Association of Oral Frailty with Physical Frailty and Malnutrition in Patients on Peritoneal Dialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Assessment of Oral Frailty
2.4. Outcomes
Assessments of Physical Frailty, Sarcopenia, Nutritional Status
2.5. Assessment Tools
2.5.1. Revised J-CHS
2.5.2. FRAIL Scale
2.5.3. AWGS2019 Criteria
2.5.4. SARC-CalF
2.5.5. MNA-SF
2.5.6. MUST
2.5.7. GLIM Criteria
2.6. Data Collection
2.7. Statistical Analysis
3. Results
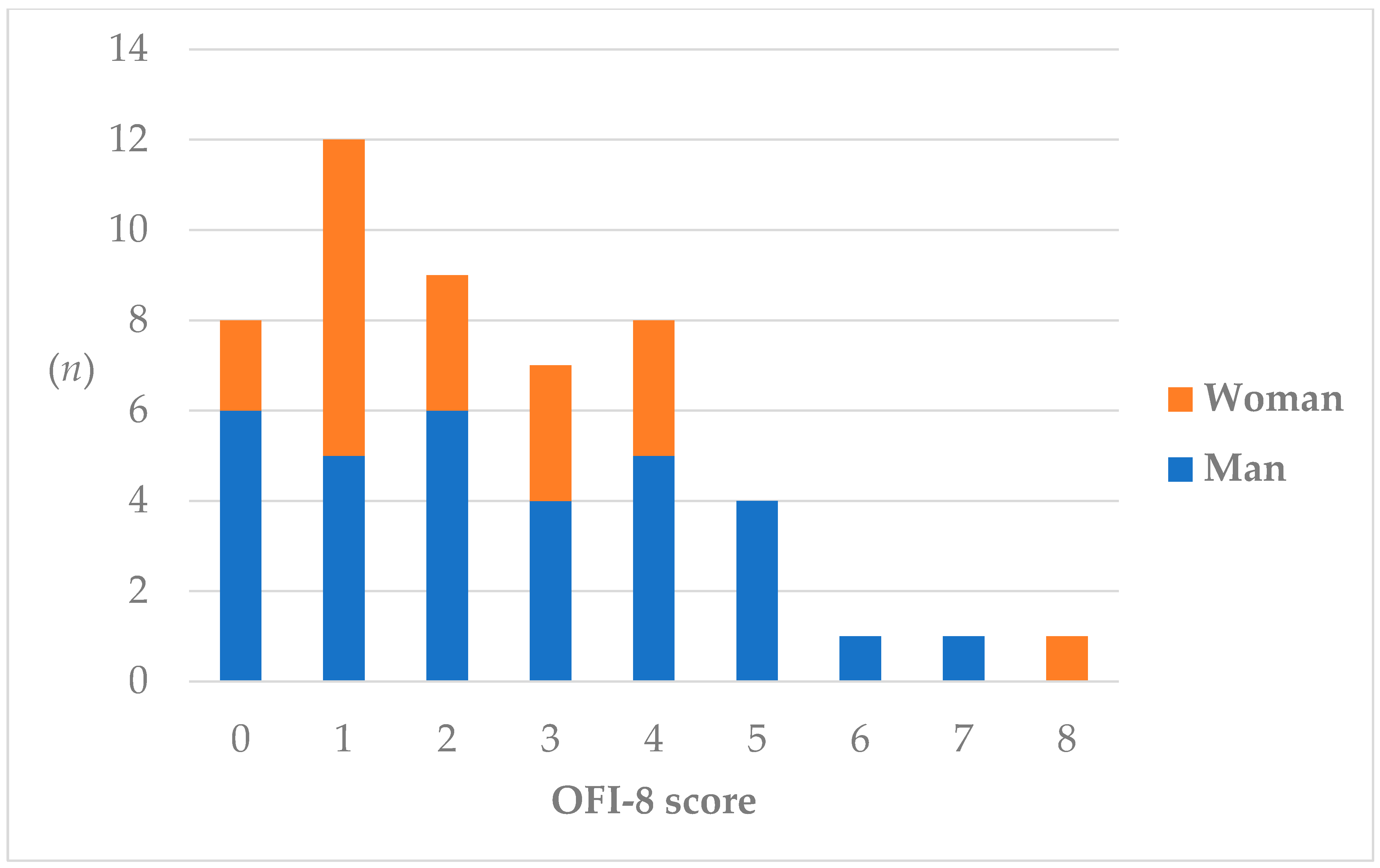
3.1. Prevalence of Oral Frailty
3.1.1. Clinical Characteristics at Baseline
