Efficacy of a Low-FODMAP Diet on the Severity of Gastrointestinal Symptoms and Quality of Life in the Treatment of Gastrointestinal Disorders—A Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
3. Results
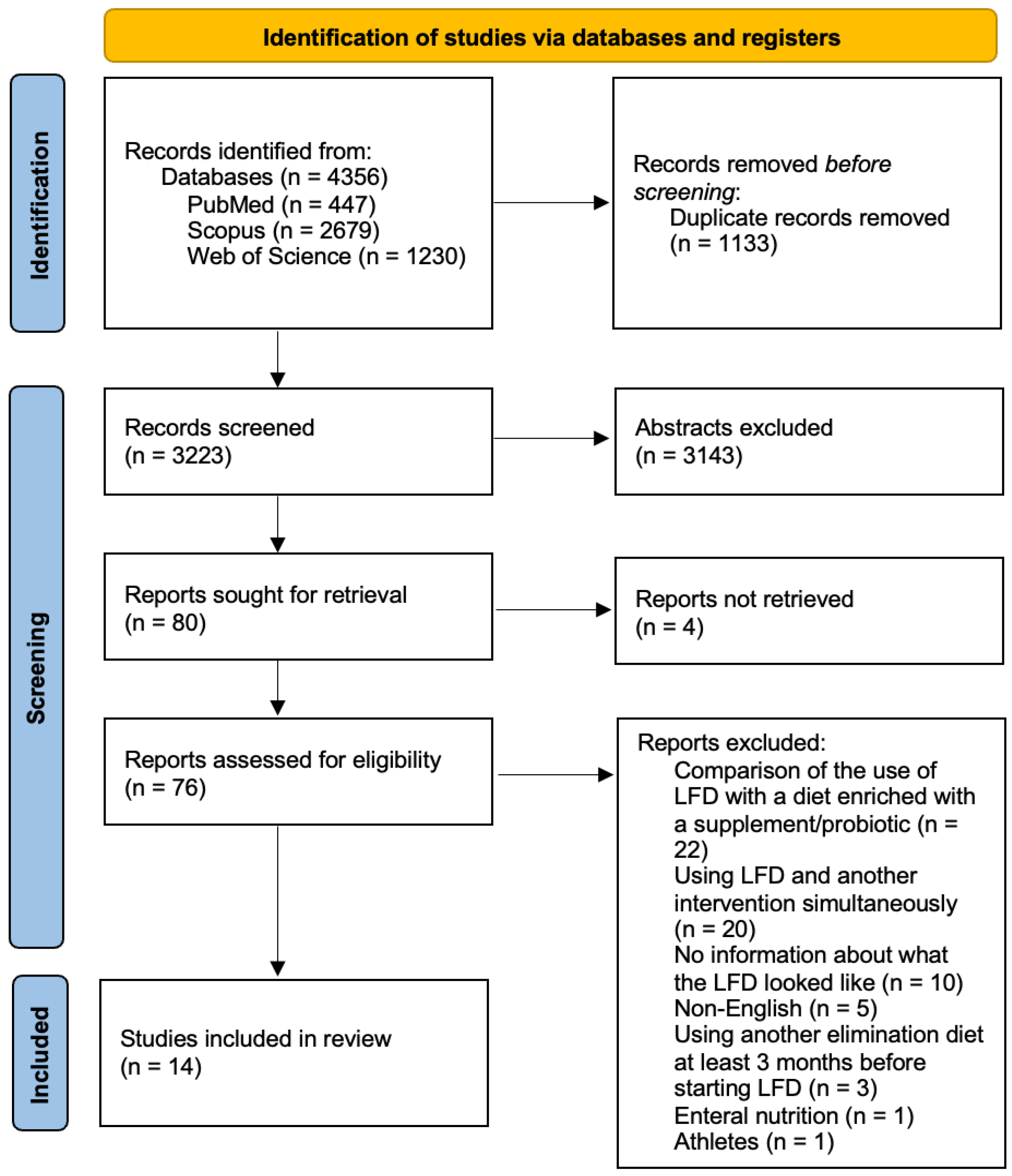
3.1. Trial Selection
3.2. Characteristics of the Trials
3.3. Study Quality
3.4. Outcomes of Interest
3.5. Effect of the LFD in IBS
3.6. Effect of the LFD in IBD
3.7. Effect of the LFD in Symptomatic PPI Refractory GERD
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- van Lanen, A.S.; de Bree, A.; Greyling, A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 3505–3522. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut 2022, 71, 1117–1126. [Google Scholar] [CrossRef]
- Khalighi Sikaroudi, M.; Soltani, S.; Ghoreishy, S.M.; Ebrahimi, Z.; Shidfar, F.; Dehnad, A. Effects of a low FODMAP diet on the symptom management of patients with irritable bowel syndrome: A systematic umbrella review with the meta-analysis of clinical trials. Food Funct. 2024, 15, 5195–5208. [Google Scholar] [CrossRef] [PubMed]
- Algera, J.P.; Demir, D.; Törnblom, H.; Nybacka, S.; Simrén, M.; Störsrud, S. Low FODMAP diet reduces gastrointestinal symptoms in irritable bowel syndrome and clinical response could be predicted by symptom severity: A randomized crossover trial. Clin. Nutr. 2022, 41, 2792–2800. [Google Scholar] [CrossRef]
- Nybacka, S.; Törnblom, H.; Josefsson, A.; Hreinsson, J.P.; Böhn, L.; Frändemark, Å.; Weznaver, C.; Störsrud, S.; Simrén, M. A low FODMAP diet plus traditional dietary advice versus a low-carbohydrate diet versus pharmacological treatment in irritable bowel syndrome (CARIBS): A single-centre, single-blind, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2024, 9, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Herfindal, A.M.; van Megen, F.; Gilde, M.K.O.; Valeur, J.; Rudi, K.; Skodje, G.I.; Lundin, K.E.A.; Henriksen, C.; Bøhn, S.K. Effects of a low FODMAP diet on gut microbiota in individuals with treated coeliac disease having persistent gastrointestinal symptoms—A randomised controlled trial. Br. J. Nutr. 2023, 130, 2061–2075. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, L.; Wang, X.; Fox, M.; Luo, L.; Du, L.; Chen, B.; Chen, X.; He, H.; Zhu, S.; et al. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet compared with traditional dietary advice for diarrhea-predominant irritable bowel syndrome: A parallel-group, randomized controlled trial with analysis of clinical and microbiological factors associated with patient outcomes. Am. J. Clin. Nutr. 2021, 113, 1531–1545. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: A randomized controlled trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2016, 66, 1241–1251. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut 2018, 67, 872–881. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Critical Appraisal Skills Programme. CASP Randomised Controlled Trial Checklist. 2020. Available online: https://Casp-Uk.Net/Casp-Tools-Checklists/ (accessed on 23 April 2025).
