Dietary Habits, Residential Air Pollution, and Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Material and Methods
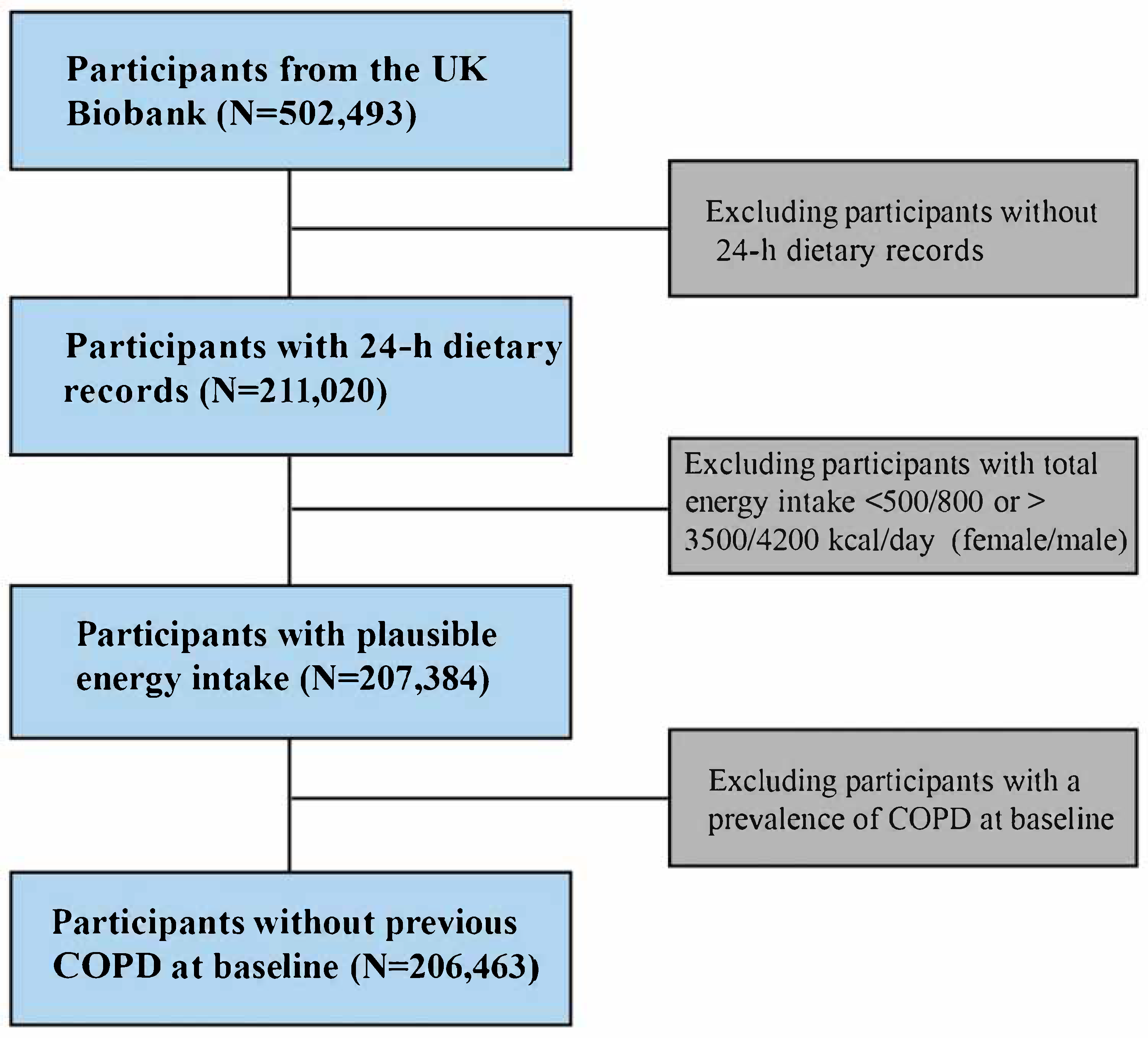
2.1. Study Design and Population
2.2. Dietary Assessment and Derivation of Dietary Pattern Scores
2.3. Ascertainment of Outcomes
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
3.2. Multiple Dietary Patterns Associated with the Risk of Total COPD and COPD-Caused Mortality
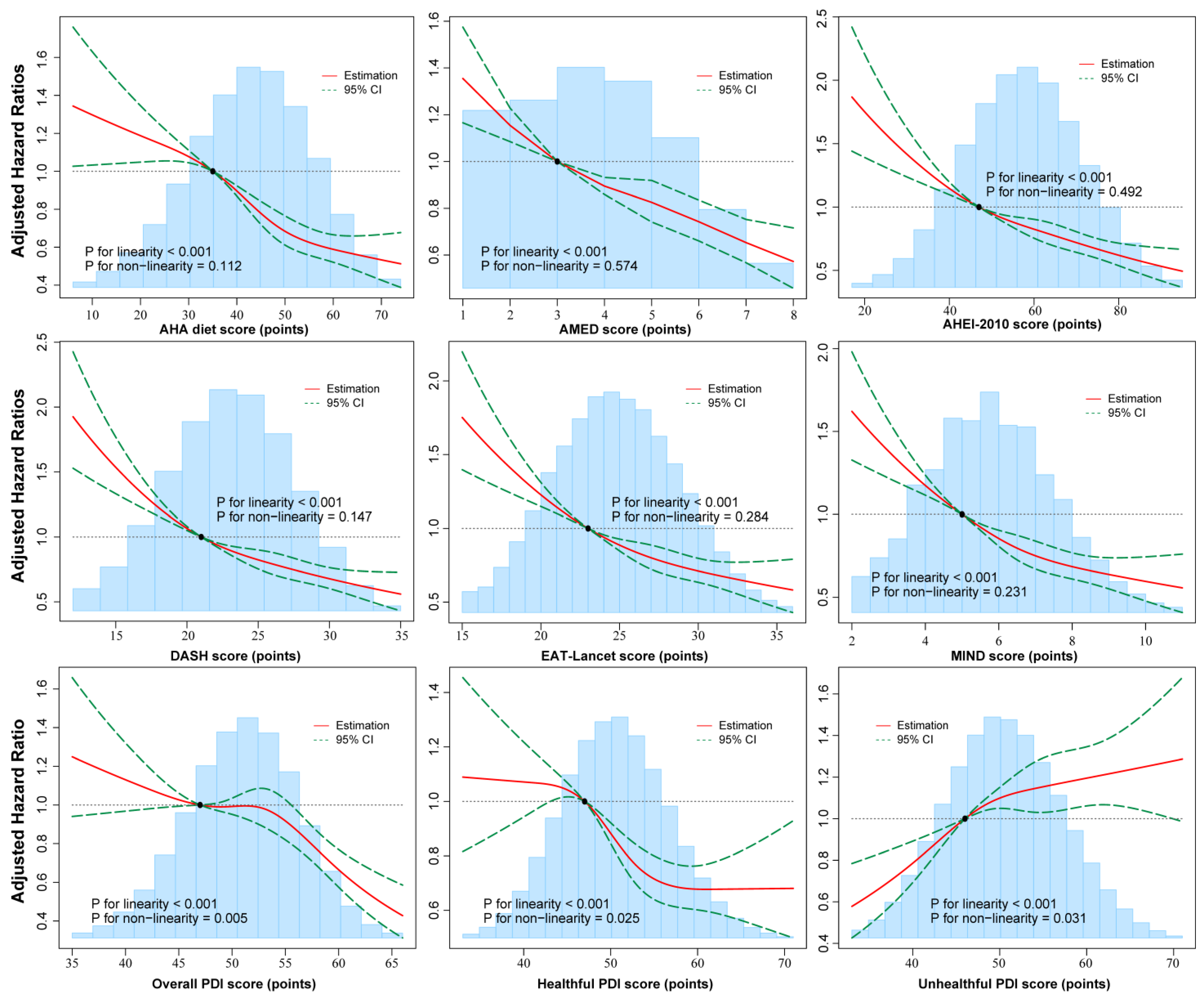
3.3. Potential Non-Linear Associations of Multiple Dietary Patterns with Total COPD and COPD-Caused Mortality
3.4. Predictive Performance of Multiple Dietary Patterns in Assessing COPD Risk
3.5. Sensitivity Analyses
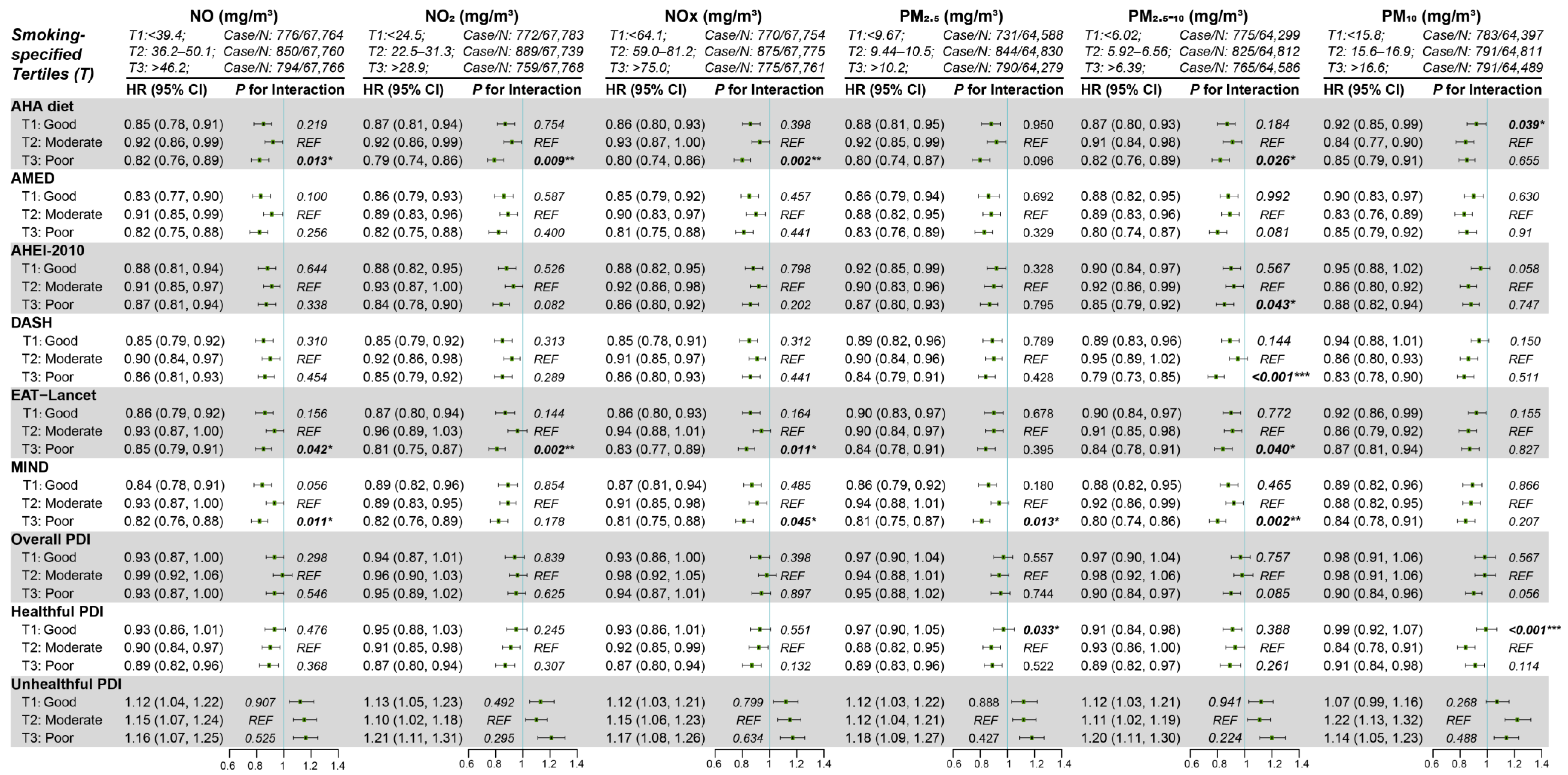
3.6. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef]
- Feng, Y.; Kang, H.; Sood, A.; Guest, D.D.; Fung, T.T.; Rowe, C.L.; Picchi, M.A.; Pankratz, V.S.; Belinsky, S.A.; Leng, S. Dietary Pattern, Sputum DNA Methylation, and Lung Health: An Epidemiological Study in People Who Ever Smoked. Chest 2024, 167, 1333–1345. [Google Scholar] [CrossRef]
- Varraso, R.; Chiuve, S.E.; Fung, T.T.; Barr, R.G.; Hu, F.B.; Willett, W.C.; Camargo, C.A. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: Prospective study. BMJ 2015, 350, h286. [Google Scholar] [CrossRef]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.; Cabezuelo, M.; Torres, L.; Viña, J.; Barber, T. Vitamin A Deficiency and the Lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Lyden, E.; Furtado, J.; Campos, H.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Serum tocopherol levels and vitamin E intake are associated with lung function in the normative aging study. Clin. Nutr. 2016, 35, 169–174. [Google Scholar] [CrossRef]
- Gonçalves, P.B.; Romeiro, N.C. Multi-target natural products as alternatives against oxidative stress in Chronic Obstructive Pulmonary Disease (COPD). Eur. J. Med. Chem. 2019, 163, 911–931. [Google Scholar] [CrossRef]
- Szmidt, M.K.; Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term dietary fiber intake and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Eur. J. Nutr. 2019, 59, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Gu, J.-M.; Qian, Y.-W.; Shao, X.-P.; Liu, Z.-Y.; Ge, Y.-Y.; Chen, G.-C. Quantity and quality of dietary carbohydrates, low-grade inflammation, and risk of chronic obstructive pulmonary disease and lung function. Clin. Nutr. 2025, 45, 53–60. [Google Scholar] [CrossRef]
