Modulation of Gut Microbiota by Whole Encapsulated Brown Seaweed (Himanthalia elongata) in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
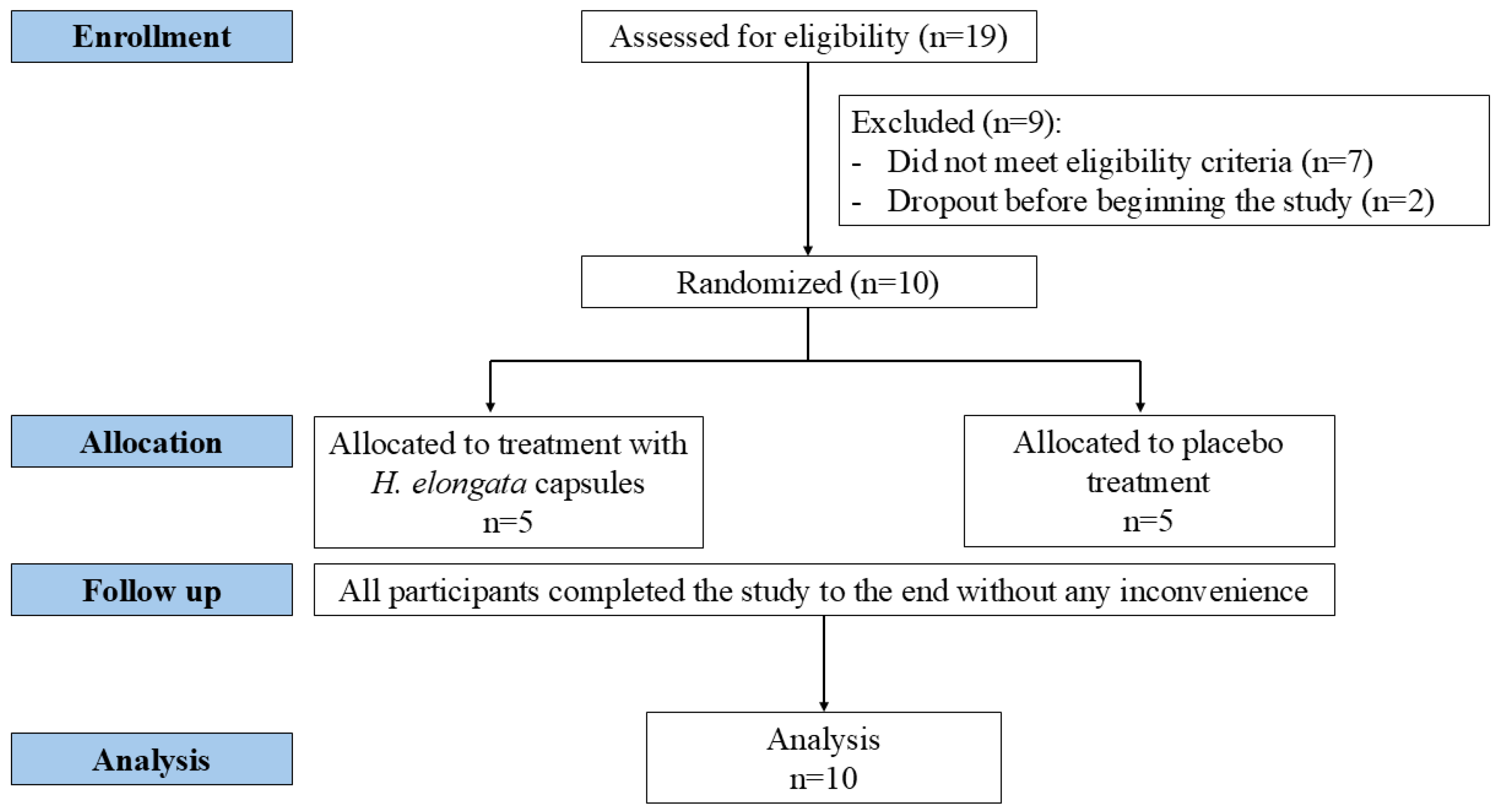
2.2. Participants, Study Design, and Intervention
2.3. Fecal Sample Collection and Dietary Intake Data
2.4. DNA Extraction and 16S Ribosomal RNA Amplicon Sequencing
2.5. Short-Chain Fatty Acid Analysis
2.6. Statistical and Bioinformatics Analysis
3. Results and Discussion
3.1. Anthropometric Characteristics of the Participants
3.2. Assessment of the Dietary Intake
3.3. Effects of Himanthalia elongata Intake on the Gut Microbiota
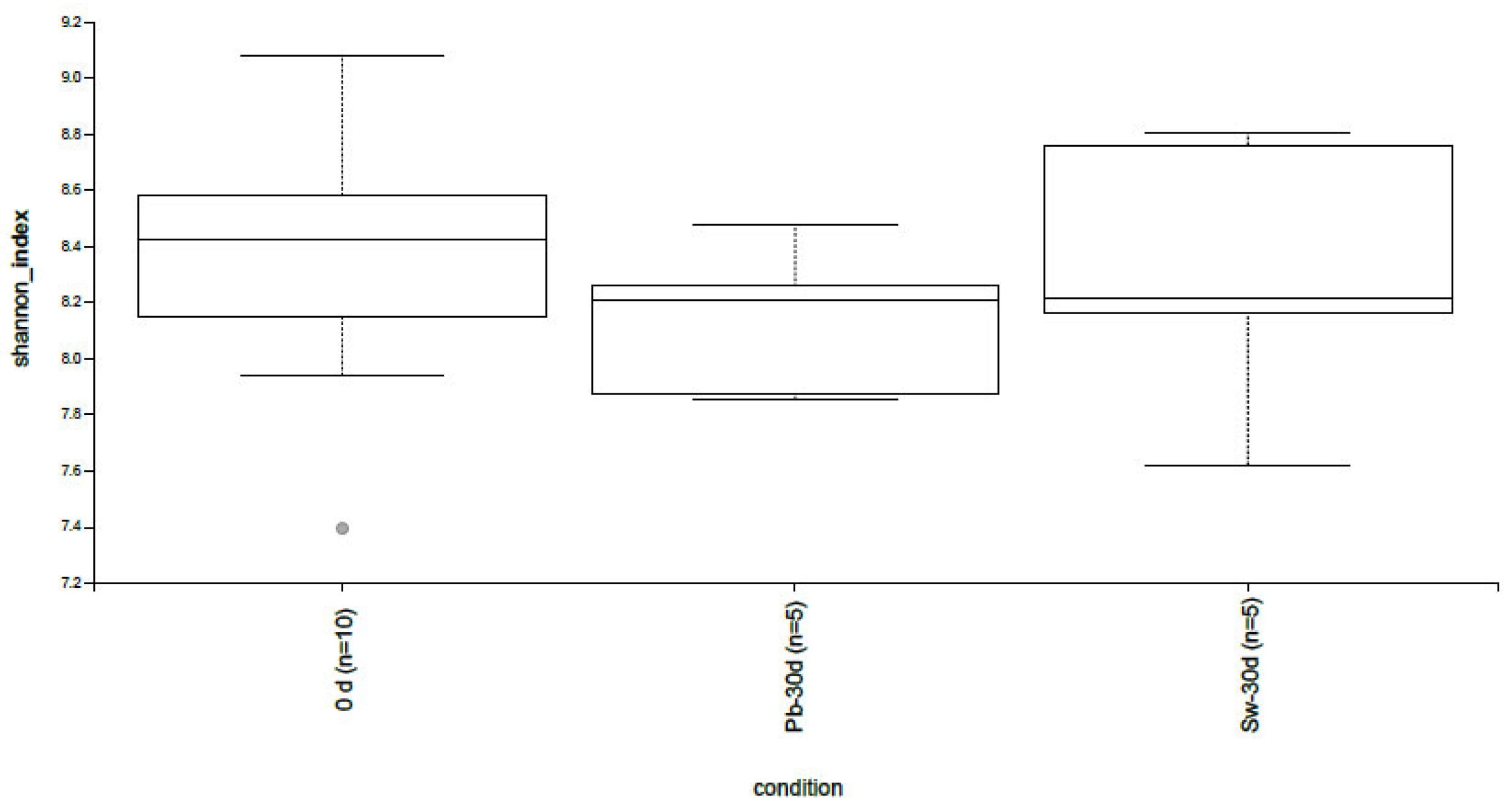
3.3.1. Alpha and Beta Diversity
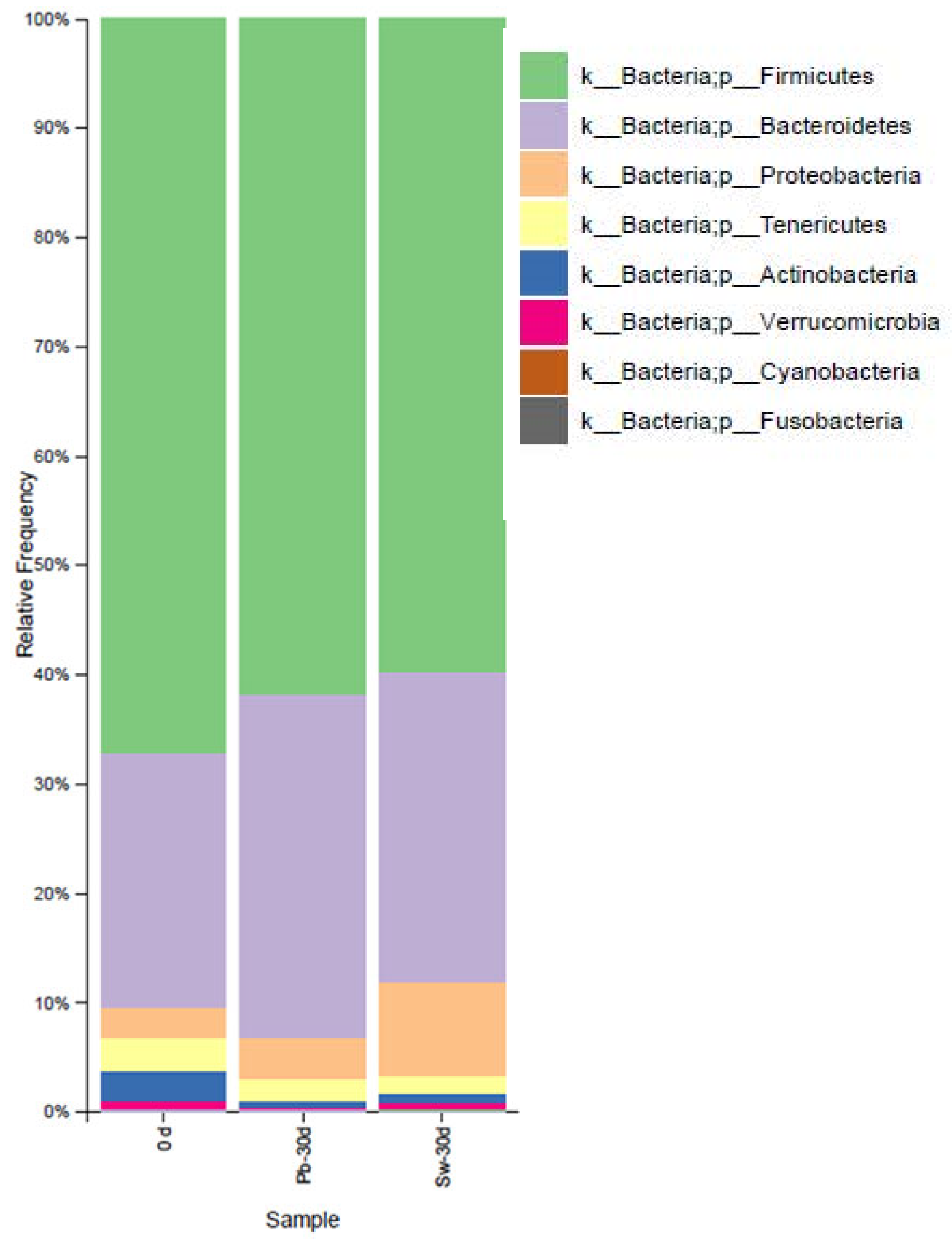
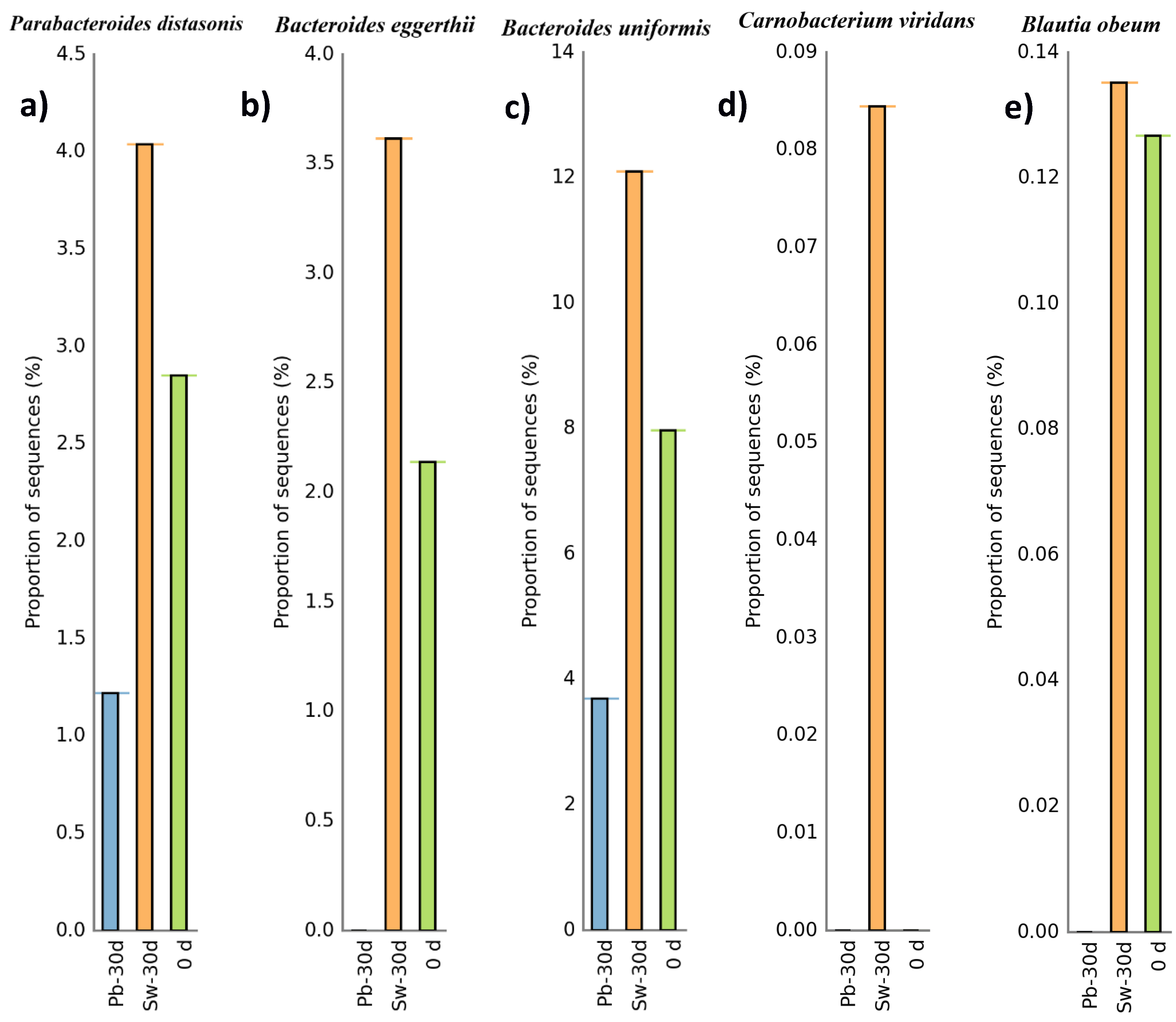
3.3.2. Evolution of Bacteria at the Phylum and Species Levels
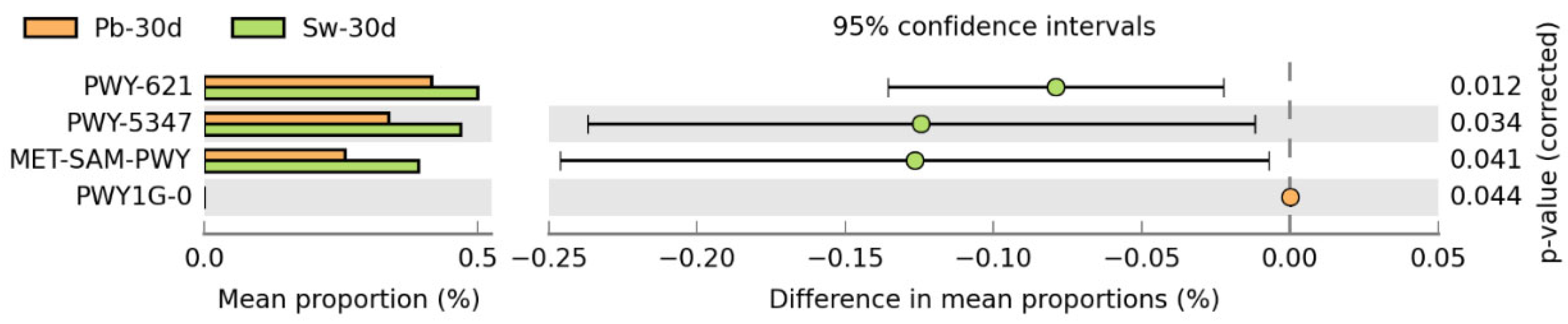
3.3.3. Metabolic Pathways
3.4. Analysis of Short-Chain Fatty Acids
4. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D.; Petitfils, C.; De Vos, W.M.; Tlg, G.; El-Omar, E.M. What defines a healthy gut microbiome? Gut 2024, 73, 1893–1908. [Google Scholar] [CrossRef]
- Alagiakrishnan, K.; Morgadinho, J.; Halverson, T. Approach to the diagnosis and management of dysbiosis. Front. Nutr. 2024, 11, 1330903. [Google Scholar] [CrossRef]
- Mazhar, M.; Zhu, Y.; Qin, L. The interplay of dietary fibers and intestinal microbiota affects type 2 diabetes by generating short-chain fatty acids. Foods 2023, 12, 1023. [Google Scholar] [CrossRef]