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients with Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188. [Google Scholar] [CrossRef]
- Ostrowska, L.; Wasiluk, D.; Lieners, C.F.J.; Gałęcka, M.; Bartnicka, A.; Tveiten, D. Igg Food Antibody Guided Elimination-Rotation Diet Was More Effective than FODMAP Diet and Control Diet in the Treatment of Women with Mixed IBS-Results from an Open Label Study. J. Clin. Med. 2021, 10, 4317. [Google Scholar] [CrossRef]
- Rej, A.; Sanders, D.S.; Shaw, C.C.; Buckle, R.; Trott, N.; Agrawal, A.; Aziz, I. Efficacy and Acceptability of Dietary Therapies in Non-Constipated Irritable Bowel Syndrome: A Randomized Trial of Traditional Dietary Advice, the Low FODMAP Diet, and the Gluten-Free Diet. Clin. Gastroenterol. Hepatol. 2022, 20, 2876–2887. [Google Scholar] [CrossRef]
- Rivière, P.; Vauquelin, B.; Rolland, E.; Melchior, C.; Roman, S.; Bruley des Varannes, S.; Mion, F.; Gourcerol, G.; Sacher-Huvelin, S.; Zerbib, F. Low FODMAPs diet or usual dietary advice for the treatment of refractory gastroesophageal reflux disease: An open-labeled randomized trial. Neurogastroenterol. Motil. 2021, 33, e14181. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Riezzo, G.; Orlando, A.; Linsalata, M.; D’Attoma, B.; Prospero, L.; Ignazzi, A.; Giannelli, G. A Comparison of the Low-FODMAPs Diet and a Tritordeum-Based Diet on the Gastrointestinal Symptom Profile of Patients Suffering from Irritable Bowel Syndrome-Diarrhea Variant (IBS-D): A Randomized Controlled Trial. Nutrients 2022, 14, 1544. [Google Scholar] [CrossRef]
- Tunali, V.; Arslan, N.Ç.; Ermiş, B.H.; Derviş Hakim, G.; Gündoğdu, A.; Hora, M.; Nalbantoğlu, Ö.U. A Multicenter Randomized Controlled Trial of Microbiome-Based Artificial Intelligence-Assisted Personalized Diet vs Low-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet: A Novel Approach for the Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2024, 119, 1901–1912. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, D.; He, T.; Liao, X.; Shao, L.; Shi, L.; Liu, L. Effect of the combined intervention of low-FODMAPs diet and probiotics on IBS symptoms in Western China: A randomized controlled trial. Food Sci. Nutr. 2024, 12, 3993–4004. [Google Scholar] [CrossRef] [PubMed]
- Bodini, G.; Zanella, C.; Crespi, M.; Lo Pumo, S.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67–68, 110542. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407. [Google Scholar] [CrossRef]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef]
- Zahedi, M.J.; Behrouz, V.; Azimi, M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled trial. J. Gastroenterol. Hepatol. 2018, 33, 1192–1199. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Juntrapirat, A.; Lakananurak, N.; Gonlachanvit, S. Effect of Structural Individual Low-FODMAP Dietary Advice vs. Brief Advice on a Commonly Recommended Diet on IBS Symptoms and Intestinal Gas Production. Nutrients 2019, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Chey, W.D.; Jackson, K.; Pillai, S.; Chey, S.W.; Han-Markey, T. A Diet Low in Fermentable Oligo-, Di-, and Monosaccharides and Polyols Improves Quality of Life and Reduces Activity Impairment in Patients with Irritable Bowel Syndrome and Diarrhea. Clin. Gastroenterol. Hepatol. 2017, 15, 1890–1899.e3. [Google Scholar] [CrossRef]
- Blanchard-Smith, J.; Bullock, I.; Dalrymple, J. NICE Guidelines: Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care. 2008. Available online: https://www.nice.org.uk/guidance/cg61 (accessed on 23 April 2025).
