Dietary n-6:n-3 PUFA Ratio Modulates Inflammation-Related Gene Expression and Influences Improvements in Biochemical Parameters in a Murine Model of Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Groups
2.2. Diets
2.3. Fasting Glucose and Insulin Tolerance Test (ITT)
2.4. Serum Biochemistry Analysis
2.5. Analysis of Gene Expression by Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. Impact of n-6:n-3 Fatty Acid Ratios on Body Weight Progression, Tissue Weights, Cytokine Expression, and Antioxidant Enzyme Profiles
3.2. Effect of the 8-Week Dietary Intervention with HFD-5:1
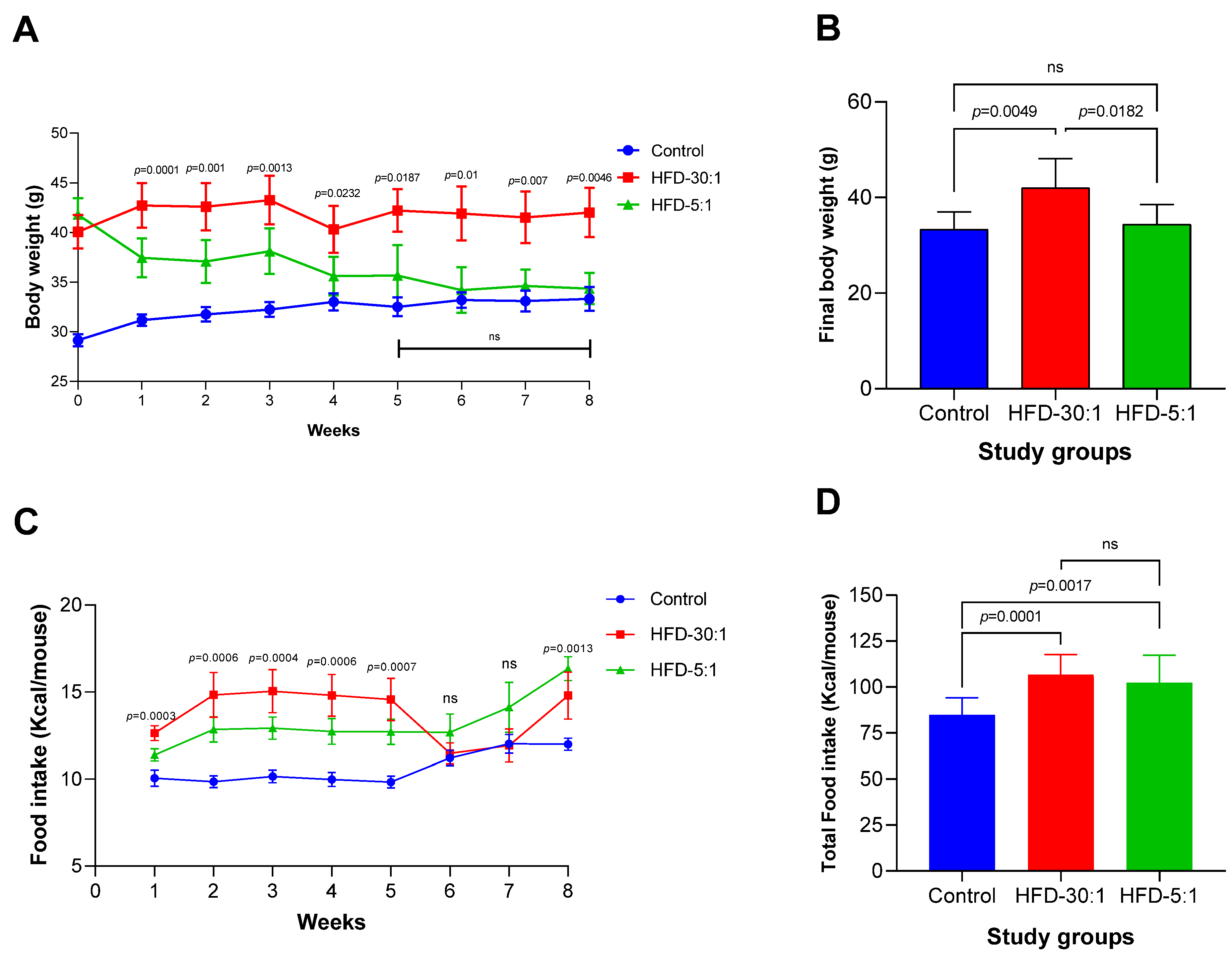
3.2.1. Body Weight and Caloric Intake
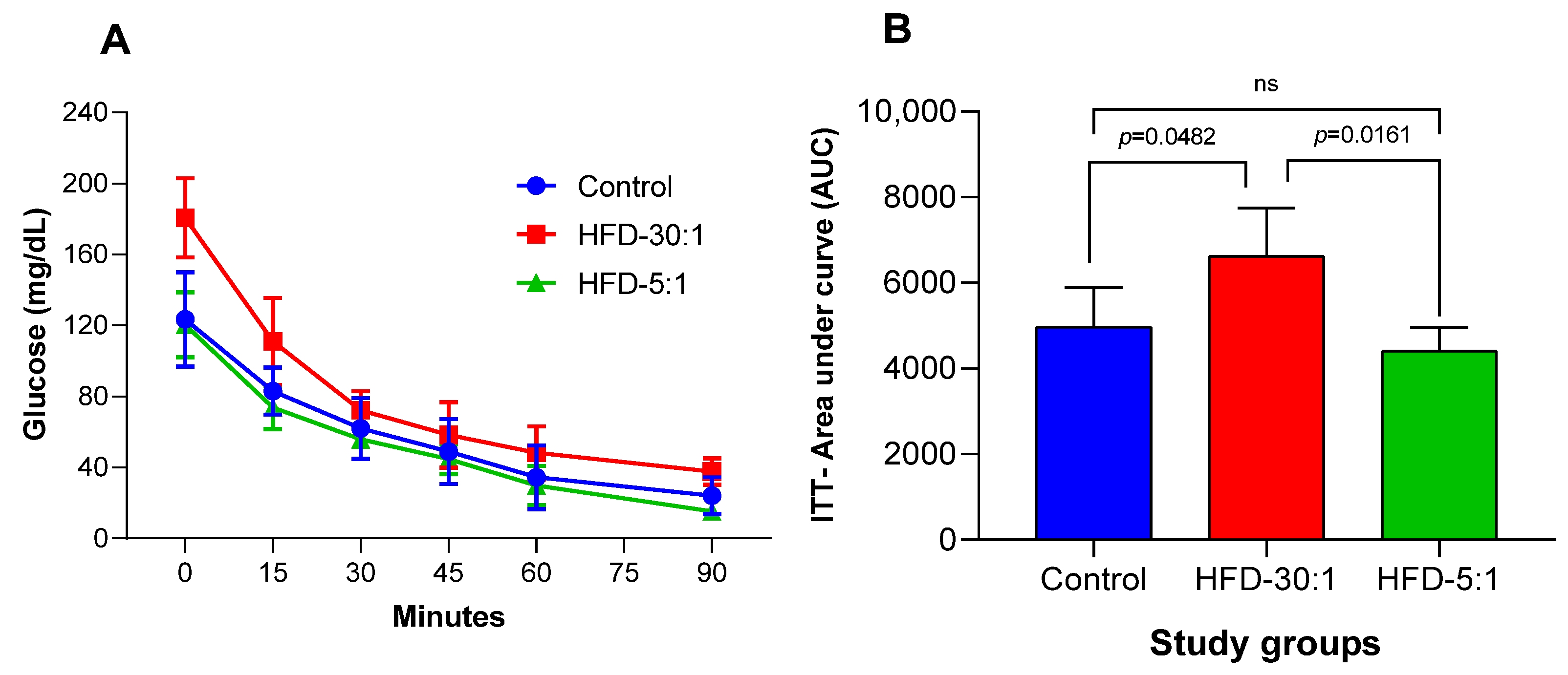
3.2.2. Insulin Tolerance Test (ITT)
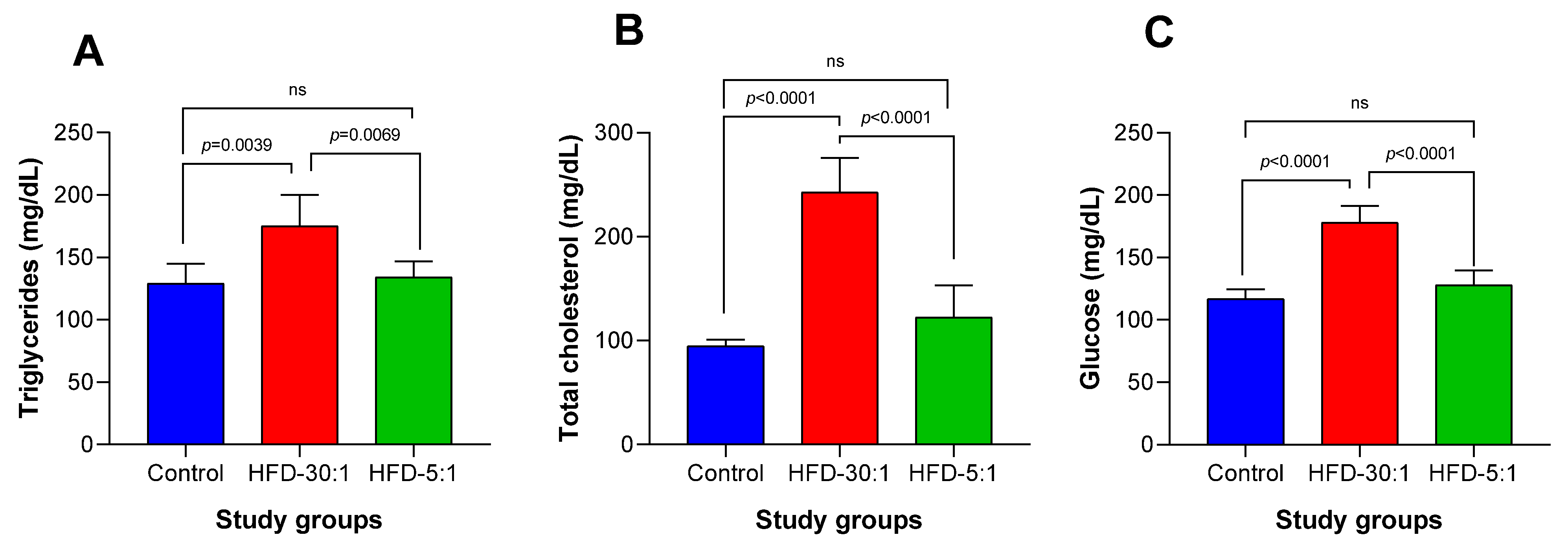
3.2.3. Biochemical Parameters
3.2.4. Cytokine Expression Profile
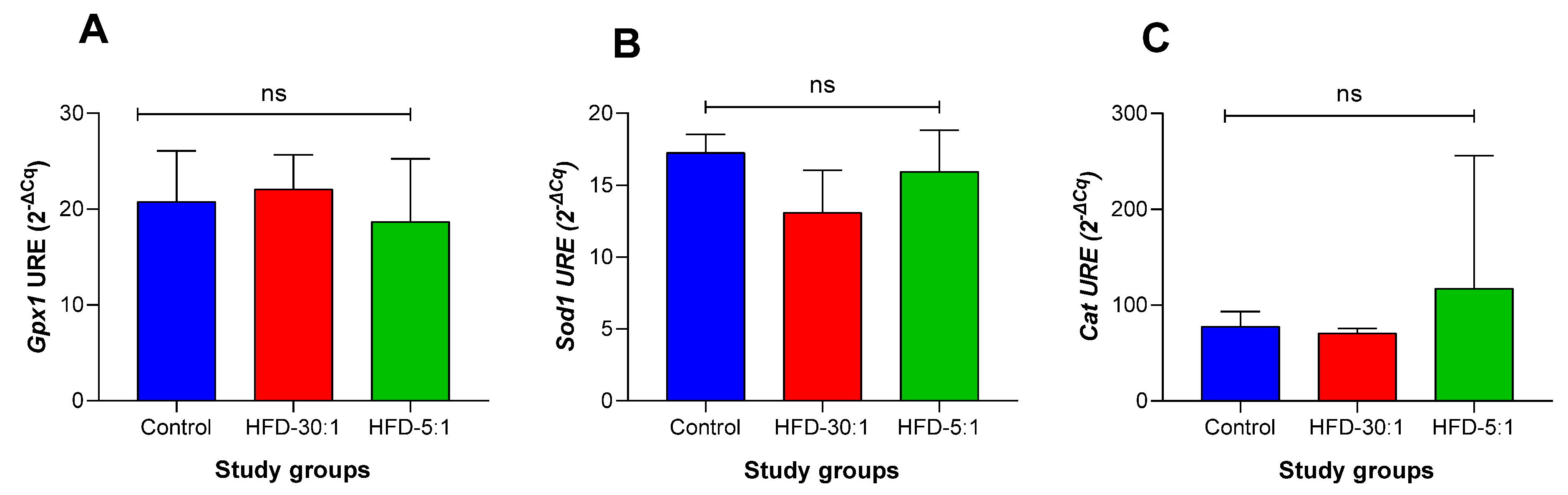
3.2.5. Antioxidant Gene Expression Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | Alpha-linolenic acid |
| ANOVA | Repeated measures analysis of variance |
| ApoB | Apolipoprotein B |
| AUC | Area under the curve |
| BMI | Body mass index |
| Cat | Catalase gene |