- Fang, S.; Chen, X.; Ye, X.; Zhou, L.; Xue, S.; Gan, Q. Effects of gut microbiome and short-chain fatty acids (SCFAs) on finishing weight of meat rabbits. Front. Microbiol. 2020, 11, 1835. [Google Scholar] [CrossRef]
- Ren, X.; Cai, S.; Zhong, Y.; Tang, L.; Xiao, M.; Li, S.; Zhu, C.; Li, D.; Mou, H.; Fu, X. Marine-derived fucose-containing carbohydrates: Review of sources, structure, and beneficial effects on gastrointestinal health. Foods 2024, 13, 3460. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary Reference Values for nutrients: Summary report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar] [CrossRef]
- Julia, C.; Martinez, L.; Allès, B.; Touvier, M.; Hercberg, S.; Méjean, C.; Kesse-Guyot, E. Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Santé study. Public Health Nutr. 2018, 21, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.K.; Renuka, R.; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: A review. Front. Microbiol. 2017, 8, 563. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.d.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-origin prebiotics based on chitin: An alternative for the future? A critical review. Foods 2020, 9, 782. [Google Scholar] [CrossRef]
- Giller, K.E.; Delaune, T.; Silva, J.V.; Descheemaeker, K.; van de Ven, G.; Schut, A.G.T.; van Wijk, M.; Hammond, J.; Hochman, Z.; Taulya, G.; et al. The future of farming: Who will produce our food? Food Sec. 2021, 13, 1073–1099. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series 916; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Pereira, L.; Cotas, J. Seaweed: A sustainable solution for greening drug manufacturing in the pursuit of sustainable healthcare. Explor. Drug Sci. 2024, 2, 50–84. [Google Scholar] [CrossRef]
- Forster, J.; Radulovich, R. Seaweed and food security. In Seaweed Sustainability: Food and Non Food Applications, 1st ed.; Tiwari, B.K., Troy, D.J., Eds.; Elsevier: London, UK, 2015; Chapter 11; pp. 289–313. [Google Scholar]
- Ma, M.; Quan, M.; Zhang, J.; Zhang, A.; Gao, P.; Shang, Q.; Yu, G. In vitro fermentation of polysaccharide from edible alga Enteromorpha clathrata by the gut microbiota of patients with ulcerative colitis. Nutrients 2023, 15, 4122. [Google Scholar] [CrossRef]
- Biancarosa, I.; Belghit, I.; Bruckner, C.G.; Liland, N.S.; Waagbø, R.; Amlund, H.; Heesch, S.; Lock, E.J. Chemical characterization of 21 species of marine macroalgae common in Norwegian waters: Benefits of and limitations to their potential use in food and feed. J. Sci. Food Agric. 2018, 98, 2035–2042. [Google Scholar] [CrossRef]
- Poole, J.; Diop, A.; Rainville, L.C.; Barnabe, S. Bioextracting polyphenols from the brown seaweed Ascophyllum nodosum from Québec’s North Shore coastline. Ind. Biotechnol. 2019, 15, 212–218. [Google Scholar] [CrossRef]