- McKenzie, Y.A.; Alder, A.; Anderson, W.; Wills, A.; Goddard, L.; Gulia, P.; Jankovich, E.; Mutch, P.; Reeves, L.B.; Singer, A.; et al. British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults. J. Hum. Nutr. Diet. 2012, 25, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.; Gibson, P.R. The Complete Low-FODMAP Diet: The Revolutionary Plan for Managing Symptoms in IBS, Crohn’s Disease, Coeliac Disease and Other Digestive Disorders, 1st ed.; The Experiment: Vermilion, OH, USA, 2013. [Google Scholar]
- Iacovou, M.; Tan, V.; Muir, J.G.; Gibson, P.R. The low FODMAP diet and its application in East and Southeast Asia. J. Neurogastroenterol. Motil. 2015, 21, 459–470. [Google Scholar] [CrossRef]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 549–575. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Maagaard, L.; Ankersen, D.V.; Vegh, Z.; Burisch, J.; Jensen, L.; Pedersen, N.; Munkholm, P. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J. Gastroenterol. 2016, 22, 4009–4019. [Google Scholar] [CrossRef]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food composition, defining cutoff values and international application. J. Gastroenterol. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef]
- Monash University. The Monash University Low FODMAP App. 2017. Available online: https://www.monashfodmap.com/ibs-central/i-have-ibs/get-the-app/ (accessed on 23 April 2025).
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG clinical guideline: Management of irritable bowel syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Karakan, T.; Gundogdu, A.; Alagözlü, H.; Ekmen, N.; Ozgul, S.; Tunali, V.; Hora, M.; Beyazgul, D.; Nalbantoglu, O.U. Artificial intelligence-based personalized diet: A pilot clinical study for irritable bowel syndrome. Gut Microbes 2022, 14, 2138672. [Google Scholar] [CrossRef] [PubMed]
- Perez y Lopez, N.; Torres-Lopez, E.; Zamarripa-Dorsey, F. Clinical response in Mexican patients with irritable bowel syndrome treated with a low diet low in fermentable carbohydrates (FODMAP). Rev. Gastroenterol. Mex. 2015, 80, 180–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Muhammad, S.A.; Amin, M.S.; Gul, A. The Efficacy of the Low-FODMAP (Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols) Diet in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Cureus 2025, 17, e77053. [Google Scholar] [CrossRef]
- Harvie, R.M.; Chisholm, A.W.; Bisanz, J.E.; Burton, J.P.; Herbison, P.; Schultz, K.; Schultz, M. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J. Gastroenterol. 2017, 23, 4632–4643. [Google Scholar] [CrossRef]
- Patrick, D.L.; Drossman, D.A.; Frederick, I.O.; DiCesare, J.; Puder, K.L. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Dig. Dis. Sci. 1998, 43, 400–411. [Google Scholar] [CrossRef]
- Peng, Z.; Yi, J.; Liu, X. A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2072. [Google Scholar] [CrossRef]
- Lamb, B.; Dhindsa, B.; Sayles, H.; Glenn, E.; Eichele, D. Systematic Review With Meta-Analysis: The Effect of the Low-FODMAP Diet on Gastrointestinal Symptoms and Quality of Life in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2021, 116, S461–S462. [Google Scholar] [CrossRef]
- Falvey, J.D.; Hoskin, T.; Meijer, B.; Ashcroft, A.; Walmsley, R.; Day, A.S.; Gearry, R.B. Disease activity assessment in IBD: Clinical indices and biomarkers fail to predict endoscopic remission. Inflamm. Bowel Dis. 2015, 21, 824–831. [Google Scholar] [CrossRef]
- Gomes, P.; du Boulay, C.; Smith, C.L.; Holdstock, G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut 1986, 27, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.B.; Lochs, H.; Löfberg, R.; Modigliani, R.; Sutherland, L.R. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002, 122, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Baars, J.E.; Nuij, V.J.; Oldenburg, B.; Kuipers, E.J.; Van Der Woude, C.J. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm. Bowel Dis. 2012, 18, 1634–1640. [Google Scholar] [CrossRef]
- Zittan, E.; Kabakchiev, B.; Kelly, O.B.; Milgrom, R.; Nguyen, G.C.; Croitoru, K.; Steinhart, A.H.; Silverberg, M.S. Development of the Harvey-Bradshaw Index-pro (HBI-PRO) Score to Assess Endoscopic Disease Activity in Crohn’s Disease. J. Crohn’s Colitis 2016, 11, 543–548. [Google Scholar] [CrossRef]
- Kostas, A.; Siakavellas, S.I.; Kosmidis, C.; Takou, A.; Nikou, J.; Maropoulos, G.; Vlachogiannakos, J.; Papatheodoridis, G.V.; Papaconstantinou, I.; Bamias, G. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 7387–7396. [Google Scholar] [CrossRef]