- Rutting, S.; Papanicolaou, M.; Xenaki, D.; Wood, L.G.; Mullin, A.M.; Hansbro, P.M.; Oliver, B.G. Dietary ω-6 polyunsaturated fatty acid arachidonic acid increases inflammation, but inhibits ECM protein expression in COPD. Respir. Res. 2018, 19, 211. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Shivappa, N.; Hébert, J.R.; Bellomi, M.; Rampinelli, C.; Bertolotti, R.; Spaggiari, L.; Palli, D.; Veronesi, G.; Gnagnarella, P. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur. J. Nutr. 2015, 55, 1069–1079. [Google Scholar] [CrossRef]
- Amir, S.; Kim, H.; Hu, E.A.; Ricardo, A.C.; Mills, K.T.; He, J.; Fischer, M.J.; Pradhan, N.; Tan, T.C.; Navaneethan, S.D.; et al. Adherence to Plant-Based Diets and Risk of CKD Progression and All-Cause Mortality: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2024, 83, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Coresh, J.; Anderson, C.A.M.; Appel, L.J.; Grams, M.E.; Crews, D.C.; Mills, K.T.; He, J.; Scialla, J.; Rahman, M.; et al. Adherence to Healthy Dietary Patterns and Risk of CKD Progression and All-Cause Mortality: Findings From the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2021, 77, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.; Baid-Agrawal, S.; Rebholz, C.M.; Nadal, J.; Schmid, M.; Schaeffner, E.; Schneider, M.P.; Meiselbach, H.; Kaesler, N.; Bergmann, M.; et al. Association Between Dietary Patterns and Kidney Function in Patients With Chronic Kidney Disease: A Cross-Sectional Analysis of the German Chronic Kidney Disease Study. J. Ren. Nutr. 2020, 30, 296–304. [Google Scholar] [CrossRef]
- Stanford, J.; Stefoska-Needham, A.; Lambert, K.; Batterham, M.J.; Charlton, K. Association between plant-based diet quality and chronic kidney disease in Australian adults. Public Health Nutr. 2024, 27, e142. [Google Scholar] [CrossRef]
- Missikpode, C.; Ricardo, A.C.; Durazo-Arvizu, R.A.; Manoharan, A.; Mattei, J.; Isasi, C.R.; Mossavar-Rahmani, Y.; Talavera, G.A.; Sotres-Alvarez, D.; Daviglus, M.L.; et al. Association of Diet Quality Indices with Longitudinal Changes in Kidney Function in U.S. Hispanics/Latinos: Findings from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Kidney360 2021, 2, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Crews, D.C.; Grams, M.E.; Steffen, L.M.; Levey, A.S.; Miller, E.R., 3rd; Appel, L.J.; Coresh, J. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am. J. Kidney Dis. 2016, 68, 853–861. [Google Scholar] [CrossRef]
- Smyth, A.; Griffin, M.; Yusuf, S.; Mann, J.F.; Reddan, D.; Canavan, M.; Newell, J.; O’Donnell, M. Diet and Major Renal Outcomes: A Prospective Cohort Study. The NIH-AARP Diet and Health Study. J. Ren. Nutr. 2016, 26, 288–298. [Google Scholar] [CrossRef]
