Abstract
Background: Adherence to the Mediterranean diet (MedDiet) offers multiple metabolic benefits. However, its relationship with maximal fat oxidation (MFO) and cardiorespiratory fitness (VO2max), alongside the potential mediating role of leptin, remains underexplored in young adults. Objective: The objective was to investigate the associations between MedDiet adherence and the body mass index (BMI), MFO, and VO2max and to evaluate whether leptin mediates these relationships. Methods: Sixty-five young adults (n = 23 women), aged 18–38, were assessed for body composition, MedDiet adherence (14-Item Mediterranean Diet Adherence Screener), MFO, and VO2max through indirect calorimetry. Plasma leptin concentrations were measured in fasting conditions. Multiple linear regression models were performed, adjusting for sex, age, and both. Mediation analyses were conducted. Results: Higher MedDiet adherence was significantly associated with lower BMI (β = −0.339, p = 0.006) and leptin values (β = 0.284, p = 0.022) and higher absolute MFO (β = 0.338, p = 0.006) and VO2max values (β = 0.462, p < 0.001). These associations remained significant in all models except BMI and leptin when adjusted for sex and sex and age. Leptin was positively associated with the BMI (β = 0.550, p < 0.001) and inversely associated with absolute MFO (β = −0.650, p < 0.001) in all models. There was a trend in the association between leptin and VO2max (β = −0.233, p = 0.061) only in the unadjusted model. Mediation analysis revealed that the leptin levels significantly mediated the associations between MedDiet adherence and BMI (β = −0.358, 95% CI [−0.677, −0.077]) and VO2max (β = 1.043, 95% CI [0.280, 1.833]). Conclusions: MedDiet adherence is associated with a lower BMI and higher MFO and VO2max in young adults. Our findings further suggest that leptin plays a mediating role in how MedDiet adherence influences the BMI and VO2max.
1. Introduction
Metabolic health is closely linked to the body’s capacity to regulate substrate utilization in response to varying energy demands. A reduced capacity for maximal fat oxidation (MFO) [1,2] and lower maximal oxygen consumption (VO2max) [3] have been identified as indicators of metabolic inefficiency. These parameters are often used to characterize metabolic inflexibility, a condition defined by a limited ability to switch between fuel sources such as fats and carbohydrates [1]. MFO in particular has been proposed as a functional marker of metabolic flexibility [4,5,6], and lower fat oxidation rates during physical activity have been associated with greater cardiometabolic risk, including insulin resistance and excessive weight gain [4,7]. Investigating lifestyle-related factors associated with MFO and VO2max may contribute to a better understanding of physiological profiles linked to metabolic function.
Dietary patterns have been increasingly studied in relation to metabolic flexibility. Among them, the Mediterranean diet (MedDiet), characterized by high consumption of fruits, vegetables, whole grains, nuts, and olive oil, along with moderate intake of fish and wine, is widely recognized for its association with favorable health indicators [8]. This dietary model has been linked to lower levels of inflammation, showing that a higher adeherence to the MedDiet reduces letpin [9], leads to healthier cardiovascular profiles by reducing the risk of coronary heart disease, stroke, myocardial infarction, and overall cardiovascular disease [10], and better glycemic regulation, leading to greater reductions in HbA1c, fasting glucose, and fasting insulin [10,11]. However, despite the broad range of reported associations, the relationship between MedDiet adherence and MFO remains insufficiently studied, representing a relevant gap in the literature.
Moreover, emerging evidence suggests a potential relationship between the MedDiet and VO2max. While a systematic review and meta-analysis indicates a general association between greater MedDiet adherence and better physical fitness across various adult populations [12], research specifically for young adults remains inconsistent. Although research specifically examining this association is still limited, one cross-sectional study in young adults reported no significant correlation between overall MedDiet adherence and VO2max. However, nut consumption, a key component of the MedDiet, was positively associated with higher VO2max levels in the same study [12]. Conversely, studies in other populations, such as obese patients with hypertrophic cardiomyopathy, have shown significant VO2max improvements when the Mediterranean diet is combined with exercise [13], and similar benefits have been noted in athletes [14]. This variability highlights the need for further investigation into the specific conditions and populations where MedDiet adherence most strongly influences cardiorespiratory fitness.
Leptin may contribute to the relationship between MedDiet adherence and physiological parameters such as MFO and VO2max. This adipocyte-derived hormone plays a key role in energy homeostasis and fat metabolism. Experimental studies suggest that leptin could influence fat oxidation through mechanisms involving mitochondrial function and substrate preference, although the exact pathways remain under investigation [15]. However, evidence in humans remains limited and inconsistent, particularly regarding its relationship with aerobic capacity [16]. Moreover, leptin levels are influenced by dietary patterns and adiposity, making it a plausible mediator of dietary effects on metabolic outcomes [17]. Based on this rationale, the present study explores whether leptin mediates the relationship between MedDiet adherence and key indicators such as the body mass index (BMI), MFO, and VO2max in young adults.
To address this knowledge gap, the present study examines the associations between adherence to the MedDiet pattern and the BMI, absolute and relative MFO capacity, VO2max, and leptin levels. Moreover, we aim to determine whether leptin mediates these associations, offering novel insights into the mechanisms linking adherence to the MedDiet pattern to improvements in BMI, metabolic flexibility, and cardiorespiratory fitness.
2. Materials and Methods
2.1. Study Design
The present investigation represents a subsample derived from the cross-sectional study of the NutAF Project (Nutritional Habits and Physical Activity Levels in Adults) [18,19,20,21,22] within a cohort of adults in the province of Cádiz (Spain). Data collection occurred between January 2016 and June 2017. The study received ethical approval from the Research Ethics Committee of Cádiz (Hospital Puerta del Mar), according to the Declaration of Helsinki. All testing was conducted in the Laboratory of Physical Activity and Exercise at the University of Cádiz (Puerto Real, Cádiz, Spain). Written informed consent was obtained from all participants following a thorough explanation of the study’s objectives and methodologies and any potential risks inherent in the measurement procedures.