- Mooiweer, E.; Severs, M.; Schipper, M.E.; Fidder, H.H.; Siersema, P.D.; Laheij, R.J.; Oldenburg, B. Low Fecal Calprotectin Predicts Sustained Clinical Remission in Inflammatory Bowel Disease Patients: A Plea for Deep Remission. J. Crohn’s Colitis 2014, 9, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Plaidum, S.; Patcharatrakul, T.; Promjampa, W.; Gonlachanvit, S. The Effect of Fermentable, Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP) Meals on Transient Lower Esophageal Relaxations (TLESR) in Gastroesophageal Reflux Disease (GERD) Patients with Overlapping Irritable Bowel Syndrome (IBS). Nutrients 2022, 14, 1755. [Google Scholar] [CrossRef]
- Lakananurak, N.; Pitisuttithum, P.; Susantitaphong, P.; Patcharatrakul, T.; Gonlachanvit, S. The Efficacy of Dietary Interventions in Patients with Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2024, 16, 464. [Google Scholar] [CrossRef]
- Lomer, M.C.E. The low FODMAP diet in clinical practice: Where are we and what are the long-term considerations? Proc. Nutr. Soc. 2024, 83, 17–27. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Rossi, M.; Kaminski, T.; Dimidi, E.; Ralph, F.S.E.; Wilson, B.; Martin, L.D.; Louis, P.; Lomer, M.C.E.; Irving, P.M.; et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14241. [Google Scholar] [CrossRef]
- Nawawi, K.N.M.; Belov, M.; Goulding, C. Low FODMAP diet significantly improves IBS symptoms: An Irish retrospective cohort study. Eur. J. Nutr. 2020, 59, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.J.; Reed, D.E.; Muir, J.G.; Vanner, S.J. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: A real-world experience. Neurogastroenterol. Motil. 2020, 32, e13730. [Google Scholar] [CrossRef]
- Dimidi, E.; McArthur, A.J.; White, R.; Whelan, K.; Lomer, M.C.E. Optimizing educational methods for the low FODMAP diet in disorders of gut-brain interaction: A feasibility randomized controlled trial. Neurogastroenterol. Motil. 2023, 35, e14640. [Google Scholar] [CrossRef]
- Chu, P.; He, Y.; Hu, F.; Wang, X. The effects of low FODMAP diet on gut microbiota regulation: A systematic review and meta-analysis. J. Food Sci. 2025, 90, e70072. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut microbiota in patients with irritable bowel syndrome—A systematic review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Rossi, M.; Kanno, T.; Parkes, G.C.; Anderson, S.; Mason, A.J.; Irving, P.M.; Lomer, M.C.; Whelan, K. β-Galactooligosaccharide in conjunction with low FODMAP diet improves irritable bowel syndrome symptoms but reduces fecal bifidobacteria. Am. J. Gastroenterol. 2020, 115, 906–915. [Google Scholar] [CrossRef]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Q. Low-FODMAP Diet for Irritable Bowel Syndrome: Insights from Microbiome. Nutrients 2025, 17, 544. [Google Scholar] [CrossRef]
| First Author, Year (Country) | Disease | Total Case/Controls | Age Case/Controls (Years; Mean ± SD) | Study Duration | Characteristics of Diets |
|---|---|---|---|---|---|
| Böhn et al., 2015 (Sweden) [21] | IBS | 38/37 | 44 (18–69)/41 (18–68) * | 4 weeks | LFD: Defined by the restriction of foods containing fermentable oligosaccharides, monosaccharides, disaccharides, and polyols. Patients were provided with a pamphlet containing detailed guidance on foods to avoid as well as alternative food options suitable for consumption. Diet frequently recommended for patients with IBS: Emphasizes the timing and manner of food consumption rather than solely focusing on specific food choices. It is based on dietary recommendations from NICE [26] and the BDA [27]. |
| Eswaran et al., 2017 (US) [25] | IBS-D | 50/42 | 41.6 ± 14.7/43.8 ± 15.2 | 4 weeks | LFD: Defined by the restriction of foods containing fermentable oligosaccharides, monosaccharides, disaccharides, and polyols. The instruction followed published materials from Monash University [28]; however, participants received educational materials developed by the University of Michigan. mNICE: Modified guidance from the NICE. Patients were advised to consume small, frequent meals, avoid trigger foods, and limit excessive alcohol and caffeine intake. The mNICE guidelines provided to study participants did not specifically exclude foods containing FODMAPs. |
