Maternal Diet Quality and Multivitamin Intake During Pregnancy Interact in the Association with Offspring Neurodevelopment at 2 Years of Age
Abstract
1. Introduction
2. Materials and Methods
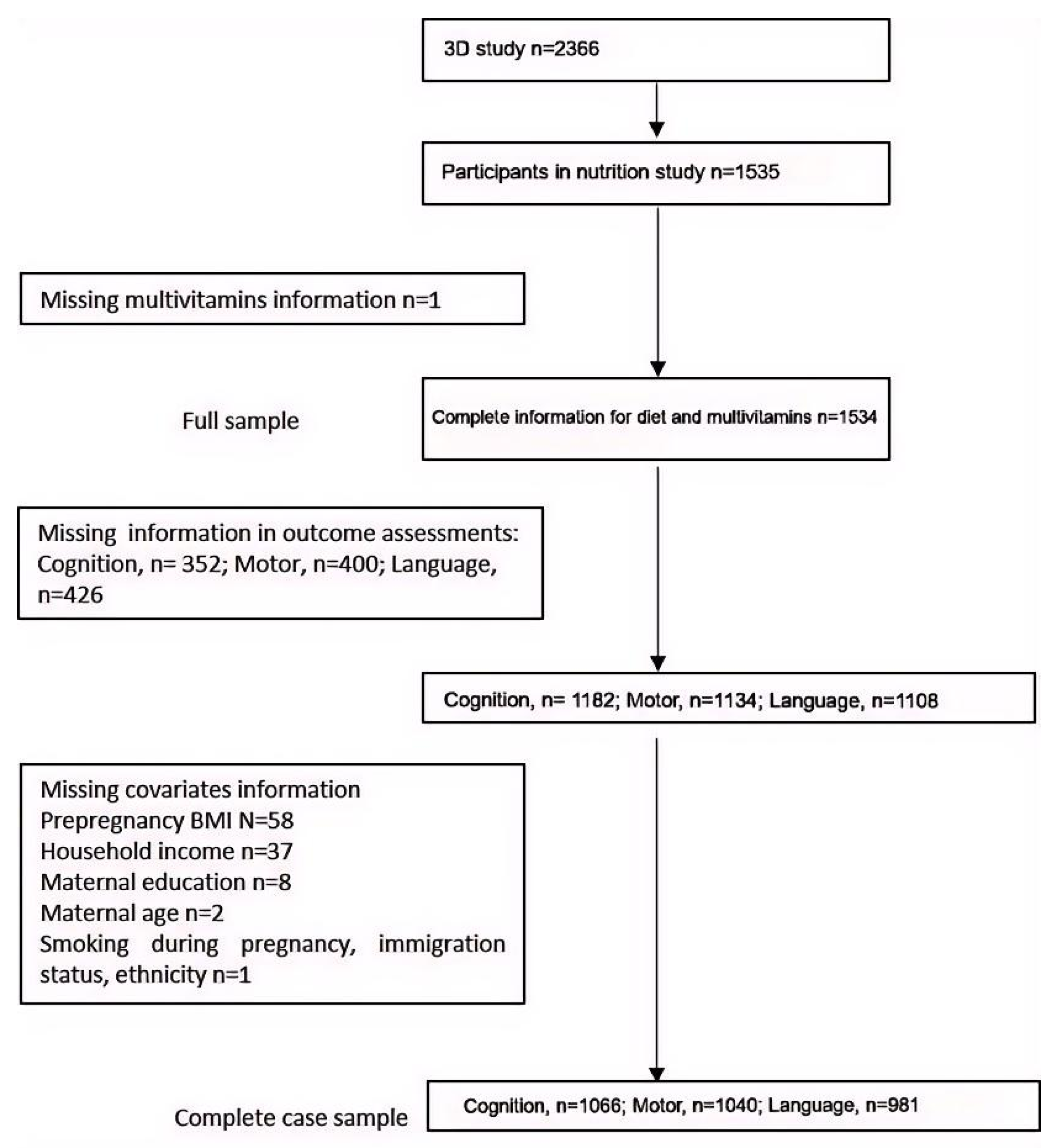
2.1. Design, Setting, and Participants
2.2. Exposures and Measurements
2.3. Outcomes and Measurements
2.4. Statistical Analyses
3. Results
3.1. Study Characteristics According to Diet Quality and Multivitamin Intake
3.2. Interactions Between Diet Quality and Multivitamin Intake During Pregnancy on Child Neurodevelopment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HEI-C | Canadian adaptation of the Healthy Eating Index |
| MML | Maternal medication log |
| MCDI | MacArthur–Bates Communicative Development Inventories |
| SD | Standard deviation |
| MD | Mean difference |
| CI | Confidence interval |
| DAG | Directed acyclic graph |
| BMI | Body mass index |
References
- Couperus, J.W.; Nelson, C.A. Early brain development and plasticity. In Blackwell Handbook of Early Childhood Development; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Pomeroy, S.L.; Ullrich, N.J. Development of the Nervous System. In Fetal and Neonatal Physiology, 3rd ed.; 2004; pp. 1675–1698. Available online: https://www.semanticscholar.org/paper/Chapter-165-%E2%80%93-Development-of-the-Nervous-System-Pomeroy-Ullrich/e206bda3f49a0b86023e64755789c179c2b5e122?utm_source=direct_link (accessed on 4 May 2021).