2.2. Participants
From the total sample of the NutAF study (n = 79), a cohort of 65 participants (n = 23 women; mean age = 22.35 ± 4.30 years) who had complete data for all variables analyzed in the present article were selected for analysis. All participants met the following inclusion and exclusion criteria: (1) age between 18 and 45 years; (2) BMI between 18.5 and 40 kg·m⁻2; (3) stable body weight (±2 kg) over the previous six months; (4) non-smoker; (5) no current or previous diagnosis of hypertension or cardiovascular disease; (6) no recent injuries or medical conditions interfering with physical testing; and (7) no adherence to specific or restrictive diets, including weight loss regimens, during the past six months. These inclusion and exclusion criteria were selected because it has been observed in the scientific literature that changes in body mass induced by exercise and diet can modify the fat oxidation capacity in young adults [23].
2.3. Procedure
All measurements were performed on a single day under standardized conditions in the morning (from 8:00 a.m. until 11:30 a.m.). Participants arrived at the laboratory in a fasted state (≥8 h) and were instructed to maintain their usual dietary and hydration habits while avoiding alcohol, caffeine, and intense physical activity in the 24 h prior to testing. Upon arrival, participants first completed the validated 14-item Mediterranean diet adherence questionnaire. Then, venous blood samples were collected to determine the plasma leptin concentrations. Subsequently, anthropometric and body composition measurements were performed using bioelectrical impedance analysis. Participants then completed the validated 14-item Mediterranean diet adherence questionnaire. Finally, an incremental exercise protocol on a cycle ergometer was used to determine maximal fat oxidation (MFO) by indirect calorimetry, followed by a previously validated VO2max test [24]. All procedures were conducted in a controlled environment (21 ± 1 °C and 50 ± 2% relative humidity), following standardized protocols previously applied in the NUTAF project.
2.3.1. Evaluation of Adherence to the Mediterranean Diet (MedDiet)
Adherence to the MedDiet was assessed using a validated 14-item Mediterranean diet adherence questionnaire [25] specifically devised for assessing MedDiet adherence in the PREDIMED study and previously validated in the Spanish population [26,27]. Each item was assigned a score of 0 or 1, with a maximum score of 14 points reflecting optimal adherence. The resulting score was classified into two categories: low adherence to the MedDiet (range: 0–7) and high adherence to the MedDiet (range: 8–14) [28]. The questionnaire was administered by trained research staff through face-to-face interviews to ensure consistency and accuracy in responses.
2.3.2. Biochemical Analysis
Fasting blood samples were collected via venipuncture and stored in Vacutainer tubes, with some containing EDTA anticoagulant. These samples were then subjected to centrifugation (2500 rpm, 15 min, 4 °C) to separate the plasma and stored at −80 °C until further analysis. Plasma leptin concentrations were determined using a MILLIPLEX® MAP Human Metabolic Hormone Magnetic Panel (HMHEMAG-34K, Millipore Sigma, Burlington, MA, USA) and the Luminex® 200™ system (Luminex Corp., Austin, TX, USA), in accordance with the manufacturer’s instructions. The intra-assay coefficients of variation were <15%.
2.3.3. Body Composition and Anthropometry
Body composition was assessed using bioelectrical impedance analysis, measuring body mass and total muscle mass with a Tanita MC-780MA multifrequency 8-electrode model (Tanita Corp, Tokyo, Japan). Standardized protocols were followed [5], including urination prior to the assessment and refraining from wearing metal objects. Anthropometric measurements were taken with the participants being barefoot and wearing light clothing. Height was measured in a standing position using a stadiometer (SECA 225; range: 60–200 cm; precision: 1 mm). Finally, the BMI was calculated by dividing the body mass (kg) by the height squared (m2). For the purposes of this study, the participants were divided into two groups based on their BMIs: normal weight (BMI: 18.5–24.9 kg/m2) and overweight or obese (BMI ≥ 25.0 kg/m2), following World Health Organization (WHO) classification [29].
2.3.4. Basal Metabolism
The study of the resting metabolic rate focused on oxygen consumption (VO2), carbon dioxide production (VCO2), and the respiratory exchange ratio (RER). Participants were instructed to remain in a supine position for 30 min, adhering to specific protocols for measuring basal metabolic rate in healthy adults [30]. Measurements were conducted via indirect calorimetry using a gas analyzer (Jaeger MasterScreen CPX® CareFusion, San Diego, CA, USA), which underwent daily calibration. During the assessment, gas analyzer readings were captured on a breath-by-breath basis and averaged every 20 s. The initial 5 min of data were excluded, with analysis performed on the subsequent 5 min, ensuring a coefficient of variation for VO2 and VCO2 not exceeding 15%. The average values of VO2 and VCO2 for the selected interval were then employed to estimate resting fat oxidation through an indirect equation [31]. This value was used as the first point on the fat oxidation curve.
2.3.5. Maximal Fat Oxidation (MFO) and Maximal Oxygen Consumption Test (VO2max)
In order to assess MFO, VO2, and VCO2, values were recorded during an incremental test performed on a cycle ergometer by utilizing a Jaeger MasterScreen CPX® gas analyzer. This procedure was based on an adapted version of a previously validated protocol [32]. The test started with a load of 30 W, which was progressively increased every 3 min until the respiratory exchange ratio (RER) reached 1.0. After 5 min for recovery, the load was further increased by 30 W per minute until exhaustion, beginning in the last load of the MFO test, which allowed for measurement of VO2max. For the obese participants, the initial load and load increments were set at 15 W. A constant cadence of 60–80 revolutions per minute was maintained, with continuous heart rate monitoring.
Fat oxidation for each stage was calculated by averaging the VO2 and VCO2 values from the last minute of each stage using Frayn’s equation [31]. The average VO2 was also used to calculate the percentage of maximal VO2 achieved. A polynomial curve was then fitted to the data for each participant, enabling comparisons of absolute MFO and MFO relativized by body mass (MFO-BM) and total muscle mass.
2.4. Statistical Analysis
Statistical analyses were conducted using SPSS version 29 (SPSS Inc., IBM, Armonk, NY, USA). A significance level of 95% (p ≤ 0.05) was established for all analyses. The normality of the distributions was assessed using the Kolmogorov–Smirnov test. Variables that deviated from a normal distribution were subjected to natural logarithmic transformation prior to further analyses. To compare the general characteristics of the sample based on BMI category (normal weight vs. overweight or obese) and adherence to the Mediterranean Diet (low vs. high), independent samples t-tests were conducted. Effect sizes were calculated using Cohen’s d and interpreted according to conventional thresholds (small: ≥0.2; medium: ≥0.5; large: ≥0.8).