| Liu et al., 2024 (China) [19] | IBS | 15/18 | 34.9 ± 12.5/35 ± 9.8 | 4 weeks | LFD: Patients are advised to limit their consumption of foods high in FODMAPs as specified in the published literature and in a mobile application produced by Monash University [29]. Conventional diet: Patients maintained their habitual Chinese diet while adhering to conventional dietary recommendations, which included consuming regular meals, ensuring adequate water intake, and reducing the consumption of fat, alcohol, caffeine, spicy foods, and other foods that may exacerbate intestinal symptoms. |
| Ostrowska et al., 2021 (Poland) [14] | IBS-M | 26/21/26 | 42.7 ± 16.7/40.6 ± 14.5/41.7 ± 13.4 | 8 weeks | LFD: Patients received personalized dietary guidance, along with educational materials containing a 7-day sample menu and a table outlining recommended and contraindicated foods in the FODMAP diet. IgG-based elimination–rotation diet: Patients underwent IgG antibody titer testing for specific foods to identify potential food hypersensitivities. Based on the test results, each patient received guidance on an elimination–rotation diet and an exemplary menu. Foods testing positive for IgG antibodies were excluded from the diet, while all IgG-negative foods were permitted within the rotation diet. Control diet: Patients were advised to follow dietary treatment as recommended by their attending gastroenterologist. They were provided with an easily digestible diet, which was a modified version of balanced nutrition for healthy individuals, meeting the same energy requirements and providing equivalent nutrient intake as a standard diet. During episodes of diarrhea, patients adhered to an easily digestible diet with restricted intake of fat and the insoluble fraction of dietary fiber. During periods of constipation, patients were advised to increase their dietary fiber intake. |
| Patcharatrakul et al., 2019 (Thailand) [24] | IBS | 30/32 | 50 ± 13.7/52 ± 14 | 4 weeks | SILFD: High-FODMAP items potentially worsening symptoms were identified from a 7-day food diary. Then patients avoided these items and modified their menus with commonly available low-FODMAP alternatives. A sample menu using low-FODMAP foods was included in the pamphlets provided to the patients. BRD: It recommended reducing foods commonly associated with gas, bloating, or abdominal pain, including fruits, vegetables, nuts, beans, and garlic, and avoiding large meals. The term FODMAP was not mentioned during the advice. |
| Rej et al., 2022 (UK) [15] | IBS-D or IBS-M | 33/33/33 | 35 ± 12/40 ± 15/36 ± 13 | 4 weeks | LFD: Defined by the limiting of foods containing fermentable oligosaccharides, monosaccharides, disaccharides, and polyols. TDA: Based on dietary recommendations from NICE and the BDA [26,30,31]. Its principles include having regular meals, never eating too little/too much, maintaining adequate hydration, reducing the intake of alcohol/caffeine/fizzy drinks and fatty/spicy/processed foods, fresh fruit to a maximum of 3 per day, fiber and other commonly consumed gas-producing foods, and addressing any perceived food intolerances. GFD: Defined by the exclusion of gluten. |
| Russo et al., 2022 (Italy) [17] | IBS-D | 21/21 | N/A | 12 weeks | LFD: Defined by the limiting of foods containing fermentable oligosaccharides, monosaccharides, disaccharides, and polyols. Participants received a structured weekly menu outlining three main meals (breakfast, lunch, dinner) and two snacks (morning and afternoon). They were provided with a booklet specifying permitted, restricted, and limited foods, based on Monash University classifications and FODMAP subgroup cut-off values [32,33,34], and a leaflet outlining where to purchase specific products. Guidance was provided on maintaining sufficient fiber intake and preparing meals without high-FODMAP ingredients like onions and garlic. Alcohol consumption was discouraged. TBD: Each patient had to consume flour, bread, breakfast biscuits, taralli (local salty biscuits), and pasta prepared exclusively with Tritordeum. A controlled diet was provided to each patient. The daily menu was breakfast, mid-morning snacks, lunch, afternoon snacks, and dinner. |