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef]
- Sayre, R.K.; Devercelli, A.E.; Neuman, M.J.; Wodon, Q. Investing in Early Childhood Development: Review of the World Bank’s Recent Experience; World Bank: Washington, DC, USA, 2015. [Google Scholar]
- Kretchmer, N.; Beard, J.L.; Carlson, S. The role of nutrition in the development of normal cognition. Am. J. Clin. Nutr. 1996, 63, 997S–1001S. [Google Scholar] [CrossRef]
- de Rooij, S.R.; Caan, M.W.; Swaab, D.F.; Nederveen, A.J.; Majoie, C.B.; Schwab, M.; Painter, R.C.; Roseboom, T.J. Prenatal famine exposure has sex-specific effects on brain size. Brain 2016, 139, 2136–2142. [Google Scholar] [CrossRef]
- de Rooij, S.R.; Mutsaerts, H.J.; Petr, J.; Asllani, I.; Caan, M.W.; Groot, P.; Nederveen, A.J.; Schwab, M.; Roseboom, T.J. Late-life brain perfusion after prenatal famine exposure. Neurobiol. Aging 2019, 82, 1–9. [Google Scholar] [CrossRef]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef]
- Dubois, L.; Diasparra, M.; Bédard, B.; Colapinto, C.K.; Fontaine-Bisson, B.; Tremblay, R.E.; Fraser, W.D. Adequacy of nutritional intake during pregnancy in relation to prepregnancy BMI: Results from the 3D Cohort Study. Br. J. Nutr. 2018, 120, 335–344. [Google Scholar] [CrossRef]
- Jessri, M.; Ng, A.P.; L’Abbé, M.R. Adapting the Healthy Eating Index 2010 for the Canadian Population: Evidence from the Canadian National Nutrition Survey. Nutrients 2017, 9, 910. [Google Scholar] [CrossRef]
- Borge, T.C.; Aase, H.; Brantsaeter, A.L.; Biele, G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: A systematic review and meta-analysis. BMJ Open 2017, 7, e016777. [Google Scholar] [CrossRef]
- Taylor, R.M.; Fealy, S.M.; Bisquera, A.; Smith, R.; Collins, C.E.; Evans, T.-J.; Hure, A.J. Effects of Nutritional Interventions during Pregnancy on Infant and Child Cognitive Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1265. [Google Scholar] [CrossRef]
- Mahmassani, H.A.; Switkowski, K.M.; Scott, T.M.; Johnson, E.J.; Rifas-Shiman, S.L.; Oken, E.; Jacques, P.F. Maternal diet quality during pregnancy and child cognition and behavior in a US cohort. Am. J. Clin. Nutr. 2022, 115, 128–141. [Google Scholar] [CrossRef]
- de Lauzon-Guillain, B.; Marques, C.; Kadawathagedara, M.; Bernard, J.Y.; Tafflet, M.; Lioret, S.; Charles, M.A. Maternal diet during pregnancy and child neurodevelopment up to age 3.5 years: The nationwide Étude Longitudinale Française depuis l’Enfance (ELFE) birth cohort. Am. J. Clin. Nutr. 2022, 116, 1101–1111. [Google Scholar] [CrossRef]
- Borge, T.C.; Biele, G.; Papadopoulou, E.; Andersen, L.F.; Jacka, F.; Eggesbø, M.; Caspersen, I.H.; Aase, H.; Meltzer, H.M.; Brantsæter, A.L. The associations between maternal and child diet quality and child ADHD—Findings from a large Norwegian pregnancy cohort study. BMC Psychiatry 2021, 21, 139. [Google Scholar] [CrossRef]