| Ccl2 | Monocyte chemoattractant protein-1 gene |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| GPR120 | G-protein-coupled receptor 120 |
| Gpx 1 | Glutathione peroxidase 1 gene |
| HFD | High-fat diet |
| HFD-5:1 | High-fat diet with n-6:n-3 ratio of 5:1 |
| HFD-30:1 | High-fat diet with n-6:n-3 ratio of 30:1 |
| IFN-γ | Interferon gamma |
| Ifng | Interferon gamma gene |
| IL-1α | Interleukin-1 alpha |
| IL-1β | Interleukin-1 beta |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| Il-13 | Interleukin-13 |
| ITT | Insulin tolerance test |
| MCP-1 | Monocyte chemoattractant protein-1 |
| n-3 | Omega-3 fatty acids |
| n-6 | Omega-6 fatty acids |
| NF-κB | Nuclear factor κappa B |
| PUFAs | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| Sod 1 | Superoxide dismutase 1 gene |
| Tgfb1 | Transforming growth factor beta 1 gene |
| TGF-β | Transforming growth factor beta |
| Tnfa | Tumor necrosis factor alpha gene |
| TNF-α | Tumor necrosis factor alpha |
| VLDL-c | Very-low-density lipoprotein cholesterol |
| WHO | World Health Organization |
References
- Sikaris, K.A. The clinical biochemistry of obesity. Clin. Biochem. Rev. 2004, 25, 165–181. [Google Scholar] [PubMed]
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 25 March 2025).