| Tunali et al., 2024 (Turkey) [18] | All subtypes of IBS | 51/70 | 37.9 ± 9.87/35.94 ± 10.13 | 6 weeks | LFD: Involved a restricted intake of foods containing fermentable oligosaccharides, monosaccharides, disaccharides, and polyols. Patients received a pamphlet outlining restricted foods and suitable alternatives. The guidelines were developed based on previously published resources from the ACG [35]. PD: It was designed based on AI-recommended foods derived from microbiome analysis results [36]. Approximately 300 foods were assessed on a scale of 0 to 10 for their impact on microbiome modulation. Foods scoring 0–3 were classified as avoidable, those scoring 4–7 as suitable for dietary diversification, and those scoring 8–10 as essential. The dietitian primarily incorporated foods with scores between 4 and 10, prioritizing high-scoring fruits. Low-scoring foods were not recommended. In the initial weeks, raw greens and legumes were restricted, with high-scoring foods gradually introduced into the diet. |
| Zahedi et al., 2018 (Iran) [23] | IBS-D | 50/51 | 37.6 ± 11.09/37.43 ± 13.27 | 6 weeks | LFD: The diet provided less than 0.5 g per meal of fermentable oligosaccharides, monosaccharides, disaccharides, and polyols [37]. Patients received a pamphlet detailing suitable and unsuitable foods, alternative options, a shopping guide, strategies for dining out, and guidance on preparing meals without onion and garlic. GDA: Incorporated dietary recommendations from the BDA [30], including limiting caffeine, alcohol, spicy foods, high-fat foods, and carbonated beverages. Additional guidelines emphasized consuming small, frequent meals, eating slowly in a calm environment, and avoiding chewing gum and sweeteners containing polyols. |
| Zhang et al., 2021 (China) [7] | IBS-D | 54/54 | 42.4 ± 10.7/44.6 ± 14.6 | 3 weeks | LFD: Patients were advised to eliminate high-FODMAP foods based on guidelines from the published literature and a Monash University mobile application [29]. TDA: Patients were instructed to reduce the intake of fatty and spicy foods, coffee, and alcohol and to follow a regular meal pattern of three meals per day. They were encouraged to avoid overeating or undereating and to eat calmly, chewing food thoroughly [21]. FODMAP-containing foods were not explicitly excluded. |
| Bodini et al., 2019 (Italy) [20] | IBD in remission or with mild disease activity | 26/29 | 41 (34–48)/47 (44–57) * | 6 weeks | LFD: Defined by the restriction of foods containing fermentable oligosaccharides, monosaccharides, disaccharides, and polyols. SD: Contained a usual FODMAP amount. All patients from both groups were encouraged to have three main meals (breakfast, lunch, and dinner) with varying types and portions of food, along with two snacks throughout the day. At each main meal, the dietitian offered patients the option to choose from at least three different menu options, each with the same caloric and FODMAP content. Additionally, patients received informational leaflets on meal preparation. |
| Cox et al., 2020 (UK) [13] | Quiescent IBD | 27/25 | 33 ± 11/40 ± 13 | 4 weeks | LFD: Defined by the restriction of foods containing fermentable oligosaccharides, monosaccharides, disaccharides, and polyols. Control (sham) diet: It was designed to offer patients an exclusion diet of comparable intensity and burden to the LFD while maintaining consistent nutrient, fiber, and FODMAP intake levels. |
| Pedersen et al., 2017 (Denmark) [22] | IBD in remission or with mild-to-moderate disease and coexisting IBS-like symptoms | 37/41 | 40 (20–70)/41 (24–69) * | 6 weeks | LFD: Defined by the restriction of foods containing fermentable oligosaccharides, monosaccharides, disaccharides, and polyols. ND: Patients were requested to follow an unchanged habitual diet. |
| Rivière et al., 2021 (France) [16] | Symptomatic PPI refractory GERD | 16/15 | 47 (37–50)/44 (40–52) * | 4 weeks | LFD: The diet was developed based on previously published studies on IBS patients [38], aiming to limit total FODMAP intake to less than 3 g/day. UDA: Usual dietary advice and lifestyle modifications include reducing the intake of high-fat foods, alcohol, caffeine, and tobacco, avoiding overeating, maintaining an upright position for at least two hours after meals, elevating the head of the bed, and refraining from eating within two hours of bedtime. |
| Authors | Parameter [Unit] | Case Group Baseline vs. After Intervention | p-Value | Control Group Baseline vs. After Intervention | p-Value | p-Value After Intervention vs. Control |
|---|---|---|---|---|---|---|
| Böhn et al. [21] | Total IBS-SSS score, mean ± SD | 324 ± 69 vs. 246 ± 127 | <0.001 | 302 ± 61 vs. 236 ± 78 | <0.001 | NS |
| Eswaran et al. [25] | Total IBS-QOL score, (95% CI) | 53.4 vs. 69.3 (10.9 to 20.8) | <0.05 | 54.3 vs. 59.4 (0.56 to 9.46) | <0.05 | <0.05 |