- Fraser, W.D.; Shapiro, G.D.; Audibert, F.; Dubois, L.; Pasquier, J.; Julien, P.; Bérard, A.; Muckle, G.; Trasler, J.; Tremblay, R.E.; et al. 3D Cohort Study: The Integrated Research Network in Perinatology of Quebec and Eastern Ontario. Paediatr. Périnat. Epidemiol. 2016, 30, 623–632. [Google Scholar] [CrossRef]
- Morisset, A.-S.; Dubois, L.; Colapinto, C.K.; Luo, Z.-C.; Fraser, W.D. Prepregnancy Body Mass Index as a Significant Predictor of Total Gestational Weight Gain and Birth Weight. Can. J. Diet. Pract. Res. 2017, 78, 66–73. [Google Scholar] [CrossRef]
- Eating Well with Canada’s Food Guide 2007. Available online: https://www.canada.ca/en/health-canada/services/canada-food-guide/about/history-food-guide/eating-well-with-canada-food-guide-2007.html (accessed on 4 May 2021).
- Yu, Y.; Feng, C.; Bédard, B.; Fraser, W.; Dubois, L. Diet quality during pregnancy and its association with social factors: 3D Cohort Study (Design, Develop, Discover). Matern. Child. Nutr. 2022, 18, e13403. [Google Scholar] [CrossRef]
- Health Canada. Canadian Nutrient File—Users Guide. 2016. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/nutrient-data/canadian-nutrient-file-compilation-canadian-food-composition-data-users-guide.html#a3 (accessed on 4 May 2021).
- Munene, L.-A.E.; Dumais, L.; Esslinger, K.; Jones-McLean, E.; Mansfield, E.; Verreault, M.-F.; Villeneuve, M.; Miller, D.; St-Pierre, S. A surveillance tool to assess diets according to Eating Well with Canada’s Food Guide. Health Rep. 2015, 26, 12–20. [Google Scholar]
- USDA Food Patterns Equivalents Database. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fped-overview/ (accessed on 4 May 2021).
- Health Canada Licensed Natural Health Products Database. Available online: https://www-canada-ca.proxy.bib.uottawa.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/applications-submissions/product-licensing/licensed-natural-health-product-database-data-extract.html (accessed on 4 May 2021).
- Michalec, D. Bayley Scales of Infant Development: Third Edition. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011; p. 215. [Google Scholar]
- Fenson, L.; Pethick, S.; Renda, C.; Cox, J.L.; Dale, P.S.; Reznick, J.S. Short-form versions of the MacArthur Communicative Development Inventories. Appl. Psycholinguist. 2000, 21, 95–116. [Google Scholar] [CrossRef]
- Balayla, J.; Sheehy, O.; Fraser, W.D.; Séguin, J.R.; Trasler, J.; Monnier, P.; MacLeod, A.A.; Simard, M.N.; Muckle, G.; Bérard, A. Neurodevelopmental Outcomes After Assisted Reproductive Technologies. Obstet. Gynecol. 2017, 129, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. Epidemiology: An Introduction; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Tennant, P.W.G.; Murray, E.J.; Arnold, K.F.; Berrie, L.; Fox, M.P.; Gadd, S.C.; Harrison, W.J.; Keeble, C.; Ranker, L.R.; Textor, J.; et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: Review and recommendations. Leuk. Res. 2021, 50, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Po-tential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, S.J.; Georgieff, M.K.; Committee On Nutrition. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal Nutrition and Neurodevelopment: A Scoping Review. Nutrients 2021, 13, 3530. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.D.; Watson, P.D.; Duff, M.C.; Cohen, N.J. The role of the hippocampus in flexible cognition and social behavior. Front. Hum. Neurosci. 2014, 8, 742. [Google Scholar] [CrossRef]