3.1.2. Risk Categories for Assessments of Physical Frailty, Sarcopenia, and Nutritional Status at Baseline
3.1.3. One-Year Changes in Body Indicators
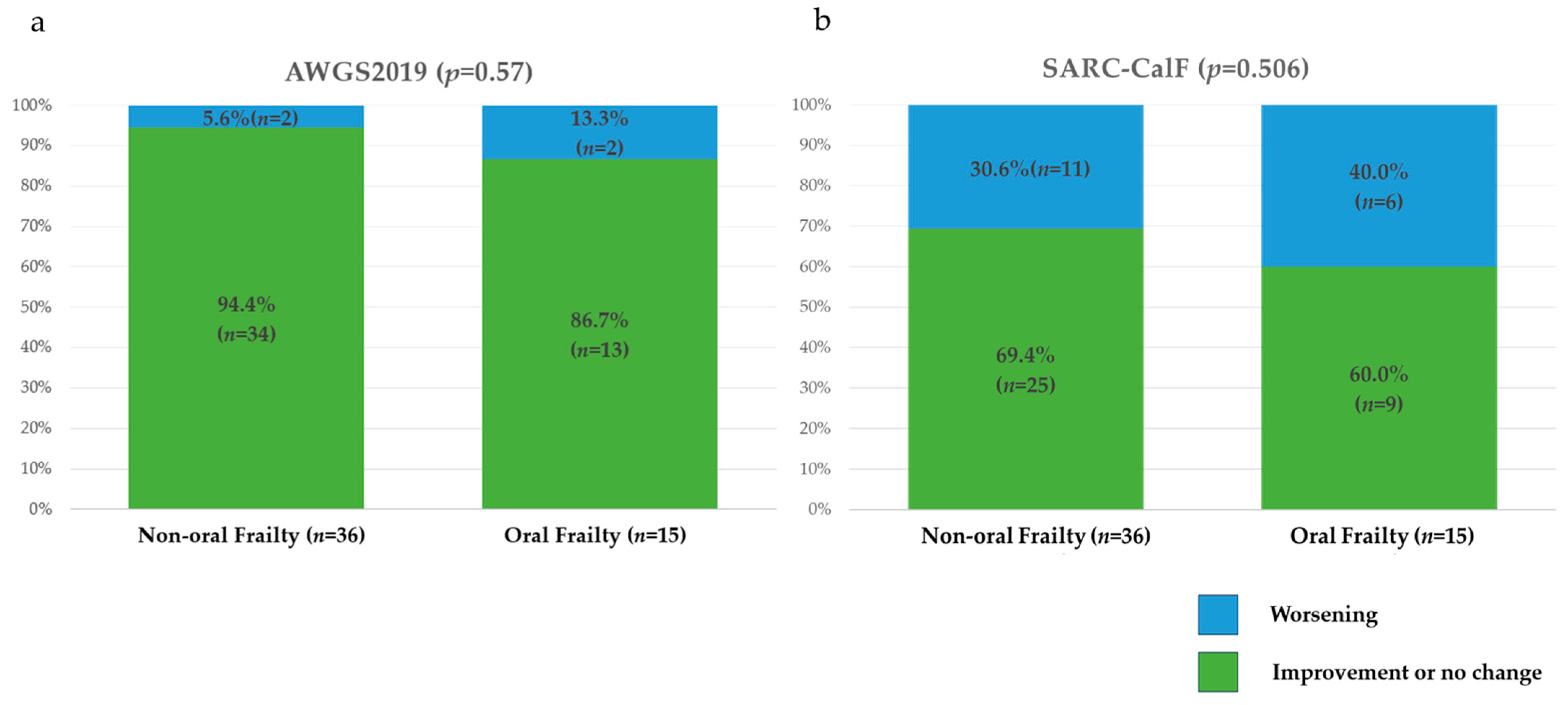
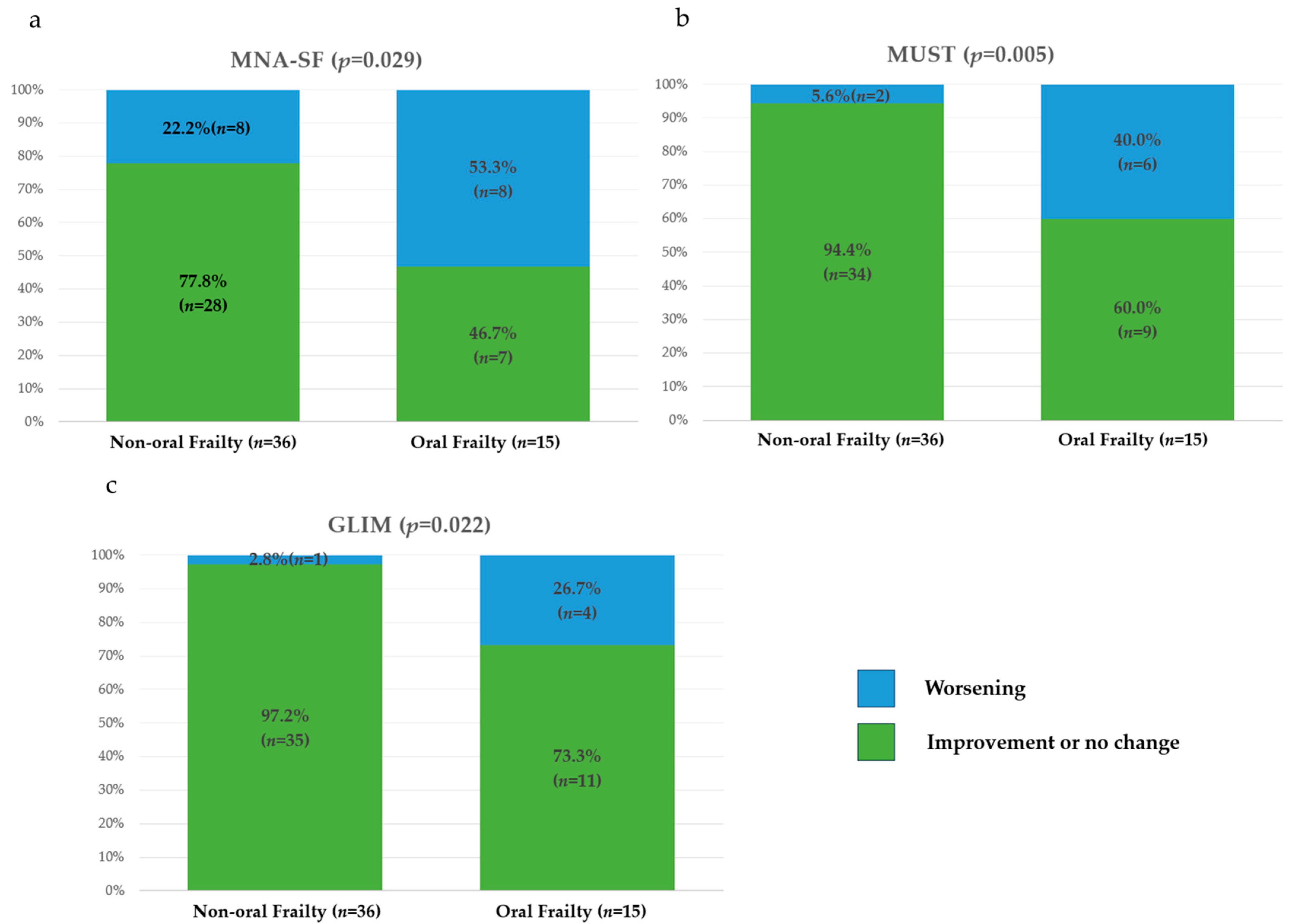
3.1.4. One-Year Changes in Risk Categories and Total Scores for Assessments of Physical Frailty, Sarcopenia, and Nutritional Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statistics Bureau of Japan Result of the Population Estimates. Monthly Report. September 2024. Available online: https://www.stat.go.jp/english/data/jinsui/tsuki/index.html (accessed on 1 February 2025).