Linear regression models were employed to explore the associations between adherence to the MedDiet as the independent variable and the BMI, absolute MFO, MFO per body mass (MFO-BM), MFO per total muscle mass (MFO-MM), VO2max, and leptin concentration as dependent variables. Four distinct linear regression models were evaluated: Model 0 (unadjusted), Model 1 (adjusted for sex), Model 2 (adjusted for age), and Model 3 (adjusted for sex and age). Moreover, linear regression models were employed to explore the associations between leptin as the independent variable and the BMI, absolute MFO, MFO-BM, MFO-MM, and VO2max as dependent variables with the same models.
Additionally, to ascertain the potential influence of leptin concentrations on adherence to the MedDiet and their impact on the absolute MFO, MFO-BM, MFO-MM, and VO2max, mediation analyses were conducted with leptin as the mediating variable. The mediating variable was similarly adjusted in alignment with the dependent variable. Linear regression models were adjusted using bootstrapping methods and implemented through Hayes’ PROCESS macro for SPSS (Armonk, NY, USA), employing a resampling procedure of 10,000 bootstrap samples [33,34]. This methodology facilitates the computation of confidence intervals for mediator effects without requiring assumptions regarding data distribution. Thus, mediation analyses were performed to assess the role of leptin concentrations as a mediator between adherence to the MedDiet and several outcomes: BMI, absolute MFO, MFO-BM, and VO2max. The analysis evaluated the total effect (Equation (2)) and the direct effect (Equations (1), (3), and (3’)), based on unstandardized regression coefficients (B) and their corresponding significance levels. Specifically, Equation (1) regressed the independent variable (MedDiet adherence) on the mediator (log-transformed leptin concentrations). Equation (2) regressed the independent variable (MedDiet adherence) on each dependent variable (BMI, absolute MFO, MFO-BM, and VO2max). Equation (3) regressed both the mediator (leptin concentrations) and the independent variable (MedDiet adherence) on each dependent variable. Acknowledging that optimal mediation analysis typically benefits from larger samples (e.g., >200 participants), we employed 10,000 sample bootstrapping to strengthen the statistical inference despite our cohort of 65, as previously performed by other authors [18]. Subsequently, indirect effects were calculated, and their 95% confidence intervals (CIs) were estimated using bootstrapping procedures (10,000 resamples) [34,35], a non-parametric method that does not assume normal distribution of the indirect effects. Mediation was considered present when the bootstrap confidence interval for the indirect effect did not include zero.
3. Results
Table 1 presents the general characteristics of the study participants and their differences according to BMI category (normal weight vs. overweight or obese) and adherence to the MedDiet (low vs. high). Participants who were overweight or obese showed higher BMI and leptin levels and lower absolute and relative MFO and VO2max levels compared with the normal-weight individuals, with large effect sizes (Cohen’s d > 0.8). Similarly, those with low adherence to the MedDiet exhibited higher leptin, lower VO2max, and lower MFO levels (particularly per body weight MFO-BM), with moderate-to-large effects.

Table 1.
General characteristics of the sample and differences by BMI category (normal weight vs. overweight or obese) and adherence to the Mediterranean Diet (MedDiet) category (low and high).
3.1. Associations of Mediterranean Diet Adherence with Body Mass Index, Maximal Fat Oxidation, VO2max, and Leptin Concentration
Table 2 presents the associations between adherence to the MedDiet and several metabolic indicators. Higher adherence to the MedDiet was significantly associated with lower BMI, higher MFO (absolute and relative), greater VO2max, and lower leptin levels in the unadjusted model. These associations remained significant after adjusting for sex. After adjusting for age or both sex and age, associations with MFO and VO2max persisted, while those with the BMI and leptin were attenuated and showed a trend (p < 0.1). Among the studied outcomes, VO2max and MFO-BM exhibited the strongest associations with MedDiet adherence, indicated by their higher standardized coefficients (β) and explained variance (R2) across models.

Table 2.
Association of adherence to the Mediterranean Diet with body mass index, maximal fat oxidation, VO2max and leptin levels.
3.2. Associations of Leptin Levels with Body Mass Index, Maximal Fat Oxidation, and VO2max
Table 3 summarizes the associations between log-transformed leptin levels and key metabolic outcomes. Higher leptin concentrations were consistently associated with higher BMI and lower absolute MFO levels across all models, even after adjustments for sex and age. Associations with MFO-BM were significant in the unadjusted and sex-adjusted models and remained as a trend after adjusting for age and for both sex and age (p < 0.1). A similar trend was observed for VO2max in the unadjusted model, although this association did not reach statistical significance after adjustment.

Table 3.
Association of leptin levels with body mass index, maximal fat oxidation, and VO2max.
3.3. Mediation Analyses: Leptin Concentration and Mediterranean Dietary Pattern with Absolute MFO, MFO Body Mass, and VO2max
Mediation models were conducted to explore whether leptin mediates the association between MedDiet adherence and specific health-related outcomes (BMI, MFO, and VO2max; Figure 1). Leptin significantly mediated the relationship between adherence to the MedDiet and the BMI, with a significant indirect effect (β = −0.358, 95% CI [−0.677, −0.077]; Figure 1A) supported by significant paths from the MedDiet to leptin and from leptin to the BMI. In contrast, no mediation effect was found for absolute MFO (β = 0.004, 95% CI [−0.003, 0.012]; Figure 1B), as leptin was not significantly associated with this outcome. For MFO relative to body mass, the indirect effect approached significance (β = 0.080, 95% CI [−0.011, 0.195]; Figure 1C), with a significant direct association and a trend observed in the leptin-to-MFO path (p = 0.0876), suggesting possible partial mediation. Finally, a significant mediation effect was found for VO2max (β = 1.043, 95% CI [0.280, 1.833]; Figure 1D), with both the MedDiet-to-leptin and leptin-to-VO2max paths reaching statistical significance.