- Maroto-Rodriguez, J.; Ortola, R.; Cabanas-Sanchez, V.; Martinez-Gomez, D.; Rodriguez-Artalejo, F.; Sotos-Prieto, M. Diet quality patterns and chronic kidney disease incidence: A UK Biobank cohort study. Am. J. Clin. Nutr. 2025, 121, 445–453. [Google Scholar] [CrossRef]
- Afthab, M.; Hambo, S.; Kim, H.; Alhamad, A.; Harb, H. Particulate matter-induced epigenetic modifications and lung complications. Eur. Respir. Rev. 2024, 33, 240129. [Google Scholar] [CrossRef]
- Okubo, H.; Shaheen, S.O.; Ntani, G.; Jameson, K.A.; Syddall, H.E.; Aihie Sayer, A.; Dennison, E.M.; Cooper, C.; Robinson, S.M. Processed meat consumption and lung function: Modification by antioxidants and smoking. Eur. Respir. J. 2014, 43, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yue, H.; Dong, Y.; Liu, T.; Li, J. Effective compound combination of Bufei Yishen formula ameliorates PM2.5-induced COPD by inhibiting mitochondrial oxidative stress through SIRT3-mediated FOXO3 deacetylation. Phytomedicine 2025, 140, 156568. [Google Scholar] [CrossRef] [PubMed]
- Marín-Hinojosa, C.; Eraso, C.C.; Sanchez-Lopez, V.; Hernández, L.C.; Otero-Candelera, R.; Lopez-Campos, J.L. Nutriepigenomics and chronic obstructive pulmonary disease: Potential role of dietary and epigenetics factors in disease development and management. Am. J. Hematol. 2021, 114, 1894–1906. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, G.; Wang, K.; Yang, J.; Shen, Y.; Yang, X.; Chen, X.; Yao, X.; Gu, X.; Qi, L.; et al. Oxidative stress: Roles in skeletal muscle atrophy. Biochem. Pharmacol. 2023, 214, 115664. [Google Scholar] [CrossRef]
- Wu, J.; Shi, Y.; Zhou, M.; Chen, M.; Ji, S.; Liu, X.; Zhou, M.; Xia, R.; Zheng, X.; Wang, W. Nutrient vitamins enabled metabolic regulation of ferroptosis via reactive oxygen species biology. Front. Pharmacol. 2024, 15, 1434088. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Young, H.J.; Guo, W.; Key, T.J. Dietary assessment in UK Biobank: An evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 2018, 7, e6. [Google Scholar] [CrossRef]
- Willett, W.; Stampfer, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef]
- Rehm, C.D.; Penalvo, J.L.; Afshin, A.; Mozaffarian, D. Dietary Intake Among US Adults, 1999–2012. JAMA 2016, 315, 2542–2553. [Google Scholar] [CrossRef]
- Liu, D.; Tan, S.; Zhou, Z.; Gu, S.; Zuo, H. Trimethylamine N-oxide, beta-alanine, tryptophan index, and vitamin B6-related dietary patterns in association with stroke risk. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1179–1188. [Google Scholar] [CrossRef]