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in Elderly People. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Kallenberg, M.H.; Kleinveld, H.A.; Dekker, F.W.; van Munster, B.C.; Rabelink, T.J.; van Buren, M.; Mooijaart, S.P. Functional and Cognitive Impairment, Frailty, and Adverse Health Outcomes in Older Patients Reaching ESRD-a Systematic Review. Clin. J. Am. Soc. Nephrol. 2016, 11, 1624–1639. [Google Scholar] [CrossRef]
- Chen, Y.T.; Lai, T.S.; Tsao, H.M.; Lai, C.F.; Yang, S.Y.; Chen, Y.M. Clinical Implications of Frailty in Peritoneal Dialysis Patients—A Prospective Observational Study. J. Formos. Med. Assoc. 2024, 123, 248–256. [Google Scholar] [CrossRef]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. J. Gerontol. Ser. A 2004, 59, 255–263. [Google Scholar] [CrossRef]
- Tanaka, T.; Hirano, H.; Ikebe, K.; Ueda, T.; Iwasaki, M.; Minakuchi, S.; Arai, H.; Akishita, M.; Kozaki, K.; Iijima, K. Commission Report Epidemiology Clinical Practice and Health. Consensus Statement on “Oral Frailty” from the Japan Geriatrics Society, the Japanese Society of Gerodontology, and the Japanese Association on Sarcopenia and Frailty. Geriatr. Gerontol. Int. 2024, 24, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Japan Dental Association Manual on Implementing Oral Frailty Countermeasures in Community Settings: Towards Integrated Implementation of Healthcare Services and Long-Term Care Need Prevention for Older Adults, 2020 Edition. Summary Leaflet. Available online: https://www.jda.or.jp/oral_frail/2020/pdf/2020-manual-all.pdf (accessed on 1 March 2025).
- Iwasaki, M.; Shirobe, M.; Motokawa, K.; Tanaka, T.; Ikebe, K.; Ueda, T.; Minakuchi, S.; Akishita, M.; Arai, H.; Iijima, K.; et al. Prevalence of Oral Frailty and Its Association with Dietary Variety, Social Engagement, and Physical Frailty: Results from the Oral Frailty 5-Item Checklist. Geriatr. Gerontol. Int. 2024, 24, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, X. An Analysis of Influencing Factors of Oral Frailty in the Elderly in the Community. BMC Oral Health 2024, 24, 260. [Google Scholar] [CrossRef]
- Nagao-Nishiwaki, R.; Nishimura, A.; Ohtsuki, M.; Kato, T.; Sudo, A. Relationship between Oral Frailty and Locomotive Syndrome in Working-Age Individuals: A Cross-Sectional Survey of Workers in Japan. BMC Oral Health 2023, 23, 711. [Google Scholar] [CrossRef]
- Ohara, Y.; Motokawa, K.; Watanabe, Y.; Shirobe, M.; Inagaki, H.; Motohashi, Y.; Edahiro, A.; Hirano, H.; Kitamura, A.; Awata, S.; et al. Association of Eating Alone with Oral Frailty among Community-Dwelling Older Adults in Japan. Arch. Gerontol. Geriatr. 2020, 87, 104014. [Google Scholar] [CrossRef]
- Hironaka, S.; Kugimiya, Y.; Watanabe, Y.; Motokawa, K.; Hirano, H.; Kawai, H.; Kera, T.; Kojima, M.; Fujiwara, Y.; Ihara, K.; et al. Association between Oral, Social, and Physical Frailty in Community-Dwelling Older Adults. Arch. Gerontol. Geriatr. 2020, 89, 104105. [Google Scholar] [CrossRef] [PubMed]
- Shirobe, M.; Watanabe, Y.; Tanaka, T.; Hirano, H.; Kikutani, T.; Nakajo, K.; Sato, T.; Furuya, J.; Minakuchi, S.; Iijima, K. Effect of an Oral Frailty Measures Program on Community-Dwelling Elderly People: A Cluster-Randomized Controlled Trial. Gerontology 2022, 68, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Ohara, Y.; Yoshida, N.; Kono, Y.; Hirano, H.; Yoshida, H.; Mataki, S.; Sugimoto, K. Effectiveness of an Oral Health Educational Program on Community-Dwelling Older People with Xerostomia. Geriatr. Gerontol. Int. 2015, 15, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, M.J.; Gahl, G.M.; Kopple, J.D.; Kamdar, A.V.; Jones, M.R.; Kessel, M.; Coburn, J.W. Protein Losses during Peritoneal Dialysis. Kidney Int. 1981, 19, 593–602. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Kosaka, S.; Ohara, Y.; Naito, S.; Iimori, S.; Kado, H.; Hatta, T.; Yanishi, M.; Uchida, S.; Tanaka, M. Association among Kidney Function, Frailty, and Oral Function in Patients with Chronic Kidney Disease: A Cross-Sectional Study. BMC Nephrol. 2020, 21, 357. [Google Scholar] [CrossRef]