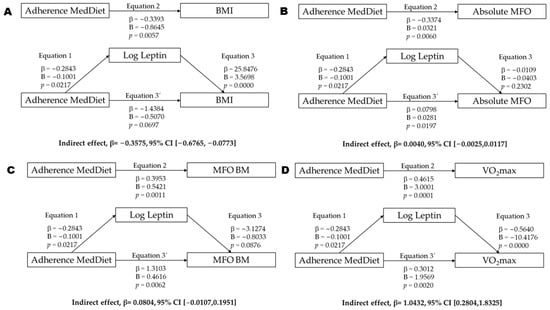
Figure 1.
Mediation analysis of leptin concentration between adherence Mediterranean diet (MedDiet) and BMI (A), absolute MFO (B), MFO relativized by body mass (C), and VO2max (D). Abbreviations: p = p value; β = standardized regression coefficient; B = unstandardized regression coefficient; BMI = body mass index (kg/m2); MFO = maximal fat oxidation; Leptin = log-transformed leptin concentrations; VO2max = maximal oxygen uptake.
4. Discussion
4.1. Main Research Findings
The present study demonstrates that higher adherence to the MedDiet is associated with improved metabolic health in young adults. Our findings revealed significant associations between greater MedDiet adherence and lower BMI, higher MFO, greater VO2max, and lower leptin levels. Furthermore, our mediation analyses suggest that leptin may partially explain the observed associations between MedDiet adherence, body composition, and aerobic capacity. These findings collectively highlight the multifaceted benefits of the MedDiet and the potential role of leptin as a mediating factor.
4.2. Associations Between Adherence to the Mediterranean Diet and the Study Variables
In agreement with our results, there is previous evidence linking the MedDiet with reduced risk of obesity, as well as improved body composition and metabolic health [36,37,38,39]. This is likely attributable to the elevated intake of fiber-rich foods, healthy fats, and antioxidants inherent in the MedDiet, which foster satiety, mitigate systemic inflammation, and enhance insulin sensitivity while also being linked to higher levels of MFO, as evidenced in prior research [40]. Our results showed a consistent positive association between MFO and the MedDiet across all models, even after adjustments for sex and age. This finding suggests that individuals with greater adherence to this dietary pattern may exhibit an enhanced capacity to utilize fat as an energy substrate during submaximal exercise, suggesting improved metabolic flexibility.
Furthermore, this enhanced metabolic flexibility could be attributed to the improvements in metabolic efficiency resulting from the consumption of healthy fats (monounsaturated and polyunsaturated), which favor the utilization of fatty acids as an energy substrate [41]. The MedDiet is rich in monounsaturated fatty acids (MUFAs), primarily from olive oil, and polyunsaturated fatty acids (PUFAs), particularly omega-3s from fish and nuts. These healthy fats are known to enhance mitochondrial biogenesis and function, thereby improving the capacity for fat oxidation within cells [42]. For instance, MUFAs have been shown to upregulate genes involved in fatty acid transport and beta-oxidation [43]. Similarly, omega-3 fatty acids can influence peroxisome proliferator-activated receptors (PPARs), which are key regulators of lipid metabolism and mitochondrial function [44]. Additionally, it has also been reported that the anti-inflammatory and antioxidant effects of the MedDiet could enhance the fat oxidation capacity and mitochondrial function [45]. The abundance of antioxidants, such as polyphenols from fruits, vegetables, and olive oil, helps to reduce oxidative stress, which can otherwise impair mitochondrial integrity and reduce metabolic efficiency [46]. Chronic low-grade inflammation, often associated with Western diets, can also negatively impact insulin sensitivity and substrate utilization. Thus, the anti-inflammatory compounds in the MedDiet may directly foster a more efficient fat-burning state [46]. Hence, the implementation of this diet could be strategically advantageous if tailored to balance healthy fats with moderate carbohydrate consumption.
Moreover, these fiber-rich foods may modulate the composition and metabolism of the gut microbiota, thereby enhancing lipid metabolism [47] and stimulating the secretion of glucagon-like peptide-1 (GLP-1), which has been associated with increased fat oxidation [48].
Initially, adherence to the MedDiet was inversely associated with the leptin concentrations. While this may seem paradoxical, given that leptin is a hormone involved in appetite suppression and fat oxidation, it is important to note that leptin is primarily secreted by adipocytes, and its circulating levels are typically elevated in individuals with obesity due to leptin resistance. In this context, higher MedDiet adherence may reflect better metabolic health and lower adiposity, leading to reduced leptin levels. Moreover, adherence to the MedDiet may enhance leptin sensitivity, promoting more efficient regulation of energy homeostasis and fat metabolism during physical activity [49].
Therefore, a dietary pattern such as the MedDiet may be associated not only with lower leptin levels but also a more favorable hormonal profile potentially linked to leptin sensitivity. Higher levels of circulating leptin, particularly in the context of obesity, are often indicative of leptin resistance, where the brain and other tissues become less responsive to its signaling, perpetuating an energy imbalance [50]. The MedDiet’s emphasis on whole, unprocessed foods, a high fiber content, healthy fats, and antioxidants could contribute to a reduction in systemic inflammation and improved insulin sensitivity, both of which are strongly linked to enhanced leptin sensitivity [51]. Specifically, reduced adiposity resulting from MedDiet adherence would directly lead to lower leptin production, while improved inflammatory markers might enhance receptor function and leptin signaling pathways [51]. This possible hormonal pathway could help contextualize the observed associations between dietary habits, energy regulation, and indicators of metabolic and cardiovascular function.
Furthermore, dietary components characteristic of the Mediterranean pattern, such as monounsaturated fats from olive oil, polyunsaturated fats from nuts and fish, and bioactive compounds like polyphenols, may improve leptin signaling and reduce leptin resistance [52,53,54].
It is also notable that in this sample, leptin levels were negatively associated with MFO, particularly absolute MFO, across all models. This suggests that higher leptin concentrations, typically linked to higher fat mass, may reflect diminished fat oxidation capacity in less metabolically flexible individuals. This finding reinforces the role of the MedDiet in supporting a favorable hormonal and metabolic profile conducive to efficient fat metabolism.