- Tyrrell, J.; Jones, S.E.; Beaumont, R.; Astley, C.M.; Lovell, R.; Yaghootkar, H.; Tuke, M.; Ruth, K.S.; Freathy, R.M.; Hirschhorn, J.N.; et al. Height, body mass index, and socioeconomic status: Mendelian randomisation study in UK Biobank. BMJ 2016, 352, i582. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.; Chau, J.Y.; Catt, M.; Bauman, A.; Trenell, M.I. Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233,110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ Open 2016, 6, e010038. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, J.; Mistry, M.N.; De La Cruz Libardi, A.; Masselot, P.; Schneider, R.; Ng, C.F.S.; Madaniyazi, L.; Gasparrini, A. Reconstructing individual-level exposures in cohort analyses of environmental risks: An example with the UK Biobank. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 1012–1017. [Google Scholar] [CrossRef]
- Gupta, R.P.; Strachan, D.P. Ventilatory function as a predictor of mortality in lifelong non-smokers: Evidence from large British cohort studies. BMJ Open 2017, 7, e015381. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Jameson, K.A.; Syddall, H.E.; Aihie Sayer, A.; Dennison, E.M.; Cooper, C.; Robinson, S.M. The relationship of dietary patterns with adult lung function and COPD. Eur. Respir. J. 2010, 36, 277–284. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef]
- Varraso, R.; Barr, R.G.; Willett, W.C.; Speizer, F.E.; Camargo, C.A. Fish intake and risk of chronic obstructive pulmonary disease in 2 large US cohorts. Am. J. Clin. Nutr. 2015, 101, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kim, R.Y.; Kohonen-Corish, M.R.J.; Chen, H.; Donovan, C.; Oliver, B.G. Particulate matter air pollution as a cause of lung cancer: Epidemiological and experimental evidence. Br. J. Cancer 2025, 132, 986–996. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the anthropocene: The EAT-lancet commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Stubbendorff, A.; Sonestedt, E.; Ramne, S.; Drake, I.; Hallström, E.; Ericson, U. Development of an EAT-Lancet index and its relation to mortality in a Swedish population. Am. J. Clin. Nutr. 2022, 115, 705–716. [Google Scholar] [CrossRef]
- Lu, X.; Wu, L.; Shao, L.; Fan, Y.; Pei, Y.; Lu, X.; Borné, Y.; Ke, C. Adherence to the EAT-Lancet diet and incident depression and anxiety. Nat. Commun. 2024, 15, 5599. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Sullivan, V.K.; Kim, H.; Caulfield, L.E.; Steffen, L.M.; Selvin, E.; Rebholz, C.M. Plant-Based Dietary Patterns and Incident Diabetes in the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 2024, 47, 803–809. [Google Scholar] [CrossRef]