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 3, CD004905. [Google Scholar] [CrossRef]
- McBryde, M.; Fitzallen, G.C.; Liley, H.G.; Taylor, H.G.; Bora, S. Academic Outcomes of School-Aged Children Born Preterm: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e202027. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 2016, 37, 17–32. [Google Scholar] [CrossRef]
- Prado, E.L.; Alcock, K.J.; Muadz, H.; Ullman, M.T.; Shankar, A.H. Maternal multiple micronutrient supplements and child cognition: A randomized trial in Indonesia. Pediatrics 2012, 130, e536–e546. [Google Scholar] [CrossRef]
- Li, Q.; Yan, H.; Zeng, L.; Cheng, Y.; Liang, W.; Dang, S.; Wang, Q.; Tsuji, I. Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: Follow-up evaluation of a double-blind, randomized, controlled trial. Pediatrics 2009, 123, e685–e692. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, Y.; Zeng, L.; Elhoumed, M.; He, G.; Li, W.; Zhang, M.; Li, W.; Li, D.; Tsegaye, S.; et al. Association of Antenatal Micronutrient Supplementation With Adolescent Intellectual Development in Rural Western China: 14-Year Follow-up From a Randomized Clinical Trial. JAMA Pediatr. 2018, 172, 832–841. [Google Scholar] [CrossRef]
- Leibovitz, Z.; Lerman-Sagie, T.; Haddad, L. Fetal Brain Development: Regulating Processes and Related Malformations. Life 2022, 12, 809. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, N.; Dumas, A.; Morisset, A.-S.; Fontaine-Bisson, B.; Cahill, N. Evaluation of the Olo Prenatal Nutrition Follow-up Care for Vulnerable Pregnant Women. Can. J. Diet. Pract. Res. 2024, 85, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.; Hor, K.; Drake, A.J. Maternal influences on fetal brain development: The role of nutrition, infection and stress, and the potential for intergenerational consequences. Early Hum. Dev. 2020, 150, 105190. [Google Scholar] [CrossRef] [PubMed]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Kolar, A.S.; Patterson, R.E.; White, E.; Neuhouser, M.L.; Frank, L.L.; Standley, J.; Potter, J.D.; Kristal, A.R. A practical method for collecting 3-day food records in a large cohort. Epidemiology 2005, 16, 579–583. [Google Scholar] [CrossRef]
- Hofler, M. The effect of misclassification on the estimation of association: A review. Int. J. Methods Psychiatr. Res. 2005, 14, 92–101. [Google Scholar] [CrossRef]
- Nohr, E.A.; Liew, Z. How to investigate and adjust for selection bias in cohort studies. Acta Obstet. Gynecol. Scand. 2018, 97, 407–416. [Google Scholar] [CrossRef]