4.3. Mediation Analysis: The Role of Leptin
Mediation analyses revealed that leptin significantly mediated the associations between MedDiet adherence and both the BMI and VO2max. These findings suggest that leptin may act as a key hormonal link between dietary patterns and metabolic outcomes, particularly in body composition and aerobic capacity. The inverse association between MedDiet adherence and leptin, combined with leptin’s positive relationship with the BMI and its trend with VO2max, supports a potential pathway in which healthier dietary habits are linked to lower leptin concentrations, which may be related to more favorable cardiorespiratory and adiposity profiles. Although no significant mediation effect was observed for MFO, a trend was noted for MFO relative to body mass, suggesting a possible partial mediating role of leptin in fat oxidation capacity. These results are in line with previous studies indicating that leptin resistance, often present in individuals with elevated leptin levels, impairs metabolic regulation and exercise capacity [55,56].
A recent study revealed that women with obesity and low fitness levels, irrespective of age, exhibit a higher MFO rate compared with their normal-weight counterparts with similar fitness levels [57]. This finding underscores the importance of adiposity as a determining factor in lipid oxidation processes. Moreover, it has been shown that individuals with elevated leptin levels have improved mitochondrial function and higher MFO compared with non-obese individuals [27].
In contrast, in this study, higher leptin levels were associated with lower absolute MFO across all models, suggesting that increased leptin may reflect greater adiposity or leptin resistance, rather than enhanced oxidative metabolism. Several studies suggest that leptin, through its role in regulating energy balance and fat metabolism, could influence fat oxidation rates [58]. Nevertheless, the trend to mediation observed for MFO relativized for muscle mass indicates that leptin may still play a compensatory role in modulating fat oxidation capacity when adjusted for the total muscle, potentially by influencing substrate utilization pathways at the muscular level. The possible mediating role of leptin in this context aligns with its established role in promoting oxidative capacity and energy expenditure.
Several studies have described an inverse association between leptin levels and cardiorespiratory fitness. In particular, Miller et al. [59] observed that plasma leptin concentrations were negatively correlated with VO2max in young adult men and women, independent of sex and body composition. This finding supports the hypothesis that higher leptin levels, often reflecting excess adiposity and potential leptin resistance, may impair aerobic performance. In line with this evidence, our study showed a negative trend in the association between leptin levels and VO2max and also found that leptin significantly mediated the relationship between MedDiet adherence and VO2max.
However, these findings should be interpreted with caution, as they reflect only a trend observed in the data and are based on a cross-sectional design, which precludes any inference of causality. Even so, our results point to a potential pathway through which adherence to the MedDiet could be associated with physiological fitness beyond traditional markers of adiposity, possibly involving leptin concentrations and sensitivity. While preliminary, these observations highlight the relevance of exploring hormonal factors that may contribute to the associations between dietary patterns and physical performance.
In addition, leptin was inversely associated with all MFO outcomes, which may reflect impaired metabolic flexibility due to leptin resistance. Therefore, higher leptin levels could promote an earlier reliance on glucose during physical effort, potentially limiting fat oxidation and contributing to a lower VO2max level. Conversely, MedDiet adherence was positively associated with MFO, suggesting that an improved fat oxidation capacity may represent an additional pathway through which dietary quality enhances aerobic performance, independent of changes in body composition.
Overall, these findings suggest that leptin may be involved in the association between MedDiet adherence, BMI, and VO2max, whereas its potential mediating role in fat oxidation appears to be limited.
4.4. Physiological and Practical Implications
The findings from the present study involve several important implications. Adherence to the MedDiet can be regarded as an effective strategy not only for improving body composition (reduction in BMI) but also for optimizing fat oxidation and cardiorespiratory performance, particularly in active populations or those at metabolic risk.
The findings underscore leptin’s pivotal role as a metabolic mediator. Enhancing leptin sensitivity through dietary interventions such as the MedDiet could serve as a key strategy for boosting energy efficiency. The mediation of VO2max by leptin suggests that the physiological benefits of the Mediterranean dietary pattern may be partially driven by endocrine modulation, particularly through improved leptin signaling involved in substrate utilization and aerobic performance. Although no significant mediation was observed for MFO outcomes, a tendency was noted for MFO relative to body mass, indicating a potential partial role of leptin in enhancing fat oxidation efficiency. These findings reinforce the idea that improvements in aerobic capacity associated with MedDiet adherence may not solely depend on changes in body composition but also underlying hormonal adaptations that influence energy metabolism during exercise.
Improvements in VO2max and MFO via leptin mediation suggest that the MedDiet could be particularly beneficial in training programs aimed at enhancing aerobic performance and utilizing fat as an energy substrate, with applications in both endurance sports and the prevention of metabolic disorders.
These findings could be of relevance to populations adhering to diets and patterns similar to the MedDiet, such as the Nordic diet [60], Dietary Approaches to Stop Hypertension (DASH) [61], and the Okinawa diet [62], by comparing their characteristics, cardiovascular health benefits, and relationships with longevity. It is notable how, despite cultural and geographical differences, all these diets share common healthy principles, such as high intake of fresh foods, low consumption of red meats, and a focus on healthy fats [63,64,65,66]. Therefore, examining their effects on maximal fat oxidation (MFO) may offer valuable insights into shared mechanisms promoting metabolic health. Future studies should investigate whether these alternative dietary patterns yield similar outcomes regarding leptin concentrations, cardiorespiratory fitness, and fat oxidation efficiency.
4.5. Study Limitations and Future Directions
Despite the significant findings, this study presents some limitations. Its cross-sectional design limits the ability to establish causal relationships. Although the sample size was sufficient to detect significant associations, it may have reduced sensitivity to identifying weaker effects in some outcomes. However, the use of a well-validated MedDiet adherence questionnaire enhances the reliability of dietary assessment, strengthening the interpretation of the associations observed. Additionally, the application of bootstrapped mediation analyses with 10,000 samples improves the robustness of the statistical inference.
Further studies are needed to determine whether these results apply to younger or older populations, individuals with diseases, or those who engage in regular physical activity.
A notable limitation of the present study is the unequal sex distribution within our cohort, with a lower proportion of women (n = 23) compared with men (n = 42). While we accounted for sex as a covariate in our statistical models to mitigate its potential influence on the associations, this imbalance might still affect the generalizability of our findings.
Moreover, body composition was measured using bioimpedance rather than more precise methods, such as dual-energy X-ray absorptiometry (DXA), which represents another limitation.