- Stockholm Resilience Centre. Nordic food systems for improved health and sustainability. In Baseline Assessment to Inform Transformation; Stockholm University: Stockholm, Sweden, 2019. [Google Scholar]
| FEV1/FVC Ratio | Quartile 1 (<0.73) | Quartile 2 (0.73–0.77) | Quartile 3 (0.77–0.80) | Quartile 4 (>0.80) | Missing Data |
|---|---|---|---|---|---|
| n = 38,499 | n = 38,486 | n = 38,229 | n = 38,757 | n = 52,492 | |
| FEV1 (L) | 2.59 (2.12, 3.15) | 2.82 (2.37, 3.39) | 2.90 (2.46, 3.47) | 3.01 (2.56, 3.60) | -- |
| FVC (L) | 3.81 (3.15, 4.61) | 3.76 (3.16, 4.53) | 3.70 (3.14, 4.43) | 3.65 (3.11, 4.37) | -- |
| Used inhaler within last hour (%) | 472 (1.23) | 230 (0.60) | 165 (0.43) | 124 (0.32) | 204 (0.53) |
| Age (years) | 60 (53, 64) | 58 (51, 63) | 56 (50, 62) | 54 (47, 60) | 57 (49, 63) |
| Male (%) | 20,253 (52.61) | 17,320 (45.00) | 16,046 (41.97) | 16,399 (42.31) | 22,573 (43.00) |
| White race (%) | 38,499 (100.00) | 38,486 (100.00) | 38,229 (100.00) | 38,757 (100.00) | 43,275 (82.44) |
| BMI (kg/m2) | 25.6 (23.3, 28.5) | 26.0 (23.6, 28.8) | 26.4 (23.9, 29.4) | 27.1 (24.4, 30.3) | 26.3 (23.6, 29.5) |
| Smoking status (%) | |||||
| Never | 18,483 (48.01) | 21,177 (55.03) | 22,200 (58.07) | 23,809 (61.43) | 31,197 (59.43) |
| Previous | 15,496 (40.25) | 14,407 (37.43) | 13,557 (35.46) | 12,756 (32.91) | 16,884 (32.16) |
| Current | 4432 (11.51) | 2835 (7.37) | 2402 (6.28) | 2110 (5.44) | 4177 (7.96) |
| Missing data | 88 (0.23) | 67 (0.17) | 70 (0.18) | 82 (0.21) | 234 (0.45) |
| Alcohol consumption (%) | |||||
| Never | 885 (2.30) | 904 (2.35) | 910 (2.38) | 1018 (2.63) | 2892 (5.51) |
| Previous | 1167 (3.03) | 1007 (2.62) | 1033 (2.70) | 1097 (2.83) | 1872 (3.57) |
| Current | 36,430 (94.63) | 36,558 (94.99) | 36,279 (94.90) | 36,630 (94.51) | 47,588 (90.66) |
| Missing data (%) | 17 (0.04) | 17 (0.04) | 7 (0.02) | 12 (0.03) | 140 (0.27) |
| Physical activity (%) | |||||
| Low | 5678 (14.75) | 5609 (14.57) | 5772 (15.10) | 6384 (16.47) | 8751 (16.67) |
| Moderate | 13,724 (35.65) | 13,891 (36.09) | 13,948 (36.94) | 14,058 (36.27) | 18,585 (35.41) |
| High | 13,426 (34.87) | 13,328 (34.63) | 12,930 (33.82) | 12,633 (32.60) | 16,324 (31.10) |
| Missing data (%) | 5671 (14.73) | 5658 (14.70) | 5579 (14.59) | 5682 (14.66) | 8832 (16.83) |
| Total energy intake (kcal/day) | 2078 (1737, 2472) | 2047 (1715, 2431) | 2022 (1693, 2397) | 2008 (1678, 2385) | 2006 (1661, 2406) |
| Townsend index | −2.36 (−3.75, −0.02) | −2.45 (−3.81, −0.23) | −2.45 (−3.78, −0.26) | −2.38 (−3.77, −0.12) | −2.01 (−3.57, 0.64) |