- Wekre, M.E.; Kåsin, K.; Underhaug, J.; Jordheim, M.; Holmelid, B. Quantification of polyphenols in seaweeds: A case study of Ulva intestinalis. Antioxidants 2019, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, S.; Maheswari, P.; Sasikala, V.; Jaya Rubika, J.; Pandiarajan, J. In vitro antioxidant study of polyphenol from red seaweeds dichotomously branched Gracilaria edulis and robust sea moss Hypnea valentiae. Toxicol. Rep. 2021, 8, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.; Kumar, M.S. Gut microbial metabolites of dietary polyphenols and their potential role in human health and diseases. J. Physiol. Biochem. 2023, 79, 695–718. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Sinisterra-Loaiza, L.; Mondragón-Portocarrero, A.; Ortiz-Viedma, J.; Cardelle-Cobas, A.; Franco Abuín, C.M.; Cepeda, A. Potential prebiotic effect of two Atlantic whole brown seaweeds, Saccharina japonica and Undaria pinnatifida, using in vitro simulation of distal colonic fermentation. Front. Nutr. 2023, 10, 1170392. [Google Scholar] [CrossRef]
- Praveen, M.A.; Parvathy, K.R.K.; Jayabalan, R.; Balasubramanian, P. Dietary fiber from Indian edible seaweeds and its in vitro prebiotic effect on the gut microbiota. Food Hydrocoll. 2019, 96, 343–353. [Google Scholar] [CrossRef]
- Kansandee, W.; Moonmangmee, S.; Vangpikul, S.; Kosawatpat, P.; Tamtin, M. Physicochemical properties and in vitro prebiotic activity of Ulva rigida polysaccharides. Biocatal. Agric. Biotechnol. 2024, 59, 103252. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, Y.; Pan, L.; Niu, Q.; Li, C.