- Miyasato, K.; Kobayashi, Y.; Ichijo, K.; Yamaguchi, R.; Takashima, H.; Maruyama, T.; Abe, M. Oral Frailty as a Risk Factor for Malnutrition and Sarcopenia in Patients on Hemodialysis: A Prospective Cohort Study. Nutrients 2024, 16, 3467. [Google Scholar] [CrossRef]
- Tanaka, T.; Hirano, H.; Ohara, Y.; Nishimoto, M.; Iijima, K. Oral Frailty Index-8 in the Risk Assessment of New-Onset Oral Frailty and Functional Disability among Community-Dwelling Older Adults. Arch. Gerontol. Geriatr. 2021, 94, 104340. [Google Scholar] [CrossRef]
- Satake, S.; Shimada, H.; Yamada, M.; Kim, H.; Yoshida, H.; Gondo, Y.; Matsubayashi, K.; Matsushita, E.; Kuzuya, M.; Kozaki, K.; et al. Prevalence of Frailty among Community-Dwellers and Outpatients in Japan as Defined by the Japanese Version of the Cardiovascular Health Study Criteria. Geriatr. Gerontol. Int. 2017, 17, 2629–2634. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A Simple Frailty Questionnaire (Frail) Predicts Outcomes in Middle Aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, T.G.; Menezes, A.M.B.; Bielemann, R.M.; Malmstrom, T.K.; Gonzalez, M.C. Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. Ser. A 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN Guidelines for Nutrition Screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Watanabe, D.; Yoshida, T.; Watanabe, Y.; Yokoyama, K.; Yamada, Y.; Kikutani, T.; Yoshida, M.; Miyachi, M.; Kimura, M. Oral Frailty Is Associated with Mortality Independently of Physical and Psychological Frailty among Older Adults. Exp. Gerontol. 2024, 191, 112446. [Google Scholar] [CrossRef]
- Yoshioka, M.; Shirayama, Y.; Imoto, I.; Hinode, D.; Yanagisawa, S.; Takeuchi, Y. Current Status of Collaborative Relationships between Dialysis Facilities and Dental Facilities in Japan: Results of a Nationwide Survey. BMC Nephrol. 2015, 16, 17. [Google Scholar] [CrossRef]
- Chen, M.; He, M.; Gu, Q.; Gao, X.; Lu, G. The Current Status and Influencing Factors of Oral Frailty in Elderly Maintenance Hemodialysis Patients Based on the Andersen Oral Health Outcome Model. BMC Oral Health 2024, 24, 1085. [Google Scholar] [CrossRef]
- Wakikawa, T.; Nishihira, A.; Fujita, J.; Sendo, W.; Matsui, N.; Shimatani, H. An Investigation into the Intraoral Sanitary Conditions in Hemodialysis Patients and Evaluation of the Relationship between Remaining Teeth and Laboratory Values Associated with Nutritional Status. J. Jpn. Assoc. Dial. Physicians 2013, 46, 535–543. [Google Scholar]
- Palmer, S.C.; Ruospo, M.; Wong, G.; Craig, J.C.; Petruzzi, M.; De Benedittis, M.; Ford, P.; Johnson, D.W.; Tonelli, M.; Natale, P.; et al. Dental Health and Mortality in People with End-Stage Kidney Disease Treated with Hemodialysis: A Multinational Cohort Study. Am. J. Kidney Dis. 2015, 66, 666–676. [Google Scholar] [CrossRef]
- Tanaka, T.; Hirano, H.; Ikebe, K.; Ueda, T.; Iwasaki, M.; Shirobe, M.; Minakuchi, S.; Akishita, M.; Arai, H.; Iijima, K. Oral Frailty Five-Item Checklist to Predict Adverse Health Outcomes in Community-Dwelling Older Adults: A Kashiwa Cohort Study. Geriatr. Gerontol. Int. 2023, 23, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shen, Y.; Leng, Y.; Zeng, Y.; Li, L.; Yang, Z.; Wang, S.; Jiang, Y. The Prevalence of Oral Frailty among Older Adults: A Systematic Review and Meta-analysis. Eur. Geriatr. Med. 2024, 15, 645–655. [Google Scholar] [CrossRef]
- Nakagawa, S.; Miura, K.; Arai, E.; Taira, K.; Watanabe, Y.; Shirobe, M.; Motokawa, K.; Ohara, Y.; Iwasaki, M.; Hirano, H.; et al. Oral Frailty, Appetite and Dietary Variety in Late-Stage Older Adults: A Cross-Sectional Study (the STudy of LAte-Stage OldeR AdulTs in Tottori; START Tottori). Geriatr. Gerontol. Int. 2024, 24, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Ohta, Y.; Furusho, N.; Kakuta, S.; Muraoka, K.; Ansai, T.; Awano, S.; Fukuhara, M.; Nakamura, H. Association between Oral Frailty and Nutritional Status among Hemodialysis Patients Aged ≥50 Years. Geriatr. Gerontol. Int. 2024, 24, 891–897. [Google Scholar] [CrossRef]