| Educational level (%) | |||||
| High | 16,100 (41.8) | 16,495 (42.9) | 16,419 (43.0) | 16,818 (43.4) | 22,372 (42.6) |
| Moderate | 12,608 (32.8) | 13,249 (34.4) | 13,226 (34.6) | 13,638 (35.2) | 16,883 (32.2) |
| Low | 9791 (25.4) | 8742 (22.7) | 8584 (22.5) | 8301 (21.4) | 13,237 (25.2) |
| CVD (%) | 2466 (6.41) | 1972 (5.12) | 1694 (4.43) | 1575 (4.06) | 3493 (6.65) |
| T2DM (%) | 1242 (3.23) | 1242 (3.23) | 1264 (3.31) | 1359 (3.51) | 2559 (4.88) |
| Hypertension (%) | 20,816 (54.1) | 19,702 (51.2) | 19,061 (49.9) | 19,173 (49.5) | 27,673 (52.7) |
| Respiratory diseases (%) | 8009 (20.8) | 4224 (11.0) | 3186 (8.33) | 2800 (7.22) | 5867 (11.2) |
| Occupation-related breathing problems (%) | 12,354 (32.1) | 12,413 (32.3) | 12,117 (31.7) | 12,086 (31.2) | 14,376 (27.4) |
| Residential air pollution (mg/m3) | |||||
| NO | 41.6 (33.4, 49.9) | 41.1 (33.1, 49.3) | 41.1 (33.1, 49.2) | 41.3 (33.5, 49.4) | 42.9 (33.8, 51.3) |
| NO2 | 25.8 (20.7, 31.1) | 25.6 (20.7, 30.7) | 25.4 (20.7, 30.7) | 25.6 (20.8, 30.8) | 26.9 (21.8, 32.1) |
| NOx | 67.5 (54.6, 80.5) | 66.8 (54.3, 79.8) | 66.7 (54.2, 79.6) | 67.0 (54.6, 79.9) | 69.9 (57.0, 83.0) |
| PM2.5 | 9.86 (9.21, 10.5) | 9.83 (9.19, 10.5) | 9.83 (9.19, 10.4) | 9.86 (9.22, 10.5) | 9.93 (9.31, 10.5) |
| PM2.5–10 | 6.11 (5.84, 6.61) | 6.10 (5.84, 6.60) | 6.11 (5.84, 6.61) | 6.11 (5.84, 6.62) | 6.16 (5.86, 6.68) |
| PM10 | 16.0 (15.2, 17.0) | 16.0 (15.2, 17.0) | 16.0 (15.2, 17.0) | 16.0 (15.2, 17.0) | 16.1 (15.4, 17.1) |
| Case/n | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| AHA diet score | |||||||
| Quintile 1 | 669/42,307 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 |
| Quintile 2 | 588/42,388 | 0.87 (0.78, 0.98) | 0.8 (0.72, 0.9) | 0.94 (0.84, 1.05) | |||
| Quintile 3 | 399/38,401 | 0.65 (0.58, 0.74) | 0.57 (0.5, 0.65) | 0.74 (0.65, 0.84) | |||
| Quintile 4 | 419/40,712 | 0.65 (0.57, 0.73) | 0.55 (0.49, 0.62) | 0.76 (0.66, 0.86) | |||
| Quintile 5 | 375/42,655 | 0.55 (0.49, 0.63) | 0.44 (0.39, 0.5) | 0.67 (0.58, 0.77) | |||
| Z-score | 2450/206,463 | 0.81 (0.78, 0.85) | <0.001 | 0.74 (0.71, 0.77) | <0.001 | 0.88 (0.84, 0.91) | <0.001 |
| AMED score | |||||||
| Quintile 1 | 565/36,727 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 |
| Quintile 2 | 455/36,541 | 0.92 (0.82, 1.05) | 0.87 (0.77, 0.99) | 0.86 (0.76, 0.98) | |||
| Quintile 3 | 525/42,919 | 0.80 (0.71, 0.90) | 0.73 (0.65, 0.83) | 0.84 (0.74, 0.95) | |||
| Quintile 4 | 466/40,233 | 0.75 (0.67, 0.85) | 0.68 (0.60, 0.76) | 0.82 (0.72, 0.93) | |||
| Quintile 5 | 439/40,233 | 0.69 (0.60, 0.79) | 0.60 (0.53, 0.69) | 0.66 (0.58, 0.76) | |||