In turn, other potential mediating variables, such as physical activity, specific macronutrient composition, or inflammatory biomarkers, were not assessed, which could provide a more comprehensive understanding of underlying mechanisms. It is also worth noting that menstrual cycle phases and estrogen levels in women were not considered, despite their substantial influence on fat oxidation and insulin sensitivity [67].
However, the assessment of fat oxidation and cardiorespiratory capacity in a controlled environment, using laboratory tests and a gas analyzer, as well as the incorporation of biochemical parameters, would enhance the study design. Finally, future research should include a larger sample size, evaluation of other metabolic hormones, and exploration of longitudinal interventions.
5. Conclusions
In conclusion, MedDiet adherence is positively associated with MFO and VO2max, highlighting a novel link between dietary quality and substrate utilization in young adults. Furthermore, leptin emerges as a key hormonal mediator in the relationships between the MedDiet and both the BMI and VO2max, suggesting that the benefits of this dietary pattern may partially operate through endocrine pathways related to energy homeostasis. These findings reinforce the MedDiet as a valuable nutritional strategy not only for metabolic health promotion and prevention of adiposity-related conditions but also for optimizing fat metabolism and cardiorespiratory fitness in physically active populations.
Building on these insights, future research should aim to investigate the precise molecular mechanisms by which MedDiet components influence leptin secretion and sensitivity. Longitudinal intervention studies are also warranted to establish causality, exploring how sustained MedDiet adherence impacts leptin levels and subsequently affects changes in body composition and cardiorespiratory fitness over time. Additionally, examining these relationships in diverse populations, including those with pre-existing metabolic conditions or across a wider age range, would strengthen the generalizability and clinical applicability of our findings.
Author Contributions
Conceptualization, P.S.-A., J.C.-P., C.C. and J.G.P.-G.; data curation, J.C.-P., D.V.-D., A.P.-B., M.R.-R., A.M.-G., A.M.-d.-O.-G., M.Á.R.-R., C.C. and J.G.P.-G.; formal analysis, P.S.-A., J.G.P.-G. and C.C.; funding acquisition, C.C. and J.G.P.-G.; resources, J.G.P.-G.; writing—original draft, P.S.-A., J.G.P.-G., J.C.-P. and C.C.; writing—review and editing, P.S.-A., J.C.-P., D.V.-D., A.P.-B., M.R.-R., A.M.-G., A.M.-d.-O.-G., M.Á.R.-R., C.C. and J.G.P.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Cadiz (grant numbers PR2016-051 and PR2019-054) as well as the Instituto de Investigación e Innovación Biomédica de Cádiz (LI19/21IN-CO09) and the Spanish Ministry of Science and Innovation (Ministerio de Ciencia e Innovación) (MCIN/AEI/10.13039/501100011033/PID2019-110063RA-I00). M.R.R. and A.M.-d.-O.-G. (UCA/REC13VPCT/2020) are supported by a predoctoral grant from the University of Cadiz. A.M.-G. is supported by a predoctoral grant from the Spanish Ministry of Education (grant number FPU20/03649).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethical Committee of the Hospital Puerta del Mar (Cadiz, Spain). The code of approval was 1590 13-B, and the date of approval was 28 September 2017.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
We would like to extend our gratitude to the research team behind the “NutAF” project, as well as the participants who made this study possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kelley, D.E.; Goodpaster, B.; Wing, R.R.; Simoneau, J.-A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol.-Endocrinol. Metab. 1999, 277, E1130–E1141. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Simoneau, J.A.; Thaete, F.L.; Kelley, D.E. Skeletal muscle utilization of free fatty acids in women with visceral obesity. J. Clin. Invest. 1995, 95, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Rauf, A.; Daglia, M. (Eds.) Nutraceuticals; De Gruyter: Boston, MA, USA, 2024; Frontmatter; pp. I–IV. Available online: https://www.degruyter.com/document/doi/10.1515/9783111317601-fm/html (accessed on 4 April 2025)ISBN 978-3-11-131730-4.
- Galgani, J.E.; Moro, C.; Ravussin, E. Metabolic flexibility and insulin resistance. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1009–E1017. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Maunder, E.; Plews, D.J.; Kilding, A.E. Contextualising Maximal Fat Oxidation During Exercise: Determinants and Normative Values. Front. Physiol. 2018, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Sureda, A.; Bibiloni, M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Román-Viñas, B.; Sanchez-Villegas, A.; Guasch-Ferré, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Asp. Med. 2019, 67, 1–55. [Google Scholar] [CrossRef]
- Santi-Cano, M.J.; Novalbos-Ruiz, J.P.; Bernal-Jiménez, M.Á.; Bibiloni, M.D.M.; Tur, J.A.; Rodriguez Martin, A. Association of Adherence to Specific Mediterranean Diet Components and Cardiorespiratory Fitness in Young Adults. Nutrients 2020, 12, 776. [Google Scholar] [CrossRef]
- Limongelli, G.; Monda, E.; D’Aponte, A.; Caiazza, M.; Rubino, M.; Esposito, A.; Palmiero, G.; Moscarella, E.; Messina, G.; Calabro’, P.; et al. Combined Effect of Mediterranean Diet and Aerobic Exercise on Weight Loss and Clinical Status in Obese Symptomatic Patients with Hypertrophic Cardiomyopathy. Heart Fail. Clin. 2021, 17, 303–313. [Google Scholar] [CrossRef]