- Lebrun, A.; Plante, A.-S.; Savard, C.; Dugas, C.; Fontaine-Bisson, B.; Lemieux, S.; Robitaille, J.; Morisset, A.-S. Tracking of Dietary Intake and Diet Quality from Late Pregnancy to the Postpartum Period. Nutrients 2019, 11, 2080. [Google Scholar] [CrossRef]
| Characteristics | Low Diet Quality 1 (HEI-C Score < 62.7) | High Diet Quality 1 (HEI-C Score ≥ 62.7) | No Multivitamin Use | Multivitamin Use | ||
|---|---|---|---|---|---|---|
| n (%)/Mean ± SD | n (%)/Mean ± SD | p | n (%)/Mean ± SD | n (%)/Mean ± SD | p | |
| Total | 767 | 767 | 161 | 1373 | ||
| Mother’s age (years) | <0.001 | 0.74 | ||||
| <25 | 61 (8.0) | 24 (3.1) | 11 (6.8) | 74 (5.4) | ||
| 25–<35 | 563 (73.6) | 565 (73.8) | 116 (72.1) | 1012 (73.9) | ||
| ≥35 | 141 (18.4) | 177 (23.1) | 34 (21.1) | 284 (20.7) | ||
| Maternal education | <0.001 | 0.78 | ||||
| Secondary school or less | 65 (8.5) | 29 (3.8) | 12 (7.5) | 82 (6.0) | ||
| College | 231 (30.4) | 159 (20.8) | 37 (23.0) | 353 (25.9) | ||
| Undergraduate university degree | 298 (39.2) | 330 (43.3) | 69 (42.8) | 559 (41.0) | ||
| Graduate university studies | 167 (21.9) | 245 (32.1) | 43 (26.7) | 369 (27.1) | ||
| Household income (CAD) | <0.001 | 0.13 | ||||
| <30,000 | 65 (8.8) | 51 (6.9) | 14 (9.0) | 102 (7.7) | ||
| 30,000–59,999 | 141 (19.0) | 113 (15.3) | 38 (24.3) | 216 (16.3) | ||
| 60,000–79,999 | 150 (20.2) | 112 (15.2) | 24 (15.4) | 238 (18.0) | ||
| 80,000–99,999 | 167 (22.5) | 165 (22.4) | 31 (19.9) | 301 (22.8) | ||
| ≥100,000 | 219 (29.5) | 296 (40.2) | 49 (31.4) | 466 (35.2) | ||
| Marital status | 0.12 | 0.96 | ||||
| Married | 306 (40.0) | 308 (40.2) | 65 (40.4) | 549 (40.0) | ||
| Common law/partner | 420 (54.8) | 435 (56.7) | 90 (55.9) | 765 (55.8) | ||
| Others | 40 (5.2) | 24 (3.1) | 6 (3.7) | 58 (4.2) | ||
| Pre-pregnancy BMI (kg/m2) | <0.001 | 0.79 | ||||
| Underweight (<18.5) | 36 (4.9) | 54 (7.4) | 10 (6.5) | 80 (6.1) | ||
| Normal weight (18.5–24.9) | 442 (60.5) | 511 (70.4) | 98 (63.6) | 855 (65.6) | ||
| Overweight (25–29.9) | 143 (19.6) | 102 (14.0) | 30 (19.5) | 215 (16.5) | ||
| Obese (≥30) | 110 (15.0) | 59 (8.2) | 16 (10.4) | 153 (11.8) | ||
| Parity | <0.001 | 0.46 | ||||
| 0 | 408 (53.2) | 482 (62.8) | 89 (55.3) | 801 (58.3) | ||
| ≥1 | 359 (46.8) | 285 (37.2) | 72 (44.7) | 572 (41.7) | ||
| Mother born in Canada | 0.97 | 0.17 | ||||
| No | 217 (28.4) | 217 (28.3) | 53 (32.9) | 381 (27.8) | ||
| Yes | 548 (71.6) | 550 (71.7) | 108 (67.1) | 990 (72.2) | ||
| Caucasian | 0.96 | 0.70 | ||||
| No | 152 (19.9) | 153 (20.0) | 30 (18.8) | 275 (20.1) | ||
| Yes | 613 (80.1) | 613 (80.0) | 130 (81.2) | 1096 (79.9) | ||
| Smoking during pregnancy | 0.007 | 0.005 | ||||
| No | 646 (84.3) | 681 (89.0) | 128 (79.5) | 1199 (87.5) | ||
| Yes | 120 (15.7) | 84 (11.0) | 33 (20.5) | 171 (12.5) | ||
| Sex of the child | 0.18 | 0.73 | ||||
| Female | 391 (51.4) | 368 (48.0) | 77 (48.4) | 682 (49.9) | ||
| Male | 369 (48.6) | 398 (52.0) | 82 (51.6) | 685 (50.1) | ||
| Child neurodevelopment assessment age (months) | 25.5 ± 2.3 | 25.4 ± 2.1 | 0.32 | 25.5 ± 2.1 | 25.4 ± 2.2 | 0.78 |