- Masaki, T.; Hanafusa, N.; Abe, M.; Joki, N.; Hoshino, J.; Taniguchi, M.; Kikuchi, K.; Hasegawa, T.; Goto, S.; Komaba, H.; et al. Annual Dialysis Data Report, JSDT Renal Data Registry. J. Jpn. Soc. Dial. Ther. 2024, 57, 543–620. [Google Scholar]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Touger-Decker, R. Diet, Cardiovascular Disease and Oral Health: Promoting Health and Reducing Risk. J. Am. Dent. Assoc. 2010, 141, 167–170. [Google Scholar] [CrossRef]
- Roberts, H.C.; Lim, S.E.R.; Cox, N.J.; Ibrahim, K. The Challenge of Managing Undernutrition in Older People with Frailty. Nutrients 2019, 11, 808. [Google Scholar] [CrossRef]
- Rani, A.N.; Reddy, M.P.; Kulkarni, S.; Srilatha, A.; Reddy, L.S. Assessment of Periodontal Status in Patients Undergoing Renal Dialysis—A Hospital-Based Study. J. Indian Soc. Periodontol. 2024, 28, 224–230. [Google Scholar] [CrossRef]
- Kir, S.; Komaba, H.; Garcia, A.P.; Economopoulos, K.P.; Liu, W.; Lanske, B.; Hodin, R.A.; Spiegelman, B.M. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab. 2016, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.A. Consequences and Management of Hyperphosphatemia in Patients with Renal Insufficiency. Kidney Int. 2005, 67, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Komaba, H.; Zhao, J.; Yamamoto, S.; Nomura, T.; Fuller, D.S.; McCullough, K.P.; Evenepoel, P.; Christensson, A.; Zhao, X.; Alrukhaimi, M.; et al. Secondary Hyperparathyroidism, Weight Loss, and Longer Term Mortality in Haemodialysis Patients: Results from the DOPPS. J. Cachexia Sarcopenia Muscle 2021, 12, 855–865. [Google Scholar] [CrossRef]
- Alcalde-Estévez, E.; Sosa, P.; Asenjo-Bueno, A.; Plaza, P.; Valenzuela, P.L.; Naves-Díaz, M.; Olmos, G.; López-Ongil, S.; Ruiz-Torres, M.P. Dietary Phosphate Restriction Prevents the Appearance of Sarcopenia Signs in Old Mice. J. Cachexia Sarcopenia Muscle 2023, 14, 1060–1074. [Google Scholar] [CrossRef]
- Woods, N.F.; LaCroix, A.Z.; Gray, S.L.; Aragaki, A.; Cochrane, B.B.; Brunner, R.L.; Masaki, K.; Murray, A.; Newman, A.B. Frailty: Emergence and Consequences in Women Aged 65 and Older in the Women’s Health Initiative Observational Study. J. Am. Geriatr. Soc. 2005, 53, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Uchida, H.A.; Kakio, Y.; Okuyama, Y.; Okuyama, M.; Umebayashi, R.; Wada, K.; Sugiyama, H.; Sugimoto, K.; Rakugi, H.; et al. The Prevalence of Frailty and Its Associated Factors in Japanese Hemodialysis Patients. Aging Dis. 2018, 9, 192–207. [Google Scholar] [CrossRef]
- Davenport, A. Comparison of Frailty, Sarcopenia and Protein Energy Wasting in a Contemporary Peritoneal Dialysis Cohort. Perit. Dial. Int. 2022, 42, 571–577. [Google Scholar] [CrossRef]
- Jegatheswaran, J.; Chan, R.; Hiremath, S.; Moorman, D.; Suri, R.S.; Ramsay, T.; Zimmerman, D. Use of the FRAIL Questionnaire in Patients With End-Stage Kidney Disease. Can. J. Kidney Health Dis. 2020, 7, 2054358120952904. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Suzuki, T. Impact of Physical Frailty on Disability in Community-Dwelling Older Adults: A Prospective Cohort Study. BMJ Open 2015, 5, e008462. [Google Scholar] [CrossRef]
- Ku, E.; Kopple, J.D.; Johansen, K.L.; McCulloch, C.E.; Go, A.S.; Xie, D.; Lin, F.; Hamm, L.L.; He, J.; Kusek, J.W.; et al. Longitudinal Weight Change During CKD Progression and Its Association With Subsequent Mortality. Am. J. Kidney Dis. 2018, 71, 657–665. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, C.H.; Tsai, J.P.; Chen, C.T.; Chen, Y.H.; Hung, S.C.; Hsu, B.G. A Comparison of SARC-F, Calf Circumference, and Their Combination for Sarcopenia Screening among Patients Undergoing Peritoneal Dialysis. Nutrients 2022, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Fortes, R.C.; Ferreira Martins, T.; Lopes Alves, M.; Cipriano, G.; França Bernardelli Cipriano, G. Performance of SARC-F and SARC-CalF Scores to Predict Risk of Mortality in Hemodialysis Patients: A Cohort Study. Sci. Rep. 2024, 14, 23262. [Google Scholar] [CrossRef]