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary polysaccharide from Enteromorpha clathrata modulates gut microbiota and promotes the growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp. Mar. Drugs. 2018, 16, 167. [Google Scholar] [CrossRef]
- Ren, X.; Liu, L.; Gamallat, Y.; Zhang, B.; Xin, Y. Enteromorpha and polysaccharides from Enteromorpha ameliorate loperamide-induced constipation in mice. Biomed. Pharmacother. 2017, 96, 1075–1081. [Google Scholar] [CrossRef]
- Seong, H.; Bae, J.H.; Seo, J.S.; Kim, S.A.; Kim, T.J.; Han, N.S. Comparative analysis of prebiotic effects of seaweed polysaccharides laminaran, porphyran, and ulvan using in vitro human fecal fermentation. J. Funct. Foods 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Organic Law 3/2018 of 5 December on the Protection of Personal Data and Guarantee of Digital Rights. BOE. No. 294. 6 December 2018, pp. 119788–119857. Available online: https://www.boe.es/eli/es/lo/2018/12/05/3 (accessed on 10 April 2025).
- Research Center for Endocrinology and Clinical Nutrition (IENVA). Diet Calculator; University of Valladolid: Valladolid, Spain, 1995. [Google Scholar]
- Sociedad Española de Nutrición Comunitaria (SENC). Guía de la Alimentación Saludable para Atención Primaria y Colectivos Ciudadanos. 2020. Available online: https://www.nutricioncomunitaria.org/es/noticia/guia-alimentacion-saludable-2020 (accessed on 21 May 2025).
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials (accessed on 16 June 2025).