| HADS: Anxiety scores, mean (95% CI) | 9.13 vs. 7.73 (−2.10 to −0.59) | <0.05 | 9.31 vs. 9.54 (−0.64 to 1.10) | NS | <0.05 | |
| HADS: Depression scores, mean (95% CI) | 4.6 vs. 3.68 (−1.57 to −0.28) | <0.05 | 5.51 vs. 4.89 (−1.42 to 0.17) | NS | NS | |
| Absenteeism (work time missed), mean ± SD | 5.59 ± 14 vs. 5.10 ± 12 | NS | 1.78 ± 5 vs. 6.47 ± 21 | NS | NS | |
| Presenteeism (impairment while working), mean ± SD | 38.4 ± 23 vs. 26.3 ± 23 | NS | 35.9 ± 25 vs. 33.04 ± 23 | NS | NS | |
| Productivity loss (overall work impairment), mean ± SD | 40.3 ± 24 vs. 28.3 ± 25 | NS | 37.5 ± 24± vs. 34.5 ± 23 | NS | NS | |
| Activity impairment, mean ± SD | 52.6 ± 22 vs. 29.7 ± 24 | N/A | 52.8 ± 25 vs. 43.3 ± 28 | N/A | NS | |
| Sleep quality, mean ± SD | 5.34 ± 2 vs. 4.38 ± 2 | <0.05 | 5.14 ± 2 vs. 4.61 ± 2 | N/A | NS | |
| Fatigue, mean ± SD | 5.06 ± 2 vs. 4.19 ± 2 | <0.05 | 5.44 ± 2 vs. 4.9 ± 3 | N/A | NS | |
| Overall sleep QOL, mean ± SD | 7.32 ± 2 vs. 6.42 ± 2 | <0.05 | 7.68 ± 2 vs. 7.49 ± 2 | NS | NS | |
| Liu et al. [19] | Total IBS-SSS score, mean ± SD | 208.56 ± 57.26 vs. 116 ± 51.37 | <0.05 | 202.80 ± 90.97 vs. 232.20 ± 113.74 | NS | <0.05 |
| Ostrowska et al. [14] | Idiopathic abdominal pain, n (%) | G1-FM: 15 (58) vs. 11 (42) G2-IP: 16 (76) vs. 2 (10) | NS <0.001 | 16 (62) vs. 14 (54) | NS | N/A |
| Abdominal pain after a meal, n (%) | G1-FM: 11 (42) vs. 6 (23) G2-IP: 14 (67) vs. 2 (10) | NS <0.001 | 14 (54) vs. 12 (46) | NS | N/A | |
| Abdominal pain during defecation, n (%) | G1-FM: 5 (19) vs. 2 (8) G2-IP: 9 (43) vs. 1 (5) | NS 0.008 | 6 (23) vs. 6 (23) | NS | N/A | |
| Sensation of incomplete defecation, n (%) | G1-FM: 13 (50) vs. 10 (39) G2-IP: 13 (62) vs. 2 (10) | NS 0.001 | 14 (54) vs. 15 (58) | NS | N/A | |
| Difficulty to defecate (constipations), n (%) | G1-FM: 11 (42) vs. 7 (27) G2-IP: 14 (67) vs. 4 (19) | NS 0.002 | 19 (73) vs. 17 (65) | NS | N/A | |
| Bloating, n (%) | G1-FM: 22 (85) vs. 7 (27) G2-IP: 19 (91) vs. 2 (10) | <0.001 <0.001 | 24 (92) vs. 22 (85) | NS | N/A | |
| Gurgling sensation, n (%) | G1-FM: 17 (65) vs. 4 (15) G2-IP: 18 (86) vs. 2 (10) | <0.001 <0.001 | 21 (81) vs. 19 (73) | NS | N/A | |
| Gastric fullness, n (%) | G1-FM: 15 (58) vs. 3 (12) G2-IP: 19 (91) vs. 2 (10) | <0.001 <0.001 | 22 (85) vs. 19 (73) | NS | N/A | |
| Patcharatrakul et al. [24] | Total IBS-SSS score 0–100, mean ± SD | 61.2 ± 21 vs. 38.5 ± 20 | <0.001 | 56.3 ± 17.8 vs. 53.5 ± 19.2 | NS | 0.006 |
| Rej et al. [15] | Total IBS-SSS score, mean ± SD | LFD: 311 ± 80 vs. 148 ± 87 GFD: 299 ± 73 vs. 180 ± 89 | N/A | 330 ± 74 vs. 199 ± 93 | N/A | NS |
| Total IBS-QOL score, mean ± SD | LFD: 51 ± 21 vs. 61 ± 24 GFD: 60 ± 26 vs. 65 ± 26 | N/A | 52 ± 18 vs. 55 ± 21 | N/A | NS | |
| HADS: Anxiety scores, mean ± SD | LFD: 10.6 ± 5.4 vs. 8.9 ± 5.1 GFD: 9.8 ± 5.4 vs. 8.4 ± 4.9 | N/A | 9.5 ± 4.4 vs. 9.5 ± 4.4 | N/A | NS | |
| HADS: Depression scores, mean ± SD | LFD: 7.6 ± 4.5 vs. 6.5 ± 5.2 GFD: 6.7 ± 4.6 vs. 6.4 ± 5 | N/A | 6.8 ± 3.3 vs. 7.6 ± 3.5 | N/A | 0.03 | |
| PHQ-12 score, mean (SD) | LFD: 8.5 ± 4 vs. 7.7 ± 3.7 GFD: 8.4 ± 3.6 vs. 7.9 ± 4.2 | N/A | 9.6 ± 4.7 vs. 8.7 ± 3.7 | N/A | NS | |
| Russo et al. [17] | Total IBS-SSS score, (95% CI) | N/A vs. −132.1 (−74.9 to −189.4), ↓ | <0.0001 | N/A vs. −130.5 (−73.2 to −187.7), ↓ | <0.0001 | NS |
| Tunali et al. [18] | Total IBS-SSS score, mean ± SD | 276.76 ± 90.15 vs. 176.86 ± 111.09 | <0.001 | 314.42 ± 92.7 vs. 210.64 ± 130.63 | <0.001 | NS |
| Total IBS-QOL score, mean ± SD | 42.65 ± 19.82 vs. 55.08 ± 23.62 | <0.001 | 45.55 ± 22.06 vs. 55.79 ± 21.85 | <0.001 | NS | |
| HADS: Anxiety scores, mean ± SD | 10.74 ± 3.95 vs. 7.86 ± 4.07 | <0.001 | 10.27 ± 4.22± vs. 8.15 ± 3.37 | <0.001 | NS | |
| HADS: Depression scores, mean ± SD | 8.33 ± 4.36 vs. 5.72 ± 4.35 | <0.001 | 7.57 ± 4.35 vs. 6.22 ± 4.10 | 0.02 | NS | |
| Zahedi et al. [23] | Total IBS-SSS score, mean ± SD | 263.75 ± 91.25 vs. 108 ± 63.82 | <0.001 | 252.5 ± 85.51 vs. 149.75 ± 51.39 | <0.001 | <0.001 |
| Total IBS-QOL score, mean ± SD | 51.03 ± 17.48 vs. 43.73 ± 8.78 | <0.001 | 50.3 ± 16.81 vs. 44.95 ± 9.19 | 0.001 | NS | |
| Zhang et al. [7] | Total IBS-SSS score, mean ± SD | 244.6 ± 76.3 vs. N/A | N/A | 231.2 ± 69.4 vs. N/A | N/A | NS |
| Total IBS-QOL score, mean ± SD | 42.2 ± 25.1 vs. 23 ± 20.4 | <0.001 | 36.5 ± 21.9 vs. 18.6 ± 17.6 | <0.001 | NS | |
| GAD-7, mean ± SD | 5.9 ± 4.9 vs. 3.7 ± 3.9 | 0.001 | 6.6 ± 4.7 vs. 3.4 ± 3.9 | <0.001 | NS | |
| PHQ-9, mean ± SD | 4.9 ± 4.2 vs. 3.5 ± 4 | 0.012 | 6 ± 4.3 vs. 3.7 ± 3.8 | <0.002 | NS |
