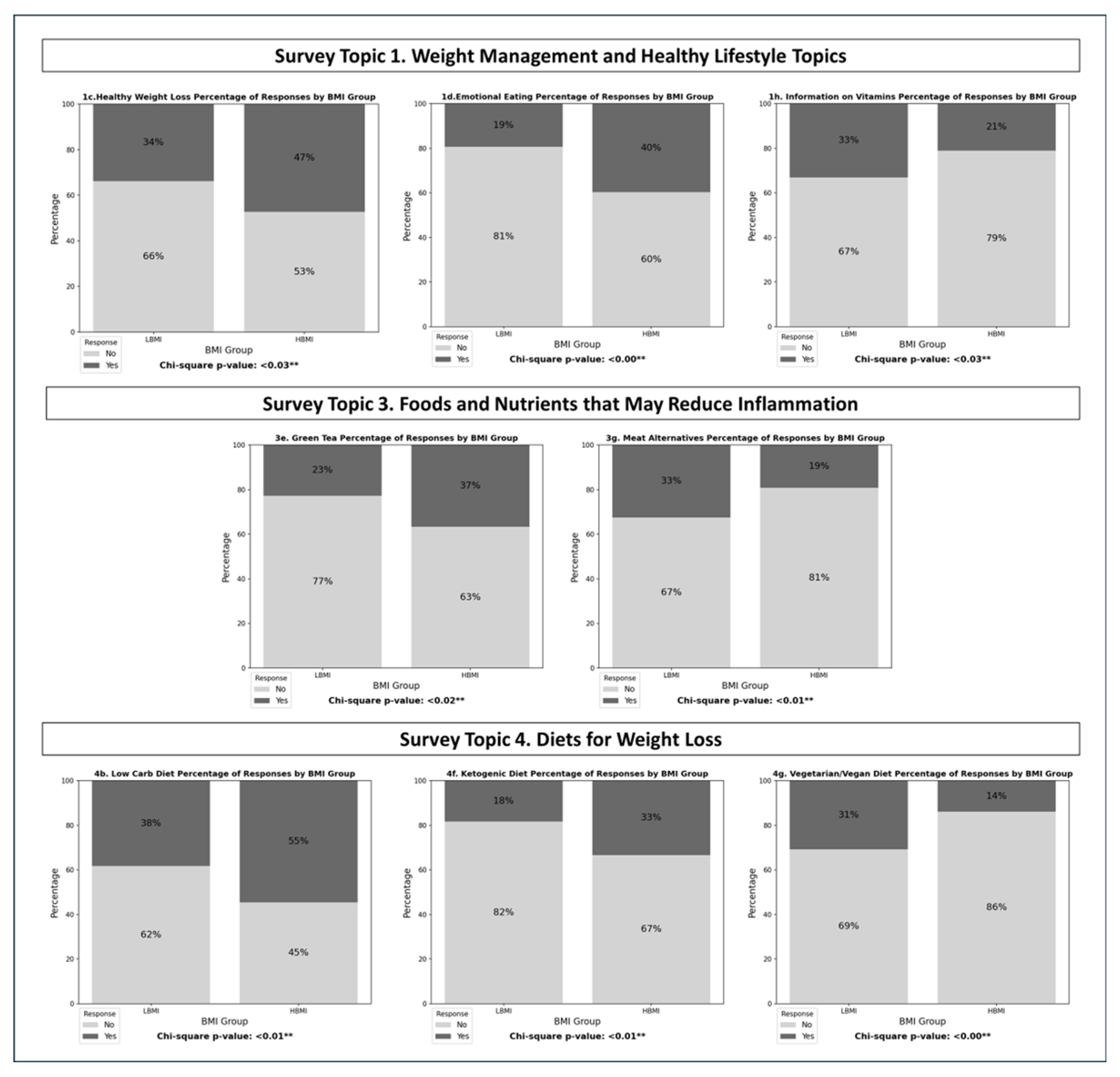
| 1c. Healthy weight loss for older adults | 82 | 42 | 91 | 81 | 0.031 |
| 1d. Ways to control emotional eating | 100 | 24 | 104 | 68 | 0.000 |
| 1e. Strategies to feel full | 89 | 35 | 109 | 63 | 0.164 |
| 1f. Information on affordable food options | 89 | 35 | 109 | 63 | 0.164 |
| 1g. Tips for healthy snacking | 82 | 42 | 123 | 49 | 0.388 |
| 1h. General info on vitamins and minerals | 83 | 41 | 136 | 36 | 0.027 |
| 1i. Awareness of food triggers | 93 | 31 | 127 | 45 | 0.927 |
| 1j. Special diet considerations | 101 | 23 | 124 | 48 | 0.085 |
| 1k. Mindful eating techniques | 97 | 27 | 146 | 26 | 0.187 |
| 1l. Ways to monitor/control caloric intake | 109 | 15 | 141 | 31 | 0.220 |
| 1m. Benefits to fasting/intermittent fasting | 107 | 17 | 136 | 36 | 0.148 |
| 1n. Impacts of organic vs. regular food | 102 | 22 | 154 | 18 | 0.102 |
| 1o. Ways to control portion size | 111 | 13 | 150 | 22 | 0.672 |
| 1p. Food labels to guide dietary decisions | 119 | 5 | 162 | 10 | 0.674 |
| 2. Vitamins, Minerals, and Supplements | |||||
| 2a. Glucosamine | 63 | 57 | 67 | 96 | 0.075 |
| 2b. Vitamin D | 56 | 64 | 87 | 76 | 0.320 |
| 2c. Omega 3 Fatty Acids | 59 | 61 | 89 | 74 | 0.433 |
| 2d. Calcium | 71 | 49 | 102 | 61 | 0.647 |
| 2e. Chondroitin | 81 | 39 | 100 | 63 | 0.347 |
| 2f. Amino Acids | 81 | 39 | 122 | 41 | 0.221 |
| 2g. Dietary Fiber | 91 | 29 | 126 | 37 | 0.884 |
| 3. Foods and Nutrients that may Reduce Inflammation | |||||
| 3a. Spices and Herbs | 38 | 85 | 59 | 108 | 0.506 |
| 3b. Fruits and Vegetables | 53 | 70 | 66 | 101 | 0.624 |
| 3c. Nuts | 78 | 45 | 97 | 70 | 0.426 |
| 3d. Olive Oil | 82 | 41 | 112 | 55 | 1.000 |
| 3e. Green Tea | 95 | 28 | 105 | 62 | 0.013 |
| 3f. Fish Oil | 86 | 37 | 132 | 35 | 0.101 |
| 3g. Plant-Based Alternatives for Meat | 83 | 40 | 135 | 32 | 0.014 |
| 4. Diets for Weight Loss | |||||
| 4a. Mediterranean Diet | 51 | 69 | 82 | 84 | 0.301 |
| 4b. Low Carbohydrate Diet | 74 | 46 | 76 | 90 | 0.011 |
| 4c. Fasting and Intermittent Fasting | 69 | 51 | 98 | 68 | 0.890 |
| 4d. High Protein Diet | 75 | 45 | 101 | 65 | 0.872 |
| 4e. DASH Diet | 91 | 29 | 114 | 52 | 0.233 |
| 4f. Ketogenic Diet | 98 | 22 | 110 | 56 | 0.006 |
| 4g. Vegetarian or Vegan Diets | 83 | 37 | 143 | 23 | 0.001 |
| Survey Topic Domain and Response | Entire Cohort (n = 296) | BMI: <30 kg/m2; n = 124 | BMI: ≥30 kg/m2; n = 172 |