| Bayley-III cognitive scores | 100.2 ± 11.2 | 100.8 ± 11.6 | 0.36 | 99.5 ± 12.0 | 100.6 ± 11.3 | 0.32 |
| Bayley-III motor scores | 101.7 ± 12.3 | 101.8 ± 12.1 | 0.97 | 103.8 ± 13.0 | 101.5 ± 12.1 | 0.07 |
| MCDI language scores | 53.6 ± 23.0 | 56.8 ± 24.1 | 0.02 | 52.9 ± 24.7 | 55.5 ± 23.5 | 0.26 |
| Maternal calorie intake (kcal per day) | 2166.5 ± 471.3 | 2224.3 ± 451.4 | 0.01 | 2178.0 ± 441.5 | 2197.5 ± 464.8 | 0.61 |
| No Multivitamin Use | Multivitamin Use | MD (95% CI) for Multivitamins Within Strata of Diet Quality | p for Interaction | |||
|---|---|---|---|---|---|---|
| Mean (SD), n | MD (95% CI), p | Mean (SD), n | MD (95% CI), p | |||
| Cognitive, unadjusted | 0.007 | |||||
| Low diet quality | 96.9 (11.4), 69 | Reference | 100.9 (11.4), 476 | 4 (1.2, 6.9), p = 0.01 1 | 4 (1.2, 6.9), p = 0.01 1 | |
| High diet quality | 102.8 (11.4), 47 | 5.9 (1.7, 10.1), p = 0.01 1 | 100.6 (11.4), 491 | 3.7 (0.9, 6.6), p = 0.01 1 | −2.1 (−5.5, 1.3), p = 0.22 2 | |
| MD (95% CI) for diet quality within strata of multivitamins | 5.9 (1.7, 10.1), p = 0.01 1 | 0.3 (−1.1, 1.7), p = 0.7 3 | ||||
| Cognitive, adjusted * | 0.018 | |||||
| Low diet quality | 94.9 (13.3), 69 | Reference | 97.9 (22.3), 476 | 3 (0.3, 5.8), p = 0.03 1 | 3 (0.3, 5.8), p = 0.03 1 | |
| High diet quality | 99 (12.5), 47 | 4.2 (0.1, 8.2), p = 0.04 1 | 96.9 (23.8), 491 | 2 (−0.7, 4.8), p = 0.15 1 | −2.1 (−5.4, 1.1), p = 0.19 2 | |
| MD (95% CI) for diet quality within strata of multivitamins | 4.2 (0.1, 8.2), p = 0.04 1 | −1 (−2.4, 0.4), p = 0.16 3 | ||||
| Language, unadjusted | 0.008 | |||||
| Low diet quality | 47.3 (23.2), 64 | Reference | 54.6 (23.2), 434 | 7.3 (1.2, 13.4), p = 0.02 1 | 7.3 (1.2, 13.4), p = 0.02 1 | |
| High diet quality | 61.9 (23.2), 42 | 14.6 (5.6, 23.6), p < 0.0005 1 | 56.2 (23.2), 441 | 8.9 (2.8, 15), p < 0.0005 1 | −5.7 (−13, 1.6), p = 0.02 2 | |
| MD (95% CI) for diet quality within strata of multivitamins | 14.6 (5.6, 23.6), p < 0.0005 1 | 1.6 (−1.5, 4.7), p = 0.31 3 | ||||
| Language, adjusted * | 0.023 | |||||
| Low diet quality | 43.9 (26.1), 64 | Reference | 48.8 (45), 434 | 4.9 (−0.7, 10.4), p = 0.09 1 | 4.9 (−0.7, 10.4), p = 0.09 1 | |
| High diet quality | 55.2 (24.8), 42 | 11.3 (3.1, 19.5), p = 0.01 1 | 50 (47.8), 441 | 6.1 (0.5, 11.7), p = 0.03 1 | −5.2 (−11.9, 1.5), p = 0.13 2 | |
| MD (95% CI) for diet quality within strata of multivitamins | 11.3 (3.1, 19.5), p = 0.01 1 | 1.2 (−1.6, 4.1), p = 0.39 3 | ||||
| No Multivitamin Use | Multivitamin Use | MD (95% CI) for Multivitamins Within Strata of Diet Quality | p for Interaction | |||
|---|---|---|---|---|---|---|
| Mean (SD), n | MD (95% CI), p | Mean (SD), n | MD (95% CI), p | |||
| Fine motor, unadjusted | 0.04 | |||||
| Low diet quality | 11.3 (2.6), 65 | Reference | 11.6 (2.6), 464 | 0.3 (−0.4, 1), p = 0.34 1 | 0.3 (−0.4, 1), p = 0.34 1 | |
| High diet quality | 12.3 (2.6), 44 | 1 (−0.1, 2), p = 0.06 1 | 11.5 (2.6), 467 | 0.2 (−0.5, 0.9), p = 0.63 1 | −0.8 (−1.6, 0), p = 0.06 2 | |