| Authors | Parameter [Unit] | Case Group Baseline vs. After Intervention | p-Value | Control Group Baseline vs. After Intervention | p-Value | p-Value After Intervention vs. Control |
|---|---|---|---|---|---|---|
| Bodini et al. [20] | HBi, median (IQR) | 4 (3–5) vs. 3 (2–3) | 0.024 | 3 (3–3) vs. 3 (2–4) | NS | NS |
| Mayo score, median (IQR) | 2 (2–3) vs. 1 (1–3) | NS | 2 (2–3) vs. 2 (1–2) | NS | NS | |
| Fecal calprotectin [mg/kg], median (IQR) | 76.6 (50–286.3) vs. 50 (50.6–81) | 0.004 | 91 (50.6–143.6) vs. 87 (50–235.6) | NS | NS | |
| IBDQ, median (IQR) | 166 (139–182) vs. 177 (155–188) | 0.05 | 181 (153–197) vs. 166 (153–200) | NS | NS | |
| Cox et al. [13] | Total IBS-SSS score, median (SEM) | 222 (76) vs. 158 (12) | N/A | 227 (81) vs. 190 (13) | N/A | NS |
| IBS-SSS 50% reduction, n (%) | N/A vs. 9 (33) | N/A | N/A vs. 1 (4) | N/A | 0.012 | |
| Adequate relief, n (%) | N/A vs. 14 (52) | N/A | N/A vs. 4 (16) | N/A | 0.007 | |
| Stool frequency (per d), mean (SEM) | 1.8 (1.3) vs. 1.7 (0.1) | N/A | 2.1 (1.0) vs. 2.1 (0.1) | N/A | 0.012 | |
| UK IBDQ, median (SEM) | N/A vs. 81.9 (1.2) | N/A | N/A vs. 78.3 (1.2) | N/A | 0.042 | |
| HBi, median (SEM) | N/A vs. 3.2 (0.4) | N/A | N/A vs. 3.4 (0.5) | N/A | NS | |
| Mayo score, median (SEM) | N/A vs. 0.2 (0.2) | N/A | N/A vs. 0.2 (0.2) | N/A | NS | |
| Fecal calprotectin [µg/g], median (IQR) | 54.8 (84.8) vs. 53.3 (84.8) | NS | 70.9 (117.3) vs. 66.9 (106.4) | NS | NS | |
| Pedersen et al. [22] | Total IBS-SSS score in all participants, median (IQR) | 210 (190–270) vs. 115 (33–169) | <0.001 | 245 (180–320) vs. 170 (91–288) | <0.001 | 0.02 |
| Total IBS-QOL score in all participants, median (IQR) | 73 (19–99) vs. 78 (51–65) | <0.01 | 77 (30–98) vs. 81 (26–99) | NS | NS | |
| Total SIBDQ score in all participants, median (IQR) | 47 (42–55) vs. 60 (51–65) | <0.01 | 50 (40–57) vs. 50 (39–60) | NS | <0.01 | |
| HBi, median (IQR) | 7 (3–8) vs. 3 (1–5) | 0.05 | 5 (4–11) vs. 6 (3–9) | NS | NS | |
| SCCAI, median (IQR) | 3 (1–4) vs. 1 (0–3) | 0.04 | 2 (1–4) vs. 2 (1–4) | NS | 0.02 | |
| Fecal calprotectin [µg/g], (95% CI) | 65 (37 to 113) vs. 53 (30 to 93) | NS | 44 (23 to 83) vs. 46 (27 to 81) | NS | NS |
| Authors | Parameter [Unit] | Case Group Baseline vs. After Intervention | p-Value | Control Group Baseline vs. After Intervention | p-Value | p-Value After Intervention vs. Control |
|---|---|---|---|---|---|---|
| Rivière et al. [16] | Total RDQ score, median (IQR) | 4.3 (3.8–4.8) vs. 3.4 (3.0–4.3) | 0.002 | 4.2 (3.6–4.8) vs. 3.9 (3.2–4.7) | 0.002 | NS |
| Total IBS-SSS score, median (IQR) | 245 (135–324) vs. 166 (93–203) | 0.04 | 290 (157–319) vs. 191 (168–274) | NS | NS | |
| Total GIQLI, median (IQR) | 73 (61–85) vs. 85 (72–94) | NS | 83 (69–89) vs. 83 (64–95) | NS | NS | |
| Total reflux events, median (IQR) | 59 (47–75) vs. 46 (35–61) | N/A | 67 (58–87) vs. 51 (28–99) | N/A | NS | |
| Total acid exposure, median (IQR) | 1.1 (0.2–2.4) vs. 0.9 (0.0–1.9) | N/A | 1.1 (0.3–2.3) vs. 1.0 (0.2–2.0) | N/A | NS | |
| Total bolus exposure, median (IQR) | 2.0 (1.6–3.3) vs. 1.4 (1.1–1.7) | N/A | 2.8 (1.7–3.8) vs. 2.9 (1.9–6.2) | N/A | NS | |
| Positive symptomatic association (%) | 7 (44) vs. 5 (31) | N/A | 9 (60) vs. 12 (80) | N/A | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuźmin, L.; Kubiak, K.; Lange, E. Efficacy of a Low-FODMAP Diet on the Severity of Gastrointestinal Symptoms and Quality of Life in the Treatment of Gastrointestinal Disorders—A Systematic Review of Randomized Controlled Trials. Nutrients 2025, 17, 2045. https://doi.org/10.3390/nu17122045
Kuźmin L, Kubiak K, Lange E. Efficacy of a Low-FODMAP Diet on the Severity of Gastrointestinal Symptoms and Quality of Life in the Treatment of Gastrointestinal Disorders—A Systematic Review of Randomized Controlled Trials. Nutrients. 2025; 17(12):2045. https://doi.org/10.3390/nu17122045
Chicago/Turabian StyleKuźmin, Laura, Katarzyna Kubiak, and Ewa Lange. 2025. "Efficacy of a Low-FODMAP Diet on the Severity of Gastrointestinal Symptoms and Quality of Life in the Treatment of Gastrointestinal Disorders—A Systematic Review of Randomized Controlled Trials" Nutrients 17, no. 12: 2045. https://doi.org/10.3390/nu17122045
APA StyleKuźmin, L., Kubiak, K., & Lange, E. (2025). Efficacy of a Low-FODMAP Diet on the Severity of Gastrointestinal Symptoms and Quality of Life in the Treatment of Gastrointestinal Disorders—A Systematic Review of Randomized Controlled Trials. Nutrients, 17(12), 2045. https://doi.org/10.3390/nu17122045