- Lobstein, T.; Brinsden, H.; Neveux, M.; Cavalcanti, O.B.; Barquera, S.; Baur, L.; Busch, V.; Buse, K.; Dietz, B.; French, A.; et al. World Obesity Atlas 2022; World Obesity Federation: London, UK, 2022. [Google Scholar]
- Jiang, S.-Z.; Lu, W.; Zong, X.-F.; Ruan, H.-Y.; Liu, Y. Obesity and hypertension. Exp. Ther. Med. 2016, 12, 2395–2399. [Google Scholar] [CrossRef] [PubMed]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Chronic and Degenerative Diseases: Obesity, Inflammation and the Immune System. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 701. [Google Scholar] [CrossRef]
- Malenica, M.; Meseldžić, N. Oxidative stress and obesity. Arh. Farm. 2022, 72, 166–183. [Google Scholar] [CrossRef]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. Curr. Diab. Rep. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Nakayama, H.; Iwahara, C.; Takamori, K. Significance of glycosphingolipid fatty acid chain length on membrane microdomain-mediated signal transduction. FEBS Lett. 2010, 584, 1642–1652. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Kusunoki, C.; Yang, L.; Yoshizaki, T.; Nakagawa, F.; Ishikado, A.; Kondo, M.; Morino, K.; Sekine, O.; Ugi, S.; Nishio, Y.; et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013, 430, 225–230. [Google Scholar] [CrossRef]
- Van Name, M.A.; Savoye, M.; Chick, J.M.; Galuppo, B.T.; Feldstein, A.E.; Pierpont, B.; Johnson, C.; Shabanova, V.; Ekong, U.; Valentinp, P.L.; et al. A low n-6 to n-3 PUFA ratio diet to treat fatty liver disease in obese youth. J. Clin. Endocrinol. Metab. 2020, 105, 2314–2321. [Google Scholar] [CrossRef]
- Yue, H.; Liu, W.; Zhang, W.; Jia, M.; Huang, F.; Du, F.; Xu, T. Dietary low ratio of n-6/n-3 polyunsaturated fatty acids improve type 2 diabetes mellitus via activating brown adipose tissue in male mice. J. Food Sci. 2021, 86, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Xu, Z.; Lv, H. Low n-6/n-3 PUFA ratio improves inflammation and myocardial ischemic reperfusion injury. Biochem. Cell Biol. 2019, 97, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Campos-Pérez, W.; González-Becerra, K.; Ramos-López, O.; Silva-Gómez, J.A.; Barrón-Cabrera, E.; Roman, S.; Panduro, A.; Martínez-López, E. Same Dietary but Different Physical Activity Pattern in Normal-weight and Overweight Mexican Subjects. J. Food Nutr. Res. 2016, 4, 729–735. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight; WHO: Geneva, Switzerland, 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 March 2025).
- Kennedy, A.J.; Ellacott, K.L.J.; King, V.L.; Hasty, A.H. Mouse models of the metabolic syndrome. DMM Dis. Model. Mech. 2010, 3, 156–166. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Xu, Y. Translational potential of mouse models of human metabolic disease. Cell 2024, 187, 4129–4143. [Google Scholar] [CrossRef]
- de Moura e Dias, M.; dos Reis, S.A.; da Conceição, L.L.; Sediyama, C.M.N.D.O.; Pereira, S.S.; de Oliveira, L.L.; Peluzio, M.D.C.G.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 56. [Google Scholar] [CrossRef]
- Cottam, M.A.; Caslin, H.L.; Winn, N.C.; Hasty, A.H. Multiomics reveals the persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat. Commun. 2022, 13, 2950. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Wang, Q.; Yi, S.; Liu, X.; Jin, H.; Xu, J.; Wen, G.; Zhu, J.; Tuo, B. The source of the fat significantly affects the results of high-fat diet intervention. Sci. Rep. 2022, 12, 4315. [Google Scholar] [CrossRef] [PubMed]