- Soldati, L.; Pivari, F.; Parodi, C.; Brasacchio, C.; Dogliotti, E.; De Simone, P.; Rossi, M.; Vezzoli, G.; Paoli, A. The benefits of nutritional counselling for improving sport performance. J. Sports Med. Phys. Fit. 2019, 59, 1878–1884. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Parolin, M.L.; Heigenhauser, G.J.F.; Dyck, D.J. Leptin increases FA oxidation in lean but not obese human skeletal muscle: Evidence of peripheral leptin resistance. Am. J. Physiol.-Endocrinol. Metab. 2002, 283, E187–E192. [Google Scholar] [CrossRef]
- Bjornstad, P.; Cree-Green, M.; Baumgartner, A.; Coe, G.; Reyes, Y.G.; Schafer, M.; Pyle, L.; Regensteiner, J.G.; Reusch, J.E.B.; Nadeau, K.J. Leptin is associated with cardiopulmonary fitness independent of body-mass index and insulin sensitivity in adolescents with type 1 diabetes: A brief report from the EMERALD study. J. Diabetes Its Complicat. 2017, 31, 850–853. [Google Scholar] [CrossRef]
- Sáinz, N.; Barrenetxe, J.; Moreno-Aliaga, M.J.; Martínez, J.A. Leptin resistance and diet-induced obesity: Central and peripheral actions of leptin. Metabolism 2015, 64, 35–46. [Google Scholar] [CrossRef]
- Montes-de-Oca-García, A.; Perez-Bey, A.; Corral-Pérez, J.; Marín-Galindo, A.; Calderon-Dominguez, M.; Velázquez-Díaz, D.; Casals, C.; Ponce-Gonzalez, J.G. Influence of Gender on Plasma Leptin Levels, Fat Oxidation, and Insulin Sensitivity in Young Adults: The Mediating Role of Fitness and Fatness. Nutrients 2023, 15, 2628. [Google Scholar] [CrossRef]
- Montes-de-Oca-García, A.; Perez-Bey, A.; Corral-Pérez, J.; Velázquez-Díaz, D.; Opazo-Díaz, E.; Fernandez-Santos, J.R.; Rebollo-Ramos, M.; Amaro-Gahete, F.J.; Cuenca-García, M.; Ponce-González, J. Maximal fat oxidation capacity is associated with cardiometabolic risk factors in healthy young adults. Eur. J. Sport Sci. 2021, 21, 907–917. [Google Scholar] [CrossRef]
- Montes-de-Oca-García, A.; Perez-Bey, A.; Velázquez-Díaz, D.; Corral-Pérez, J.; Opazo-Díaz, E.; Rebollo-Ramos, M.; Gómez-Gallego, F.; Cuenca-García, M.; Casals, C.; Ponce-González, J.G. Influence of ACE Gene I/D Polymorphism on Cardiometabolic Risk, Maximal Fat Oxidation, Cardiorespiratory Fitness, Diet and Physical Activity in Young Adults. Int. J. Environ. Res. Public Health 2021, 18, 3443. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Ramos, M.; Velázquez-Díaz, D.; Corral-Pérez, J.; Barany-Ruiz, A.; Pérez-Bey, A.; Fernández-Ponce, C.; García-Cózar, F.J.; Ponce-González, J.G.; Cuenca-García, M. Capacidad aeróbica, dieta mediterránea y riesgo cardiometabólico en adultos. Endocrinol. Diabetes Nutr. 2020, 67, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Montes-de-Oca-García, A.; Corral-Pérez, J.; Velázquez-Díaz, D.; Perez-Bey, A.; Rebollo-Ramos, M.; Marín-Galindo, A.; Gómez-Gallego, F.; Calderon-Dominguez, M.; Casals, C.; Ponce-González, J.G. Influence of Peroxisome Proliferator-Activated Receptor (PPAR)-gamma Coactivator (PGC)-1 alpha gene rs8192678 polymorphism by gender on different health-related parameters in healthy young adults. Front. Physiol. 2022, 13, 885185. [Google Scholar] [CrossRef]
- Besnier, F.; Lenclume, V.; Gérardin, P.; Fianu, A.; Martinez, J.; Naty, N.; Porcherat, S.; Boussaid, K.; Schneebeli, S.; Jarlet, E.; et al. Individualized Exercise Training at Maximal Fat Oxidation Combined with Fruit and Vegetable-Rich Diet in Overweight or Obese Women: The LIPOXmax-Réunion Randomized Controlled Trial. PLoS ONE 2015, 10, e0139246. [Google Scholar] [CrossRef]
- Achten, J.; Gleeson, M.; Jeukendrup, A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef]
- García-Conesa, M.-T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Fernández-Jarne, E.; Serrano-Martínez, M.; Wright, M.; Gomez-Gracia, E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur. J. Clin. Nutr. 2004, 58, 1550–1552. [Google Scholar] [CrossRef]
- Ara, I.; Larsen, S.; Stallknecht, B.; Guerra, B.; Morales-Alamo, D.; Andersen, J.L.; Ponce-González, J.G.; Guadalupe-Grau, A.; Galbo, H.; Calbet, J.A.L.; et al. Normal mitochondrial function and increased fat oxidation capacity in leg and arm muscles in obese humans. Int. J. Obes. 2011, 35, 99–108. [Google Scholar] [CrossRef]
- Shvartz, E.; Reibold, R.C. Aerobic fitness norms for males and females aged 6 to 75 years: A review. Aviat. Space Environ. Med. 1990, 61, 3–11. [Google Scholar]
- World Health Organization. Obesity—Preventing and Managing the Global Epidemic: Report on a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; ISBN 978-92-4-120894-9. [Google Scholar]
- Bircher, S.; Knechtle, B. Relationship between Fat Oxidation and Lactate Threshold in Athletes and Obese Women and Men. J. Sports Sci. Med. 2004, 3, 174–181. [Google Scholar]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, A.E. Optimizing fat oxidation through exercise and diet. Nutrition 2004, 20, 716–727. [Google Scholar] [CrossRef]
- Hayes, A.F. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Commun. Monogr. 2009, 76, 408–420. [Google Scholar] [CrossRef]
- Bolin, J.H. Hayes, Andrew F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: The Guilford Press. J. Educ. Meas. 2014, 51, 335–337. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Grosso, G.; Marventano, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Missbach, B.; König, J.; Hoffmann, G. Adherence to a Mediterranean diet and risk of diabetes: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 1292–1299. [Google Scholar] [CrossRef]
- Esposito, K.; Kastorini, C.-M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean Diet and Weight Loss: Meta-Analysis of Randomized Controlled Trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; Merchan-Ramirez, E.; Labayen, I.; Ruiz, J.R. Relationships between diet and basal fat oxidation and maximal fat oxidation during exercise in sedentary adults. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1087–1101. [Google Scholar] [CrossRef]