- Food and Nutrition Board (FNB). Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- López-Santamarina, A.; Roade-Pérez, P.; Mondragón-Portocarrero, A.C.; Cardelle-Cobas, A.; Cepeda, A.; Miranda, J.M. Effects of a green seaweed from the Atlantic coast (Ulva lactuca) on gut microbiota, using an in vitro colon model. Food Biosci. 2025, 63, 105596. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhover, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Poslusna, K.; Ruprich, J.; de Vries, J.H.; Jakubikova, M.; van’t Veer, P. Misreporting of energy and micronutrient intake estimated by food records and 24-hour recalls, control and adjustment methods in practice. Br. J. Nutr. 2009, 101 (Suppl. S2), S73–S85. [Google Scholar] [CrossRef]
- Schwab, U.; Lauritzen, L.; Tholstrup, T.; Haldorssoni, T.; Riseurs, U.; Uusitupa, M.; Becker, W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014, 58, 25145. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Hao Zhu, T.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Dalamaga, M.; Tsigalou, C. Diet patterns, gut microbiota and metabolic disorders: Perspectives and challenges. Metabol. Open 2024, 23, 100310. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G. Seaweeds: A sustainable feed source for livestock and aquaculture. Trends Food Sci. Technol. 2015, 45, 145–153. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from seaweeds: An ocean of opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef]
- Zhang, X.; Su, C.; Cao, C.; Gong, G.; Huang, L.; Wang, Z.; Song, S.; Zhu, B. Gut microbiota of individuals could be balanced by a 14-day supplementation with Laminaria japonica and differed in metabolizing alginate and galactofucan. Front. Nutr. 2022, 9, 881464. [Google Scholar] [CrossRef]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Personalized Microbiome Class Students; et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Roca-Saavedra, P.; Mendez-Vilabrille, V.; Miranda, J.M.; Nebot, C.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Food additives, contaminants and other minor components: Effects on human gut microbiota—A review. J. Physiol. Biochem. 2018, 74, 69–83. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Cardelle-Cobas, A.; Del Carmen Mondragon, A.; Sinisterra-Loaiza, L.; Miranda, J.M.; Cepeda, A. Evaluation of the potential prebiotic effect of Himanthalia elongata, an Atlantic brown seaweed, in an in vitro model of the human distal colon. Food Res. Int. 2022, 156, 111156. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wong, C.C.; Jia, Z.; Liu, W.; Liu, C.; Ji, F.; Pan, Y.; Wang, F.; Wang, G.; Zhao, L.; et al. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat. Microbiol. 2023, 8, 1534–1548. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Tillotson, G.; MacKenzie, T.N.; Warren, C.A.; Wexler, H.M.; Goldstein, E.J.C. Bacteroides and related species: The keystone taxa of the human gut microbiota. Anaerobe 2024, 85, 102819. [Google Scholar] [CrossRef]
- Zhang, S.; Nie, Q.; Sun, Y.; Zuo, S.; Chen, C.; Li, S.; Yang, J.; Hu, J.; Zhou, X.; Yu, Y.; et al. Bacteroides uniformis degrades β-glucan and enhances Lactobacillus growth through cross-feeding. Microbiome 2024, 12, 62. [Google Scholar] [CrossRef]
- Leisner, J.J.; Laursen, B.G.; Prévost, H.; Drider, D.; Dalgaard, P. Carnobacterium: Positive and negative effects in the environment and in foods. FEMS Microbiol. Rev. 2007, 31, 592–613. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhi, F. Lower level of Bacteroides in the gut microbiota is associated with inflammatory bowel disease: A meta-analysis. BioMed Res. Int. 2016, 2016, 5828959. [Google Scholar] [CrossRef]