- He, M.Q.; Wang, J.Y.; Wang, Y.; Sui, J.; Zhang, M.; Ding, X.; Zhao, Y.; Chen, Z.; Ren, X.; Shi, B. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Dis. Transl. Med. 2020, 6, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strains of mice: Kunming, c57bl/6, balb/c and icr. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef]
- Varani, J. Healthful Eating, the Western Style Diet and Chronic Disease. Approaches Poult. Dairy Vet. Sci. 2017, 1, 44–46. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef]
- van der Heijden, R.A.; Sheedfar, F.; Morrison, M.C.; Hommelberg, P.P.H.; Kor, D.; Kloosterhuis, N.J.; Gruben, N.; Youssef, S.A.; de Bruin, A.; Hofker, M.H.; et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging 2015, 7, 256–268. [Google Scholar] [CrossRef]
- Rojas-Cruz, A.F.; Martín-Jiménez, C.A.; González, J.; González-Giraldo, Y.; Pinzón, A.M.; Barreto, G.E.; Aristizábal-Pachón, A.F. Palmitic Acid Upregulates Type I Interferon–Mediated Antiviral Response and Cholesterol Biosynthesis in Human Astrocytes. Mol. Neurobiol. 2023, 60, 4842–4854. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Yi, J.; Lan, X.; Lu, K.; Du, X.; Guo, Z.; Guo, Y.; Geng, M.; Li, D.; et al. IFN-γ contributes to the hepatic inflammation in HFD-induced nonalcoholic steatohepatitis by STAT1β/TLR2 signaling pathway. Mol. Immunol. 2021, 134, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Hooks, J.J.; Moutsopoulos, H.M.; Geis, S.A.; Stahl, N.I.; Decker, J.L.; Notkins, A.L. Immune Interferon in the Circulation of Patients with Autoimmune Disease. N. Engl. J. Med. 1979, 301, 5–8. [Google Scholar] [CrossRef]
- Casanova, J.L.; MacMicking, J.D.; Nathan, C.F. Interferon-γ and infectious diseases: Lessons and prospects. Science 2024, 384, eadl2016. [Google Scholar] [CrossRef] [PubMed]
- O’ROurke, R.W.; White, A.E.; Metcalf, M.D.; Winters, B.R.; Diggs, B.S.; Zhu, X.; Marks, D.L. Systemic inflammation and insulin sensitivity in obese IFN-γ knockout mice. Metabolism 2012, 61, 1152–1161. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Hosseini, H.; Tajik, M.H.; Meshkani, R.; Sadeghi, A. Resveratrol relieves HFD-induced insulin resistance in skeletal muscle tissue through antioxidant capacity enhancement and the Nrf2-Keap1 signaling pathway. Mol. Biol. Rep. 2024, 51, 516. [Google Scholar] [CrossRef]
- Mendes, I.K.S.; Matsuura, C.; Aguila, M.B.; Daleprane, J.B.; Martins, M.A.; Mury, W.V.; Brunini, T.M.C. Weight loss enhances hepatic antioxidant status in a NAFLD model induced by high-fat diet. Appl. Physiol. Nutr. Metab. 2018, 43, 23–29. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef]
- Kim, C.W.; Addy, C.; Kusunoki, J.; Anderson, N.N.; Deja, S.; Fu, X.; Burgess, S.C.; Li, C.; Ruddy, M.; Chakravarthy, M.; et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: A bedside to bench investigation. Cell Metab. 2017, 26, 394–406.e6. [Google Scholar] [CrossRef]
- Shearer, G.C.; Savinova, O.V.; Harris, W.S. Fish oil and plasma triglycerides. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 843–851. [Google Scholar] [CrossRef]