- Wang, L.; Xue, C.; Zhu, B.; Zhou, F.; Luo, Q. The Association between Changes in Muscle Mass and Function and Mortality in Individuals Receiving Peritoneal Dialysis. Perit. Dial. Int. 2025, 45, 84–92. [Google Scholar] [CrossRef]
- Rashid, I.; Sahu, G.; Tiwari, P.; Willis, C.; Asche, C.V.; Bagga, T.K.; Ghule, P.; Bland, A. Malnutrition as a Potential Predictor of Mortality in Chronic Kidney Disease Patients on Dialysis: A Systematic Review and Meta-Analysis. Clin. Nutr. 2024, 43, 1760–1769. [Google Scholar] [CrossRef]
- Kittiskulnam, P.; Chuengsaman, P.; Kanjanabuch, T.; Katesomboon, S.; Tungsanga, S.; Tiskajornsiri, K.; Praditpornsilpa, K.; Eiam-Ong, S. Protein-Energy Wasting and Mortality Risk Prediction Among Peritoneal Dialysis Patients. J. Ren. Nutr. 2021, 31, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, E.; Vanden Wyngaert, K.; Van Craenenbroeck, A.H.; Van Biesen, W.; Eloot, S. The Screening Score of Mini Nutritional Assessment (MNA) Is a Useful Routine Screening Tool for Malnutrition Risk in Patients on Maintenance Dialysis. PLoS ONE 2020, 15, e0229722. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Li, Y.; Wang, Y.; Chang, L.; Deng, L.; Li, Y.; Jiang, H.; Zhou, P. Prevalence of Malnutrition Based on Global Leadership Initiative in Malnutrition Criteria for Completeness of Diagnosis and Future Risk of Malnutrition Based on Current Malnutrition Diagnosis: Systematic Review and Meta-Analysis. Front. Nutr. 2023, 10, 1174945. [Google Scholar] [CrossRef]
- Avesani, C.M.; Sabatino, A.; Guerra, A.; Rodrigues, J.; Carrero, J.J.; Rossi, G.M.; Garibotto, G.; Stenvinkel, P.; Fiaccadori, E.; Lindholm, B. A Comparative Analysis of Nutritional Assessment Using Global Leadership Initiative on Malnutrition Versus Subjective Global Assessment and Malnutrition Inflammation Score in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2022, 32, 476–482. [Google Scholar] [CrossRef]
- Wang, D.; Yin, J.; Liao, W.; Feng, X.; Zhang, F. GLIM Criteria for Definition of Malnutrition in Peritoneal Dialysis: A New Aspect of Nutritional Assessment. Ren. Fail. 2024, 46, 2337290. [Google Scholar] [CrossRef]
- Stratton, R.J.; King, C.L.; Stroud, M.A.; Jackson, A.A.; Elia, M. ‘Malnutrition Universal Screening Tool’ Predicts Mortality and Length of Hospital Stay in Acutely Ill Elderly. Br. J. Nutr. 2006, 95, 325–330. [Google Scholar] [CrossRef]
- Iyota, K.; Mizutani, S.; Kishimoto, H.; Oku, S.; Tani, A.; Yatsugi, H.; Chu, T.; Liu, X.; Kashiwazaki, H. Effect of Isometric Tongue Lifting Exercise on Oral Function, Physical Function, and Body Composition in Community-Dwelling Older Individuals: A Pilot Study. Gerontology 2022, 68, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, R.; Masuda, Y.; Ogawa, K.; Tanaka, T.; Kanazawa, M.; Suzuki, K.; Stading, M.; Iijima, K.; Matsuo, K. Impact of the Comprehensive Awareness Modification of Mouth, Chewing and Meal (CAMCAM) Program on the Attitude and Behavior Towards Oral Health and Eating Habits as Well as the Condition of Oral Frailty: A Pilot Study. J. Nutr. Health Aging 2023, 27, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, H.; Moriishi, M.; Tsuchiya, S. Five Years’ Experience of Combination Therapy: Peritoneal Dialysis with Hemodialysis. Adv. Perit. Dial. 2002, 18, 62–67. [Google Scholar] [PubMed]


| Variable | All Patients | Non-Oral Frailty Group | Oral Frailty Group | p Value |
|---|---|---|---|---|
| Patients, n (male, %) | 51 (62.8) | 36 (58.3) | 15 (73.3) | 0.313 |
| Age, years | 59.1 ± 12.8 | 56.1 ± 10.8 | 66.3 ± 14.7 | 0.009 |
| Duration of dialysis, months | 60 (34–147) | 56 (25–141) | 63 (44–171) | 0.380 |