- Delday, M.; Mulder, I.; Logan, E.T.; Grant, G. Bacteroides thetaiotaomicron ameliorates colon inflammation in preclinical models of Crohn’s disease. IBD 2019, 25, 85–96. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. App. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Li, T.T.; Chen, X.; Huo, D.; Arifuzzaman, M.; Qiao, S.; Jin, W.B.; Shi, H.; Li, X.V.; JRI Live Cell Bank Consortium; Iliev, I.D.; et al. Microbiota metabolism of intestinal amino acids impacts host nutrient homeostasis and physiology. Cell Host Microbe 2024, 32, 661–675.e10. [Google Scholar] [CrossRef]
- Yao, W.; Kong, Q.; You, L.; Zhong, S.; Hileuskaya, K. Polysaccharides from brown seaweed: Physicochemical properties, absorption in the intestine, and beneficial effects on intestinal barrier. Food Front. 2023, 4, 1547–1560. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ramos, D.; Lopitz-Otsoa, F.; Lu, S.C.; Mato, J.M.S. Adenosylmethionine: A multifaceted regulator in cancer pathogenesis and therapy. Cancers 2025, 17, 535. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.W.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation-current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Sig. Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fiber and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short-chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short-chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
| Participant | Group | BMI (kg/m2) | Waist Circumference (cm) |
|---|---|---|---|
| P1 | Placebo | 33.9 | 110.2 |
| P2 | Placebo | 32.9 | 107.6 |
| P3 | Placebo | 32.1 | 105.1 |
| P4 | Placebo | 25.1 | 88.4 |
| P5 | Placebo | 27.9 | 87.1 |
| P6 | H. elongata | 26.3 | 86.5 |
| P7 | H. elongata | 30.1 | 101.2 |
| P8 | H. elongata | 27.2 | 95.4 |
| P9 | H. elongata | 28.4 | 97.6 |
| P10 | H. elongata | 30.5 | 102.5 |
| Mean | SD | Median | Q1 | Q3 | IQR | |
|---|---|---|---|---|---|---|
| Water 1 | 2427.1 | 393.0 | 2361.7 | 2120.8 | 2699.8 | 2120.8–2699.8 |
| Energy 2 | 1724.8 | 888.8 | 1463.4 | 1061.5 | 2401.1 | 1061.5–2401.1 |
| Protein 1 | 83.8 | 35.2 | 69.0 | 64.4 | 95.6 | 64.4–95.6 |
| Total fat 1 | 76.8 | 48.0 | 67.5 | 49.2 | 90.5 | 49.2–90.5 |
| Carbohydrates 1 | 174.9 | 98.9 | 148.0 | 89.9 | 255.1 | 89.9–255.1 |
| Sugars 1 | 70.6 | 41.5 | 56.4 | 46.7 | 66.5 | 46.7–66.5 |
| Starch 1 | 104.3 | 77.4 | 89.2 | 43.6 | 127.3 | 43.6–127.3 |
| Dietary fiber 1 | 17.2 | 9.9 | 18.0 | 9.6 | 19.2 | 9.6–19.2 |
| Saturated fat 1 | 27.6 | 20.2 | 24.1 | 12.3 | 36.9 | 12.3–36.9 |
| Monounsaturated fat 1 | 28.8 | 19.0 | 22.4 | 19.6 | 32.9 | 19.6–32.9 |
| – | 11.7 | 5.5 | 12.5 | 9.5 | 12.8 | 9.5–12.8 |
| Cholesterol 3 | 257.7 | 170.4 | 249.0 | 130.9 | 414.6 | 130.9–414.6 |
| Alcohol 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| Mean | SD | Median | Q1 | Q3 | IQR | |
|---|---|---|---|---|---|---|
| Water 1 | 2256.9 | 877.9 | 1988.1 | 1913.2 | 2971.1 | 1913.2–2971.1 |
| Energy 2 | 1947.0 | 918.9 | 1734.4 | 1392.9 | 2143.1 | 1392.9–2143.1 |
| Protein 1 | 100.0 | 39.6 | 93.1 | 73.2 | 107.7 | 73.2–107.7 |
| Total fat 1 | 84.8 | 49.2 | 75.8 | 42.4 | 98.5 | 42.4–98.5 |
| Carbohydrates 1 | 190.1 | 103.1 | 171.9 | 108.8 | 273.4 | 108.8–273.4 |
| Sugars 1 | 67.6 | 55.0 | 44.4 | 32.9 | 65.2 | 32.9–65.2 |
| Starch 1 | 122.6 | 69.2 | 119.7 | 63.4 | 158.5 | 63.4–158.5 |
| Dietary fiber 1 | 14.7 | 5.6 | 14.9 | 10.7 | 18.7 | 10.7–18.7 |
| Saturated fat 1 | 30.5 | 19.9 | 23.8 | 15.8 | 37.6 | 15.8–37.6 |
| Monounsaturated fat 1 | 34.1 | 20.4 | 29.7 | 18.0 | 44.9 | 18.0–44.9 |
| Polyunsaturated fat 1 | 11.8 | 7.5 | 9.5 | 5.0 | 16.0 | 5.0–16.0 |
| Cholesterol 3 | 365.9 | 224.2 | 391.8 | 170.9 | 480.5 | 170.9–480.5 |
| Alcohol 1 | 3.4 | 8.0 | 0.0 | 0.0 | 0.0 | - |
| Mean | SD | Median | Q1 | Q3 | IQR | |
|---|---|---|---|---|---|---|
| Calcium 1 | 1151.4 | 724.5 | 951.5 | 801.4 | 1058.1 | 801.4–1058.1 |
| Iron 1 | 9.8 | 4.7 | 9.6 | 6.3 | 10.6 | 6.3–10.6 |
| Iodine 2 | 342.0 | 210.7 | 350.5 | 185.0 | 499.7 | 185.0–499.7 |
| Magnesium 1 | 295.8 | 93.4 | 253.2 | 247.5 | 330.7 | 247.5–330.7 |