- Tanaka, N.; Zhang, X.; Sugiyama, E.; Kono, H.; Horiuchi, A.; Nakajima, T.; Kanbe, H.; Tanaka, E.; Gonzalez, F.J.; Aoyama, T. Eicosapentaenoic acid improves hepatic steatosis independent of PPARα activation through inhibition of SREBP-1 maturation in mice. Biochem. Pharmacol. 2010, 80, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.M.; Ma, D.W. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef]
- Maitin, V.; Andreo, U.; Guo, L.; Fisher, E.A. Docosahexaenoic acid impairs the maturation of very low density lipoproteins in rat hepatic cells. J. Lipid Res. 2014, 55, 75–84. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Fisher, E.A. N-3 fatty acids stimulate intracellular degradation of apoprotein B in rat hepatocytes. J. Clin. Investig. 1993, 91, 1380–1389. [Google Scholar] [CrossRef]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol review: A metabolically important molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in wild plants, nuts and seeds. Asia Pac. J. Clin. Nutr. 2002, 11, S163–S173. [Google Scholar] [CrossRef]
- Ullah, A.; Singla, R.K.; Batool, Z.; Cao, D.; Shen, B. Pro- and anti-inflammatory cytokines are the game-changers in childhood obesity-associated metabolic disorders (diabetes and non-alcoholic fatty liver diseases). Rev. Endocr. Metab. Disord. 2024, 25, 783–803. [Google Scholar] [CrossRef]
- Ji, Y.; Sun, S.; Xia, S.; Yang, L.; Li, X.; Qi, L. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J. Biol. Chem. 2012, 287, 24378–24386. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Menezes-Garcia, Z.; Henriques, M.C.C.; Soriani, F.M.; Pinho, V.; Faria, A.M.C.; Santiago, A.F.; Cara, D.C.; Souza, D.G.; Teixeira, M.M.; et al. Acute and sustained inflammation and metabolic dysfunction induced by high refined carbohydrate-containing diet in mice. Obesity 2013, 21, e396–e406. [Google Scholar] [CrossRef]
- Denys, A.; Hichami, A.; Khan, N.A. n-3 PUFAs modulate T-cell activation via protein kinase C-alpha and -epsilon and the NF-kappaB signaling pathway. J. Lipid Res. 2005, 46, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Sundseth, S.S.; Jones, S.A.; Brown, P.J.; Wisely, G.B.; Koble, C.S.; Devchand, P.; Wahli, W.; Willson, T.M.; Lenhard, J.M.; et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA 1997, 94, 4318–4323. [Google Scholar] [CrossRef] [PubMed]
- Lefils-Lacourtablaise, J.; Socorro, M.; Géloën, A.; Daira, P.; Debard, C.; Loizon, E.; Guichardant, M.; Dominguez, Z.; Vidal, H.; Lagarde, M.; et al. The eicosapentaenoic acid metabolite 15-deoxy-δ(12,14)-prostaglandin J3 increases adiponectin secretion by adipocytes partly via a PPARγ-dependent mechanism. PLoS ONE 2013, 8, e63997. [Google Scholar] [CrossRef]
- da Silva, E.P., Jr.; Nachbar, R.T.; Levada-Pires, A.C.; Hirabara, S.M.; Lambertucci, R.H. Omega-3 fatty acids differentially modulate enzymatic anti-oxidant systems in skeletal muscle cells. Cell Stress Chaperones 2016, 21, 87–95. [Google Scholar] [CrossRef]

| Components | Control | HFD-30:1 | HFD-5:1 | |||
|---|---|---|---|---|---|---|
| D12450H | D21022504 | D21022505 | ||||
| g | % kcal | g | % kcal | g | % kcal | |
| Proteins | 19 | 20 | 24 | 20 | 24 | 20 |
| Carbohydrates | 67 | 70 | 41 | 35 | 41 | 35 |
| Fat | 4 | 10 | 24 | 45 | 24 | 45 |
| Total | 100 | 100 | 100 | |||
| Kcal/g | 3.8 | 4.7 | 4.7 | |||
| Ingredients | g | kcal | g | kcal | g | kcal |