|---|---|---|---|
| 1. Weight Management and Healthy Lifestyle Topics | 10 responses | 4 responses | 6 responses |
| Reduce sugar cravings | 1 (10%) | 1 (25%) | - |
| Foods with natural anti-inflammatory properties | 1 (10%) | 1 (25%) | - |
| Alcohol and OA | 1 (10%) | 1 (25%) | - |
| Easy meals and snacks for increased energy | 1 (10%) | 1 (25%) | - |
| Treatment for eating disorders | 3 (30%) | - | 3 (50%) |
| Easy-to-prepare meals | 1 (10%) | - | 1 (17%) |
| Losing weight while managing multiple health issues | 1 (10%) | - | 1 (17%) |
| Medications that cause weight gain | 1 (10%) | - | 1 (17%) |
| 2. Vitamins, Minerals, and Supplements | 20 responses | 11 responses | 9 responses |
| Collagen | 2 (10%) | 2 (18%) | - |
| THC | 2 (10%) | 2 (18%) | - |
| CBD | 2 (10%) | 2 (18%) | - |
| Hyaluronic Acid | 2 (10%) | 2 (18%) | - |
| B Vitamins | 3 (15%) | - | 3 (25%) |
| OTC Pain Relief | 2 (10%) | - | 2 (17%) |
| 3. Foods and Nutrients that Reduce Inflammation | 5 responses | 2 responses | 3 responses |
| Chocolate | 1 (20%) | 1 (50%) | - |
| Alternate foods if you have intestinal issues | 1 (20%) | 1 (50%) | - |
| Butter substitutes | 1 (20%) | - | 1 (33%) |
| Shellfish | 1 (20%) | - | 1 (33%) |
| Tart Cherry | 1 (20%) | - | 1 (33%) |
| 4. Diets for Weight Loss | 10 responses | 3 responses | 7 responses |
| MIND | 1 (10%) | 1 (33%) | - |
| Weight Watchers | 1 (10%) | 1 (33%) | - |
| Reduce sugar | 1 (10%) | 1 (33%) | - |
| Diets to improve health condition | 3 (33%) | - | 3 (43%) |
| Balanced eating plan | 3 (33%) | - | 3 (43%) |
| Southern recipes | 1 (10%) | - | 1 (14%) |
| 5. Other Nutrition Topics Related to Joint Health You Would like to Learn About? | 35 responses | 10 responses | 25 responses |
| Foods to manage inflammation and pain | 3 (9%) | 3 (30%) | - |
| Diets for joint health in those who deal with other chronic health conditions (IBS, GERD, etc.) | 3 (9%) | 3 (30%) | - |
| Foods to prevent joint deterioration/ improve joint health | 3 (9%) | 1 (10%) | 2 (8%) |
| Various foods on joint health (e.g., Asian diet, bone broth, white flour, Coke, Diet colas) | 6 (18%) | - | 6 (24%) |
| Foods to manage inflammation and pain | 6 (18%) | - | 6 (24%) |
| Foods that contribute to increased weight and pain | 2 (6%) | - | 2 (8%) |