| Zinc 1 | 7.9 | 3.9 | 5.8 | 5.2 | 10.1 | 5.2–10.1 |
| Natrium 1 | 2277.7 | 1043.3 | 1894.7 | 1495.6 | 2806.3 | 1495.6–2806.3 |
| Potassium 1 | 2878.3 | 915.8 | 2456.6 | 2206.6 | 3656.8 | 2206.6–3656.8 |
| Phosphorous 1 | 1581.8 | 540.2 | 1641.7 | 1308.8 | 1711.7 | 1308.8–1711.7 |
| Selenium 2 | 83.5 | 34.1 | 98.7 | 68.2 | 106.4 | 68.2–106.4 |
| Vitamin B1 1 | 1.2 | 0.7 | 1.0 | 0.8 | 1.2 | 0.8–1.2 |
| Vitamin B2 1 | 1.7 | 0.8 | 1.5 | 1.3 | 1.5 | 1.3–1.5 |
| Vitamin B3 1 | 30.8 | 14.9 | 27.6 | 25.2 | 30.7 | 25.2–30.7 |
| Vitamin B6 1 | 1.6 | 0.3 | 1.5 | 1.4 | 1.9 | 1.4–1.9 |
| Folic acid 2 | 147.6 | 65.0 | 129.4 | 93.6 | 187.1 | 93.6–187.1 |
| Vitamin B12 2 | 4.9 | 3.9 | 3.8 | 1.8 | 8.5 | 1.8–8.5 |
| Vitamin C 1 | 73.7 | 30.4 | 68.7 | 57.0 | 91.3 | 57.0–91.3 |
| Vitamin A 2 | 878.7 | 494.7 | 756.4 | 655.2 | 918.5 | 655.2–918.5 |
| Retinol 2 | 485.0 | 338.6 | 533.4 | 257.5 | 738.2 | 257.5–738.2 |
| Carotene 2 | 2442.1 | 2550.0 | 2188.4 | 182.3 | 4060.7 | 182.3–4060.7 |
| Vitamin D 2 | 4.1 | 4.8 | 1.8 | 0.8 | 8.2 | 0.8–8.2 |
| Vitamin E 1 | 5.5 | 3.6 | 4.3 | 2.8 | 7.4 | 2.8–7.4 |
| Mean | SD | Median | Q1 | Q3 | IQR | |
|---|---|---|---|---|---|---|
| Calcium 1 | 944.5 | 505.3 | 831.9 | 775.9 | 831.9 | 775.9–831.9 |
| Iron 1 | 11.4 | 5.2 | 10.1 | 8.8 | 10.1 | 8.8–10.1 |
| Iodine 2 | 269.1 | 119.6 | 245.1 | 216.0 | 245.1 | 216.0–245.1 |
| Magnesium 1 | 287.1 | 97.6 | 272.2 | 208.4 | 272.2 | 208.4–272.2 |
| Zinc 1 | 12.1 | 5.9 | 12.7 | 8.9 | 12.7 | 8.9–12.7 |
| Natrium 1 | 2973.2 | 1235.6 | 2629.2 | 2041.4 | 2629.2 | 2041.4–2629.2 |
| Potassium 1 | 3134.0 | 1470.8 | 2943.8 | 2127.7 | 2943.8 | 2127.7–2943.8 |
| Phosphorous 1 | 1518.5 | 566.5 | 1410.1 | 1090.8 | 1410.1 | 1090.8–1410.1 |
| Selenium 2 | 98.2 | 59.8 | 87.5 | 58.5 | 87.5 | 58.5–87.5 |
| Vitamin B1 1 | 1.5 | 0.7 | 1.3 | 1.2 | 1.3 | 1.2–1.3 |
| Vitamin B2 1 | 1.6 | 0.8 | 1.5 | 1.1 | 1.5 | 1.1–1.5 |
| Vitamin B3 1 | 38.4 | 18.5 | 31.7 | 24.5 | 31.7 | 24.5–31.7 |
| Vitamin B6 1 | 2.0 | 1.0 | 1.6 | 1.4 | 1.6 | 1.4–1.6 |
| Folic acid 2 | 179.5 | 75.2 | 167.3 | 129.3 | 167.3 | 129.3–167.3 |
| Vitamin B12 2 | 6.8 | 5.2 | 5.4 | 4.3 | 5.4 | 4.3–5.4 |
| Vitamin C 1 | 86.4 | 56.9 | 79.9 | 38.3 | 79.9 | 38.3–79.9 |
| Vitamin A 2 | 745.9 | 732.6 | 416.4 | 211.4 | 416.4 | 211.4–416.4 |
| Retinol 2 | 245.1 | 198.6 | 226.1 | 129.7 | 226.1 | 129.7–226.1 |
| Carotene 2 | 3239.8 | 4277.8 | 957.0 | 336.0 | 957.0 | 336.0–957.0 |
| Vitamin D 2 | 3.6 | 4.5 | 1.6 | 0.5 | 1.6 | 0.5–1.6 |
| Vitamin E 1 | 5.8 | 3.7 | 4.7 | 3.5 | 4.7 | 3.5–4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Santamarina, A.; Cardelle-Cobas, A.; Mondragon, A.d.C.; Cepeda, A.; Rodriguez, J.A.; Miranda, J.M. Modulation of Gut Microbiota by Whole Encapsulated Brown Seaweed (Himanthalia elongata) in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2025, 17, 2047. https://doi.org/10.3390/nu17122047
Lopez-Santamarina A, Cardelle-Cobas A, Mondragon AdC, Cepeda A, Rodriguez JA, Miranda JM. Modulation of Gut Microbiota by Whole Encapsulated Brown Seaweed (Himanthalia elongata) in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Trial. Nutrients. 2025; 17(12):2047. https://doi.org/10.3390/nu17122047
Chicago/Turabian StyleLopez-Santamarina, Aroa, Alejandra Cardelle-Cobas, Alicia del Carmen Mondragon, Alberto Cepeda, Jose A. Rodriguez, and Jose Manuel Miranda. 2025. "Modulation of Gut Microbiota by Whole Encapsulated Brown Seaweed (Himanthalia elongata) in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Trial" Nutrients 17, no. 12: 2047. https://doi.org/10.3390/nu17122047
APA StyleLopez-Santamarina, A., Cardelle-Cobas, A., Mondragon, A. d. C., Cepeda, A., Rodriguez, J. A., & Miranda, J. M. (2025). Modulation of Gut Microbiota by Whole Encapsulated Brown Seaweed (Himanthalia elongata) in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Trial. Nutrients, 17(12), 2047. https://doi.org/10.3390/nu17122047