| Casein | 200 | 800 | 200 | 800 | 200 | 800 |
| L-Cysteine | 3 | 12 | 3 | 12 | 3 | 12 |
| Cornstarch | 452.2 | 1809 | 72.8 | 291 | 72.8 | 291 |
| Maltodextrin 10 | 75 | 300 | 100 | 400 | 100 | 400 |
| Sucrose | 172.8 | 691 | 172.8 | 691 | 172.8 | 691 |
| Cellulose | 50 | 0 | 50 | 0 | 50 | 0 |
| Lard | 20 | 180 | 157 | 1413 | 157 | 1413 |
| Menhaden oil | 0 | 0 | 0 | 0 | 20.5 | 185 |
| Safflower oil | 0 | 0 | 45.5 | 410 | 0 | 0 |
| Soy oil | 25 | 225 | 0 | 0 | 25 | 225 |
| Mineral mix S10026 | 10 | 0 | 10 | 0 | 10 | 0 |
| Dicalcium phosphate | 13 | 0 | 13 | 0 | 13 | 0 |
| Calcium carbonate | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 |
| Potassium citrate monohydrate | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 |
| Vitamin mix | 10 | 0 | 10 | 0 | 10 | 0 |
| Choline bitartrate | 2 | 0 | 2 | 0 | 2 | 0 |
| Total | 1055.05 | 4057 | 858.15 | 4057 | 858.15 | 4057 |
| PUFA and cholesterol content | ||||||
| n-6 (g/4057 kcal) | 17.9 | 74.6 | 52.8 | |||
| n-3 (g/4057 kcal) | 2.1 | 2.3 | 10.6 | |||
| EPA | 0.0 | 0.0 | 2.9 | |||
| DHA | 0.0 | 0.0 | 2.1 | |||
| n-6:n-3 | 8.4 | 30.2 | 5.0 | |||
| Linoleic acid (g/kg) | 16.9 | 82.1 | 60.2 | |||
| Fat cholesterol (mg/4057 kcal) | 14.4 | 117.4 | 233.3 | |||
| Total cholesterol (mg/4057 kcal) | 14.4 | 233.4 | 233.3 | |||
| Tissue | Control | HFD-30:1 | HFD-5:1 | p-Value |
|---|---|---|---|---|
| Epididymal adipose (g) | 1.1 ± 0.4 | 1.3 ± 0.5 | 1.0 ± 0.4 | 0.4 |
| Liver (g) | 1.4 ± 0.1 a | 2.1 ± 0.4 b | 1.6 ± 0.4 a | 0.01 |
| Relative liver weight | 4.2 ± 0.6 a | 5.5 ± 1.4 b | 4.0 ± 0.2 a | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Guerra, A.; Cambron-Mora, D.; Rodriguez-Echevarria, R.; Hernández-Bello, J.; Campos-Pérez, W.; Canales-Aguirre, A.A.; Pérez-Robles, M.; Martinez-Lopez, E. Dietary n-6:n-3 PUFA Ratio Modulates Inflammation-Related Gene Expression and Influences Improvements in Biochemical Parameters in a Murine Model of Diet-Induced Obesity. Nutrients 2025, 17, 1996. https://doi.org/10.3390/nu17121996
Gutierrez-Guerra A, Cambron-Mora D, Rodriguez-Echevarria R, Hernández-Bello J, Campos-Pérez W, Canales-Aguirre AA, Pérez-Robles M, Martinez-Lopez E. Dietary n-6:n-3 PUFA Ratio Modulates Inflammation-Related Gene Expression and Influences Improvements in Biochemical Parameters in a Murine Model of Diet-Induced Obesity. Nutrients. 2025; 17(12):1996. https://doi.org/10.3390/nu17121996
Chicago/Turabian StyleGutierrez-Guerra, Alejandro, Diego Cambron-Mora, Roberto Rodriguez-Echevarria, Jorge Hernández-Bello, Wendy Campos-Pérez, Alejandro A. Canales-Aguirre, Mariana Pérez-Robles, and Erika Martinez-Lopez. 2025. "Dietary n-6:n-3 PUFA Ratio Modulates Inflammation-Related Gene Expression and Influences Improvements in Biochemical Parameters in a Murine Model of Diet-Induced Obesity" Nutrients 17, no. 12: 1996. https://doi.org/10.3390/nu17121996
APA StyleGutierrez-Guerra, A., Cambron-Mora, D., Rodriguez-Echevarria, R., Hernández-Bello, J., Campos-Pérez, W., Canales-Aguirre, A. A., Pérez-Robles, M., & Martinez-Lopez, E. (2025). Dietary n-6:n-3 PUFA Ratio Modulates Inflammation-Related Gene Expression and Influences Improvements in Biochemical Parameters in a Murine Model of Diet-Induced Obesity. Nutrients, 17(12), 1996. https://doi.org/10.3390/nu17121996