| Nutritional choices can help for low density bones and prevention of fractures | 2 (6%) | - | 2 (8%) |
| 6. What other resources about joint health and nutrition you would you like? | 21 responses | 7 responses | 14 responses |
| Review articles—evidence-based information | 7 (33%) | 3 (43%) | 4 (29%) |
| Appointments with other specialists/support group (rheumatologist, post gastric bypass specialist, eating disorder counseling, nutritionist, support groups) | 6 (29%) | - | 6 (43%) |
| 7. Summary of additional information noted but not related to nutrition. | 25 responses | 13 responses | 12 responses |
| Additional non-medical treatments (physical therapy, exercise, massage, etc.) | 6 (24%) | 6 (46%) | - |
| Relationship between OA and PCOS | 2 (8%) | 2 (15%) | - |
| Exercise and joint mobility (with joint pain) | 2 (8%) | - | 2 (15%) |
| Is losing weight fast to reduce stress on joints better than losing weight slowly and nutritiously? | 2 (8%) | - | 2 (15%) |
| 8. Irrelevant Responses | 15 responses | 6 responses | 9 responses |
| Responses that were given but deemed not relevant. | 15 (100%) | 6 (100%) | 9 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buck, A.N.; Gross, D.C.; Kim, J.; Rauff, E.L.; Dinallo, J.M.; Abbate, L.M.; Schwartz, T.A.; Beresic, N.J.; Newman, C.B.; Shultz, S.P. BMI-Specific Nutritional Education Priorities for Weight Management in Osteoarthritis. Nutrients 2025, 17, 2056. https://doi.org/10.3390/nu17132056
Buck AN, Gross DC, Kim J, Rauff EL, Dinallo JM, Abbate LM, Schwartz TA, Beresic NJ, Newman CB, Shultz SP. BMI-Specific Nutritional Education Priorities for Weight Management in Osteoarthritis. Nutrients. 2025; 17(13):2056. https://doi.org/10.3390/nu17132056
Chicago/Turabian StyleBuck, Ashley N., Danae C. Gross, Jieun (Jenna) Kim, Erica L. Rauff, Jennifer M. Dinallo, Lauren M. Abbate, Todd A. Schwartz, Nicholas J. Beresic, Connie B. Newman, and Sarah P. Shultz. 2025. "BMI-Specific Nutritional Education Priorities for Weight Management in Osteoarthritis" Nutrients 17, no. 13: 2056. https://doi.org/10.3390/nu17132056
APA StyleBuck, A. N., Gross, D. C., Kim, J., Rauff, E. L., Dinallo, J. M., Abbate, L. M., Schwartz, T. A., Beresic, N. J., Newman, C. B., & Shultz, S. P. (2025). BMI-Specific Nutritional Education Priorities for Weight Management in Osteoarthritis. Nutrients, 17(13), 2056. https://doi.org/10.3390/nu17132056