| Z-score | 2450/206,463 | 0.83 (0.80, 0.87) | <0.001 | 0.76 (0.73, 0.79) | <0.001 | 0.87 (0.83, 0.91) | <0.001 |
| AHEI-2010 score | |||||||
| Quintile 1 | 655/40,483 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 |
| Quintile 2 | 523/44,079 | 0.73 (0.65, 0.82) | 0.75 (0.66, 0.84) | 0.82 (0.73, 0.92) | |||
| Quintile 3 | 464/39,434 | 0.73 (0.64, 0.82) | 0.69 (0.62, 0.78) | 0.81 (0.72, 0.91) | |||
| Quintile 4 | 450/41,873 | 0.66 (0.59, 0.75) | 0.63 (0.56, 0.72) | 0.80 (0.70, 0.90) | |||
| Quintile 5 | 358/40,594 | 0.54 (0.48, 0.62) | 0.45 (0.4, 0.51) | 0.70 (0.61, 0.79) | |||
| Z-score | 2450/206,463 | 0.81 (0.78, 0.84) | <0.001 | 0.75 (0.72, 0.78) | <0.001 | 0.90 (0.86, 0.93) | <0.001 |
| DASH score | |||||||
| Quintile 1 | 755/46,191 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 |
| Quintile 2 | 365/29,724 | 0.75 (0.66, 0.85) | 0.66 (0.58, 0.75) | 0.82 (0.72, 0.93) | |||
| Quintile 3 | 606/52,116 | 0.71 (0.64, 0.79) | 0.58 (0.52, 0.65) | 0.82 (0.73, 0.91) | |||
| Quintile 4 | 317/31,590 | 0.61 (0.54, 0.7) | 0.49 (0.43, 0.55) | 0.75 (0.66, 0.86) | |||
| Quintile 5 | 407/46,842 | 0.53 (0.47, 0.6) | 0.4 (0.35, 0.45) | 0.69 (0.61, 0.79) | |||
| Z-score | 2450/206,463 | 0.8 (0.77, 0.83) | <0.001 | 0.71 (0.68, 0.74) | <0.001 | 0.88 (0.85, 0.92) | <0.001 |
| EAT-Lancet score | |||||||
| Quintile 1 | 712/44,399 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 |
| Quintile 2 | 463/35,129 | 0.82 (0.73, 0.92) | 0.76 (0.68, 0.85) | 0.9 (0.8, 1.01) | |||
| Quintile 3 | 443/39,723 | 0.69 (0.62, 0.78) | 0.62 (0.55, 0.69) | 0.8 (0.71, 0.91) | |||
| Quintile 4 | 532/50,390 | 0.66 (0.59, 0.73) | 0.57 (0.51, 0.64) | 0.81 (0.73, 0.91) | |||
| Quintile 5 | 300/36,822 | 0.51 (0.44, 0.58) | 0.43 (0.38, 0.5) | 0.68 (0.60, 0.79) | |||
| Z-score | 2450/206,463 | 0.78 (0.75, 0.81) | <0.001 | 0.75 (0.72, 0.78) | <0.001 | 0.89 (0.85, 0.93) | <0.001 |
| MIND score | |||||||
| Quintile 1 | 700/40,525 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 |
| Quintile 2 | 533/40,865 | 0.75 (0.67, 0.84) | 0.70 (0.63, 0.79) | 0.88 (0.78, 0.98) | |||
| Quintile 3 | 500/46,329 | 0.62 (0.55, 0.7) | 0.56 (0.5, 0.62) | 0.76 (0.68, 0.85) | |||
| Quintile 4 | 353/38,613 | 0.53 (0.46, 0.6) | 0.46 (0.41, 0.53) | 0.70 (0.61, 0.8) | |||
| Quintile 5 | 364/40,131 | 0.52 (0.46, 0.59) | 0.44 (0.38, 0.5) | 0.72 (0.63, 0.82) | |||
| Z-score | 2450/206,463 | 0.75 (0.72, 0.78) | <0.001 | 0.72 (0.7, 0.76) | <0.001 | 0.88 (0.84, 0.91) | <0.001 |
| Overall PDI score | |||||||
| Quintile 1 | 522/41,525 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 |