| Height, m | 1.65 ± 0.07 | 1.66 ± 0.07 | 1.64 ± 0.08 | 0.396 |
| Weight, kg | 65.3 ± 14.5 | 64.9 ± 14.1 | 66.2 ± 15.9 | 0.772 |
| BMI, kg/m2 | 23.8 ± 4.4 | 23.5 ± 4.1 | 24.6 ± 5.1 | 0.440 |
| SMI, kg/m2 | 7.7 ± 1.5 | 7.8 ± 1.7 | 7.5 ± 0.9 | 0.547 |
| CC, cm | 35.5 ± 4.4 | 35.8 ± 4.0 | 35.0 ± 5.4 | 0.574 |
| Grip strength, kg | 25.9 ± 8.5 | 26.5 ± 7.7 | 24.6 ± 10.5 | 0.487 |
| Gait speed, m/s | 1.1 (0.8–1.2) | 1.1 (1.0–1.2) | 0.8 (0.7–1.1) | <0.001 |
| Number of teeth, <20 (%) | 8 (15.7) | 2 (5.6) | 6 (40.0) | 0.005 |
| Systolic blood pressure, mmHg | 140 ± 16 | 142 ± 17 | 137 ± 15 | 0.389 |
| Diastolic blood pressure, mmHg | 82 ± 12 | 85 ± 10 | 74 ± 11 | 0.002 |
| Heart rate, bpm | 82 ± 14 | 85 ± 14 | 75 ± 14 | 0.031 |
| Serum urea nitrogen, mg/dL | 52.3 ± 12.1 | 52.6 ± 12.7 | 51.6 ± 10.8 | 0.780 |
| Creatinine, mg/dL | 9.6 ± 3.4 | 10.0 ± 3.7 | 8.8 ± 2.7 | 0.247 |
| Hemoglobin, g/dL | 11.0 ± 1.1 | 11.0 ± 1.2 | 11.3 ± 0.8 | 0.251 |
| Albumin, g/dL | 3.2 ± 0.5 | 3.3 ± 0.5 | 3.0 ± 0.5 | 0.090 |
| Corrected calcium, mg/dL | 9.1 ± 0.7 | 9.1 ± 0.8 | 9.0 ± 0.5 | 0.468 |
| Phosphate, mg/dL | 5.4 ± 1.4 | 5.4 ± 1.3 | 5.4 ± 1.6 | 0.907 |
| Intact PTH, pg/mL | 212 (148–377) | 183 (132–297) | 275 (198–631) | 0.045 |
| CRP, mg/dL | 0.11 (0.1–0.48) | 0.1 (0.1–0.29) | 0.48 (0.2–1.73) | 0.011 |
| TIBC, µg/dL | 256 ± 58 | 263 ± 62 | 240 ± 46 | 0.206 |
| Total cholesterol, mg/dL | 174 ± 37 | 179 ± 39 | 160 ± 25 | 0.090 |
| APD/CAPD, (n) | 44/7 | 32/3 | 12/4 | 0.174 |
| Dialysate volume, mL/day | 6271 ± 2425 | 6428 ± 2352 | 5893 ± 2637 | 0.479 |
| Number of dialysate exchanges, times/day | 4 (3–5) | 4 (3–5) | 4 (3–4) | 0.492 |
| HD combination, n (%) | 8 (15.7) | 4 (11.1) | 4 (26.7) | 0.213 |
| Cause of ESKD, n (%) | ||||
| Diabetic nephropathy | 21 | 14 (27.5) | 7 (13.7) | 0.607 |
| Hypertension | 13 | 8 (15.7) | 5 (9.8) | 0.407 |
| Chronic glomerular nephritis | 2 | 2 (3.9) | 0 (0) | 1.000 |
| IgA nephropathy | 5 | 4 (7.8) | 1 (2.0) | 1.000 |
| Polycystic kidney disease | 6 | 5 (9.8) | 1 (2.0) | 0.657 |
| Others | 4 | 3 (5.9) | 1 (2.0) | 1.000 |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 23 (45.1) | 15 (41.7) | 8 (53.3) | 0.446 |
| Hypertension | 50 (98.0) | 35 (97.2) | 15 (100) | 1.000 |
| Dyslipidemia | 33 (64.7) | 22 (61.1) | 11 (73.3 | 0.527 |
| CVD | 12 (23.5) | 3 (8.3) | 9 (60.3) | <0.001 |
| PD-related peritonitis | 24 (47.1) | 17 (47.2) | 7 (46.7) | 0.971 |
| Smoking, n (%) | 27(52.9) | 17 (47.2) | 10 (66.7) | 0.205 |
| Drinking, n(%) | 15 (29.4) | 10 (27.8) | 5 (33.3) | 0.692 |
| Employment, n (%) | 31 (60.8) | 27 (75.0) | 4 (26.7) | 0.002 |
| Variables | Univariate | Multivariate (R2 = 0.495) | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |||
| Lower | Upper | Lower | Upper | |||||
| Male sex (vs. female) | 1.96 | 0.52 | 7.37 | 0.317 | ||||
| Age (per 1-year increase) | 1.07 | 1.02 | 1.14 | 0.016 | ||||
| Duration of dialysis (per month) | 1.00 | 0.99 | 1.01 | 0.398 | ||||
| BMI (per 1 kg/m2 increase) | 1.06 | 0.92 | 1.21 | 0.433 | ||||
| SMI (per 1 kg/m2 increase) | 0.87 | 0.57 | 1.34 | 0.540 | ||||
| CC (per 1 cm increase) | 0.96 | 0.84 | 1.04 | 0.566 | ||||
| Grip strength (per 1 kg increase) | 0.97 | 0.91 | 1.03 | 0.479 | ||||
| Gait speed (per 1 m/s increase) | 0.01 | 0.0004 | 0.20 | 0.003 | ||||
| Fewer teeth (<20 teeth vs. ≥20 teeth) | 11.3 | 1.95 | 65.9 | 0.007 | ||||
| Serum urea nitrogen (per 1 mg/dL increase) | 0.99 | 0.94 | 1.01 | 0.775 | ||||
| Creatinine (per 1 mg/dL increase) | 0.90 | 0.75 | 1.08 | 0.243 | ||||
| Hemoglobin (per 1 g/dL increase) | 1.43 | 0.78 | 2.60 | 0.249 | ||||
| Albumin (per 1 g/dL increase) | 0.31 | 0.08 | 3.23 | 0.095 | ||||
| Corrected calcium (per 1 mg/dL increase) | 0.70 | 0.28 | 1.80 | 0.462 | ||||
| Phosphate (per 1 mg/dL increase) | 1.03 | 0.66 | 1.60 | 0.905 | ||||
| Intact PTH (per 1 pg/dL increase) | 1.00 | 1.00 | 1.01 | 0.022 | ||||
| CRP (per 1 mg/dL increase) | 7.96 | 1.88 | 33.8 | 0.005 | 12.2 | 2.01 | 74.2 | 0.007 |