- Fletcher, G.; Eves, F.F.; Glover, E.I.; Robinson, S.L.; Vernooij, C.A.; Thompson, J.L.; Wallis, G.A. Dietary intake is independently associated with the maximal capacity for fat oxidation during exercise. Am. J. Clin. Nutr. 2017, 105, 864–872. [Google Scholar] [CrossRef]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef]
- Watanabe, N.; Komiya, Y.; Sato, Y.; Watanabe, Y.; Suzuki, T.; Arihara, K. Oleic acid up-regulates myosin heavy chain (MyHC) 1 expression and increases mitochondrial mass and maximum respiration in C2C12 myoblasts. Biochem. Biophys. Res. Commun. 2020, 525, 406–411. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Pollicino, F.; Veronese, N.; Dominguez, L.J.; Barbagallo, M. Mediterranean diet and mitochondria: New findings. Exp. Gerontol. 2023, 176, 112165. [Google Scholar] [CrossRef]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived Antioxidants and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef]
- Serrano, J.; Cassanye, A.; Martín-Gari, M.; Granado-Serrano, A.; Portero-Otín, M. Effect of Dietary Bioactive Compounds on Mitochondrial and Metabolic Flexibility. Diseases 2016, 4, 14. [Google Scholar] [CrossRef]
- Pannacciulli, N.; Bunt, J.C.; Koska, J.; Bogardus, C.; Krakoff, J. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. Am. J. Clin. Nutr. 2006, 84, 556–560. [Google Scholar] [CrossRef]
- Dinu, M.; Colombini, B.; Pagliai, G.; Cesari, F.; Gori, A.; Giusti, B.; Marcucci, R.; Sofi, F. Effects of a dietary intervention with Mediterranean and vegetarian diets on hormones that influence energy balance: Results from the CARDIVEG study. Int. J. Food Sci. Nutr. 2020, 71, 362–369. [Google Scholar] [CrossRef]
- Myers, M.G.; Leibel, R.L.; Seeley, R.J.; Schwartz, M.W. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol. Metab. 2010, 21, 643–651. [Google Scholar] [CrossRef]
- Dayi, T.; Ozgoren, M. Effects of the mediterranean diet on the components of metabolic syndrome. J. Prev. Med. Hyg. 2022, 63, E56. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Algarra, I.; Gaforio, J.J. Differential Immunometabolic Effects of High-Fat Diets Containing Coconut, Sunflower, and Extra Virgin Olive Oils in Female Mice. Mol. Nutr. Food Res. 2022, 66, 2200082. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Z.; Qi, S.; Fang, T.; Zhu, H.; Santos, H.O.; Khani, V.; Wong, C.H.; Qiu, Z. Walnut intake may increase circulating adiponectin and leptin levels but does not improve glycemic biomarkers: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2020, 52, 102505. [Google Scholar] [CrossRef]
- Rausch, J.; Gillespie, S.; Orchard, T.; Tan, A.; McDaniel, J.C. Systematic review of marine-derived omega-3 fatty acid supplementation effects on leptin, adiponectin, and the leptin-to-adiponectin ratio. Nutr. Res. 2021, 85, 135–152. [Google Scholar] [CrossRef]
- García-Carrizo, F.; Nozhenko, Y.; Palou, A.; Rodríguez, A.M. Leptin Effect on Acetylation and Phosphorylation of Pgc1α in Muscle Cells Associated With Ampk and Akt Activation in High-Glucose Medium. J. Cell. Physiol. 2016, 231, 641–649. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Kim, Y.-B.; Peroni, O.D.; Fryer, L.G.D.; Müller, C.; Carling, D.; Kahn, B.B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef]
- Frandsen, J.; Hansen, I.M.D.; Wismann, J.F.; Olsen, M.H.; Brage-Andersen, M.R.; Sahl, R.E.; Hansen, M.; Ingersen, A.; Modvig, J.L.; Schmücker, M.; et al. Maximal Fat Oxidation Rate Is Higher in Fit Women and Unfit Women with Obesity, Compared to Normal-weight Unfit Women. J. Clin. Endocrinol. Metab. 2021, 106, E4389–E4399. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Toda, C.; Okamoto, S. Regulatory role of leptin in glucose and lipid metabolism in skeletal muscle. Indian J. Endocr. Metab. 2012, 16, 562. [Google Scholar] [CrossRef]
- Miller, G.D.; Frost, R.; Olive, J. Relation of plasma leptin concentrations to sex, body fat, dietary intake, and peak oxygen uptake in young adult women and men. Nutrition 2001, 17, 105–111. [Google Scholar] [CrossRef]
- Adamsson, V.; Reumark, A.; Cederholm, T.; Vessby, B.; Risérus, U.; Johansson, G. What is a healthy Nordic diet? Foods and nutrients in the NORDIET study. Food Nutr. Res. 2012, 56, 18189. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. New Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Willcox, D.C.; Willcox, B.J.; Todoriki, H.; Suzuki, M. The Okinawan Diet: Health Implications of a Low-Calorie, Nutrient-Dense, Antioxidant-Rich Dietary Pattern Low in Glycemic Load. J. Am. Coll. Nutr. 2009, 28, 500S–516S. [Google Scholar] [CrossRef]
- Krznarić, Ž.; Karas, I.; Ljubas Kelečić, D.; Vranešić Bender, D. The Mediterranean and Nordic Diet: A Review of Differences and Similarities of Two Sustainable, Health-Promoting Dietary Patterns. Front. Nutr. 2021, 8, 683678. [Google Scholar] [CrossRef]
- Willcox, D.C.; Scapagnini, G.; Willcox, B.J. Healthy aging diets other than the Mediterranean: A focus on the Okinawan diet. Mech. Ageing Dev. 2014, 136–137, 148–162. [Google Scholar] [CrossRef]
- Damigou, E.; Kosti, R.I.; Downs, S.M.; Naumovski, N.; Panagiotakos, D. Comparing The Mediterranean and The Japanese Dietary Pattern in Relation to Longevity—A Narrative Review. Endocr. Metab. Immune Disord.-Drug Targets 2024, 24, 1746–1755. [Google Scholar] [CrossRef]
- Manoharan, L.; Roth, B.; Bang, C.; Stenlund, H.; Ohlsson, B. An Okinawan-Based Nordic Diet Leads to Profound Effects on Gut Microbiota and Plasma Metabolites Linked to Glucose and Lipid Metabolism. Nutrients 2023, 15, 3273. [Google Scholar] [CrossRef]
- Goossens, G.H.; Jocken, J.W.E.; Blaak, E.E. Sexual dimorphism in cardiometabolic health: The role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol. 2021, 17, 47–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).