| Quintile 2 | 474/37,372 | 1.01 (0.89, 1.14) | 0.95 (0.84, 1.08) | 1.08 (0.95, 1.22) | |||
| Quintile 3 | 544/44,877 | 0.96 (0.85, 1.09) | 0.87 (0.77, 0.98) | 1.04 (0.92, 1.18) | |||
| Quintile 4 | 508/40,539 | 1 (0.88, 1.12) | 0.88 (0.78, 0.99) | 1.10 (0.97, 1.24) | |||
| Quintile 5 | 402/42,150 | 0.76 (0.66, 0.86) | 0.65 (0.57, 0.74) | 0.86 (0.75, 0.98) | |||
| Z-score | 2450/206,463 | 0.92 (0.88, 0.95) | <0.001 | 0.87 (0.83, 0.9) | <0.001 | 0.95 (0.92, 0.99) | 0.025 |
| Healthful PDI score | |||||||
| Quintile 1 | 574/41,574 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 |
| Quintile 2 | 601/44,376 | 0.98 (0.87, 1.1) | 0.89 (0.8, 1) | 1.00 (0.89, 1.12) | |||
| Quintile 3 | 465/37,624 | 0.89 (0.79, 1.01) | 0.78 (0.69, 0.89) | 0.97 (0.86, 1.10) | |||
| Quintile 4 | 407/42,166 | 0.7 (0.61, 0.79) | 0.59 (0.52, 0.67) | 0.75 (0.65, 0.85) | |||
| Quintile 5 | 403/40,723 | 0.71 (0.63, 0.81) | 0.6 (0.53, 0.68) | 0.82 (0.72, 0.94) | |||
| Z-score | 2450/206,463 | 0.87 (0.84, 0.91) | <0.001 | 0.81 (0.78, 0.85) | <0.001 | 0.91 (0.87, 0.95) | <0.001 |
| Unhealthful PDI score | |||||||
| Quintile 1 | 408/40,958 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 | 1 (Ref.) | <0.001 |
| Quintile 2 | 511/46,503 | 1.1 (0.97, 1.26) | 1.2 (1.05, 1.36) | 1.19 (1.04, 1.36) | |||
| Quintile 3 | 449/34,414 | 1.31 (1.15, 1.5) | 1.44 (1.26, 1.65) | 1.38 (1.21, 1.59) | |||
| Quintile 4 | 572/43,973 | 1.31 (1.15, 1.48) | 1.54 (1.36, 1.75) | 1.39 (1.22, 1.59) | |||
| Quintile 5 | 510/40,615 | 1.26 (1.11, 1.44) | 1.59 (1.4, 1.81) | 1.34 (1.16, 1.54) | |||
| Z-score | 2450/206,463 | 1.1 (1.06, 1.15) | <0.001 | 1.21 (1.16, 1.26) | <0.001 | 1.13 (1.08, 1.18) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Ma, J.; Cui, X.-L.; Zhang, Y.; Liu, T.; Chen, L.-H. Dietary Habits, Residential Air Pollution, and Chronic Obstructive Pulmonary Disease. Nutrients 2025, 17, 2029. https://doi.org/10.3390/nu17122029
Liu D, Ma J, Cui X-L, Zhang Y, Liu T, Chen L-H. Dietary Habits, Residential Air Pollution, and Chronic Obstructive Pulmonary Disease. Nutrients. 2025; 17(12):2029. https://doi.org/10.3390/nu17122029
Chicago/Turabian StyleLiu, Dong, Junyi Ma, Xia-Lin Cui, Yunnan Zhang, Tong Liu, and Li-Hua Chen. 2025. "Dietary Habits, Residential Air Pollution, and Chronic Obstructive Pulmonary Disease" Nutrients 17, no. 12: 2029. https://doi.org/10.3390/nu17122029
APA StyleLiu, D., Ma, J., Cui, X.-L., Zhang, Y., Liu, T., & Chen, L.-H. (2025). Dietary Habits, Residential Air Pollution, and Chronic Obstructive Pulmonary Disease. Nutrients, 17(12), 2029. https://doi.org/10.3390/nu17122029