| TIBC (per 1 µg/dL increase) | 0.99 | 0.98 | 1.01 | 0.208 | ||||
| Total cholesterol (per 1 mg/dL increase) | 0.98 | 0.97 | 1.00 | 0.093 | ||||
| APD (vs. CAPD) | 0.25 | 0.05 | 1.30 | 0.099 | ||||
| Diabetes mellitus (yes vs. no) | 1.23 | 0.37 | 4.12 | 0.743 | ||||
| Hyperlipidemia (yes vs. no) | 1.75 | 0.47 | 6.59 | 0.408 | ||||
| History of CVD (yes vs. no) | 16.5 | 3.43 | 79.3 | <0.001 | 16.6 | 2.17 | 127.6 | 0.007 |
| History of PD-related peritonitis (yes vs. no) | 0.98 | 0.29 | 3.27 | 0.971 | ||||
| Smoking (current smoker vs. non-smoker) | 2.24 | 0.64 | 7.86 | 0.21 | ||||
| Drinking (yes vs. no) | 1.30 | 0.36 | 4.76 | 0.692 | ||||
| Unemployment (yes vs. employed) | 8.25 | 2.10 | 32.5 | 0.003 | 5.82 | 0.87 | 39.0 | 0.069 |
| Assessment | All Patients | Non-Oral Frailty Group | Oral Frailty Group | p Value |
|---|---|---|---|---|
| Revised J-CHS, % | 0.007 | |||
| Normal | 21.6 | 25 | 13.3 | |
| Pre-frailty | 51 | 61.1 | 26.7 | |
| Frailty | 27.5 | 13.9 | 60 | |
| FRAIL scale, % | 0.022 | |||
| Normal | 9.8 | 13.9 | 0 | |
| Pre-frailty | 56.9 | 63.9 | 40 | |
| Frailty | 33.3 | 22.2 | 60 | |
| AWGS2019, % | 0.46 | |||
| Normal | 88.2 | 91.7 | 80 | |
| Sarcopenia | 3.9 | 2.8 | 6.7 | |
| Severe sarcopenia | 7.8 | 5.6 | 13.3 | |
| SARC-CalF, % | 0.047 | |||
| Normal | 80.4 | 88.9 | 60 | |
| Sarcopenia | 19.6 | 11.1 | 40 | |
| MNA-SF, % | 0.475 | |||
| Normal | 7.8 | 5.6 | 13.3 | |
| Risk of malnutrition | 49 | 47.2 | 53.3 | |
| Malnutrition | 43.1 | 47.2 | 33.3 | |
| MUST, % | 0.728 | |||
| Low risk | 64.7 | 61.1 | 73.3 | |
| Moderate risk | 23.5 | 25 | 20 | |
| High risk | 11.8 | 13.9 | 6.7 | |
| GLIM, % | 0.746 | |||
| Normal | 66.7 | 63.9 | 73.3 | |
| Malnutrition | 33.3 | 36.1 | 26.7 |
| Variable | All Patients | Non-Oral Frailty Group | Oral Frailty Group | p Value |
|---|---|---|---|---|
| SMI, kg/m2 | −0.02 ± 0.78 | 0.14 ± 0.65 | −0.41 ± 0.93 | 0.018 |
| CC, cm | 0.26 ± 1.81 | 0.75 ± 1.54 | −0.91 ± 1.92 | 0.002 |
| Grip strength, kg | 0.44 ± 4.05 | 0.90 ± 3.90 | −0.68 ± 4.32 | 0.207 |
| Gait speed, m/s | −0.03 ± 0.15 | −0.02 ± 0.15 | −0.09 ± 0.11 | 0.127 |
| Weight, kg | −0.01 ± 3.24 | 1.18 ± 2.75 | −2.87 ± 2.49 | <0.001 |
| BMI, kg/m2 | −0.04 ± 1.22 | 0.40 ± 1.01 | −1.10 ± 1.00 | <0.001 |
| Outcome | Adjusted Mean Difference | SE | 95% CI | p Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| SMI, kg/m2 | –0.382 | 0.335 | –1.060 | 0.293 | 0.261 |
| CC, cm | –1.550 | 0.774 | –3.110 | 0.006 | 0.051 |
| Grip strength, kg | 2.77 | 1.52 | –0.285 | 5.83 | 0.075 |
| Gait speed, m/s | 0.046 | 0.159 | –0.274 | 0.366 | 0.772 |
| Weight, kg | –2.730 | 1.16 | –5.060 | –0.386 | 0.023 |
| BMI, kg/m2 | –1.090 | 0.439 | –1.970 | –0.203 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, Y.; Matsuoka, T.; Yamaguchi, R.; Ichijo, K.; Suzuki, M.; Saito, T.; Igarashi, K.; Sato, T.; Takashima, H.; Abe, M. Association of Oral Frailty with Physical Frailty and Malnutrition in Patients on Peritoneal Dialysis. Nutrients 2025, 17, 1950. https://doi.org/10.3390/nu17121950
Kobayashi Y, Matsuoka T, Yamaguchi R, Ichijo K, Suzuki M, Saito T, Igarashi K, Sato T, Takashima H, Abe M. Association of Oral Frailty with Physical Frailty and Malnutrition in Patients on Peritoneal Dialysis. Nutrients. 2025; 17(12):1950. https://doi.org/10.3390/nu17121950
Chicago/Turabian StyleKobayashi, Yu, Tomomi Matsuoka, Ryo Yamaguchi, Kiyomi Ichijo, Miya Suzuki, Tomoyuki Saito, Kimihiro Igarashi, Tokiko Sato, Hiroyuki Takashima, and Masanori Abe. 2025. "Association of Oral Frailty with Physical Frailty and Malnutrition in Patients on Peritoneal Dialysis" Nutrients 17, no. 12: 1950. https://doi.org/10.3390/nu17121950
APA StyleKobayashi, Y., Matsuoka, T., Yamaguchi, R., Ichijo, K., Suzuki, M., Saito, T., Igarashi, K., Sato, T., Takashima, H., & Abe, M. (2025). Association of Oral Frailty with Physical Frailty and Malnutrition in Patients on Peritoneal Dialysis. Nutrients, 17(12), 1950. https://doi.org/10.3390/nu17121950