| MD (95% CI) for diet quality within strata of multivitamins | 1 (−0.1, 2), p = 0.06 1 | −0.2 (−0.5, 0.2), p = 0.34 3 | ||||
| Fine motor, adjusted * | 0.17 | |||||
| Low diet quality | 11.2 (3.2), 65 | Reference | 11.3 (5.4), 464 | 0.2 (−0.5, 0.8), p = 0.62 1 | 0.2 (−0.5, 0.8), p = 0.62 1 | |
| High diet quality | 11.7 (3), 44 | 0.5 (−0.5, 1.5), p = 0.31 1 | 11.1 (5.7), 467 | −0.1 (−0.7, 0.6), p = 0.88 1 | −0.6 (−1.4, 0.2), p = 0.17 2 | |
| MD (95% CI) for diet quality within strata of multivitamins | 0.5 (−0.5, 1.5), p = 0.31 1 | −0.2 (−0.6, 0.1), p = 0.2 3 | ||||
| Gross motor, unadjusted | 0.112 | |||||
| Low diet quality | 9 (2.3), 65 | Reference | 8.9 (2.3), 464 | −0.2 (−0.7, 0.4), p = 0.6 1 | −0.2 (−0.7, 0.4), p = 0.60 1 | |
| High diet quality | 9.8 (2.3), 44 | 0.7 (−0.1, 1.6), p = 0.09 1 | 8.9 (2.3), 467 | 0.7 (−0.2, 1.7), p = 0.11 1 | −0.9 (−1.6, −0.2), p = 0.01 2 | |
| MD (95% CI) for diet quality within strata of multivitamins | 0.7 (−0.1, 1.6), p = 0.09 1 | 0 (−0.3, 0.3), p = 0.98 3 | ||||
| Gross motor, adjusted * | 0.163 | |||||
| Low diet quality | 9 (2.8), 65 | Reference | 8.8 (4.7), 464 | −0.2 (−0.8, 0.4), p = 0.43 1 | −0.2 (−0.8, 0.4), p = 0.43 1 | |
| High diet quality | 9.7 (2.6), 44 | 0.7 (−0.2, 1.5), p = 0.13 1 | 8.8 (4.9), 467 | −0.2 (−0.8, 0.4), p = 0.49 1 | −0.9 (−1.6, −0.2), p = 0.01 2 | |
| MD (95% CI) for diet quality within strata of multivitamins | 0.7 (−0.2, 1.5), p = 0.13 1 | 0 (−0.3, 0.3), p = 0.87 3 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Liu, H.; Feng, C.; Seguin, J.R.; Hardy, I.S.; Sun, W.; Ramsay, T.; Little, J.; Potter, B.; Simard, M.-N.; et al. Maternal Diet Quality and Multivitamin Intake During Pregnancy Interact in the Association with Offspring Neurodevelopment at 2 Years of Age. Nutrients 2025, 17, 2020. https://doi.org/10.3390/nu17122020
Yu Y, Liu H, Feng C, Seguin JR, Hardy IS, Sun W, Ramsay T, Little J, Potter B, Simard M-N, et al. Maternal Diet Quality and Multivitamin Intake During Pregnancy Interact in the Association with Offspring Neurodevelopment at 2 Years of Age. Nutrients. 2025; 17(12):2020. https://doi.org/10.3390/nu17122020
Chicago/Turabian StyleYu, Yamei, Han Liu, Cindy Feng, Jean R. Seguin, Isabelle S. Hardy, Wenguang Sun, Tim Ramsay, Julian Little, Beth Potter, Marie-Noëlle Simard, and et al. 2025. "Maternal Diet Quality and Multivitamin Intake During Pregnancy Interact in the Association with Offspring Neurodevelopment at 2 Years of Age" Nutrients 17, no. 12: 2020. https://doi.org/10.3390/nu17122020
APA StyleYu, Y., Liu, H., Feng, C., Seguin, J. R., Hardy, I. S., Sun, W., Ramsay, T., Little, J., Potter, B., Simard, M.-N., Muckle, G., MacLeod, A., Fraser, W. D., & Dubois, L. (2025). Maternal Diet Quality and Multivitamin Intake During Pregnancy Interact in the Association with Offspring Neurodevelopment at 2 Years of Age. Nutrients, 17(12), 2020. https://doi.org/10.3390/nu17122020









