Does Identifying with Another Face Alter Body Image Disturbance in Women with an Eating Disorder? An Enfacement Illusion Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
2.2.1. Stimulation Videos
2.2.2. Morph Videos
2.3. Measures
2.3.1. Demographic and Clinical Information
2.3.2. Subjective Enfacement
2.3.3. Objective Enfacement
2.3.4. Facial Attractiveness and Adiposity
2.3.5. Head and Body Dissatisfaction
2.3.6. Free-Text Responses
2.4. Procedure
2.5. Statistical Analyses
Power Considerations
3. Results
3.1. Assumptions Checking
3.2. Susceptibility to Enfacement
3.2.1. Subjective Enfacement
3.2.2. Objective Enfacement
3.3. Face and Body Image Changes Post-Enfacement
3.3.1. Facial Attractiveness and Adiposity
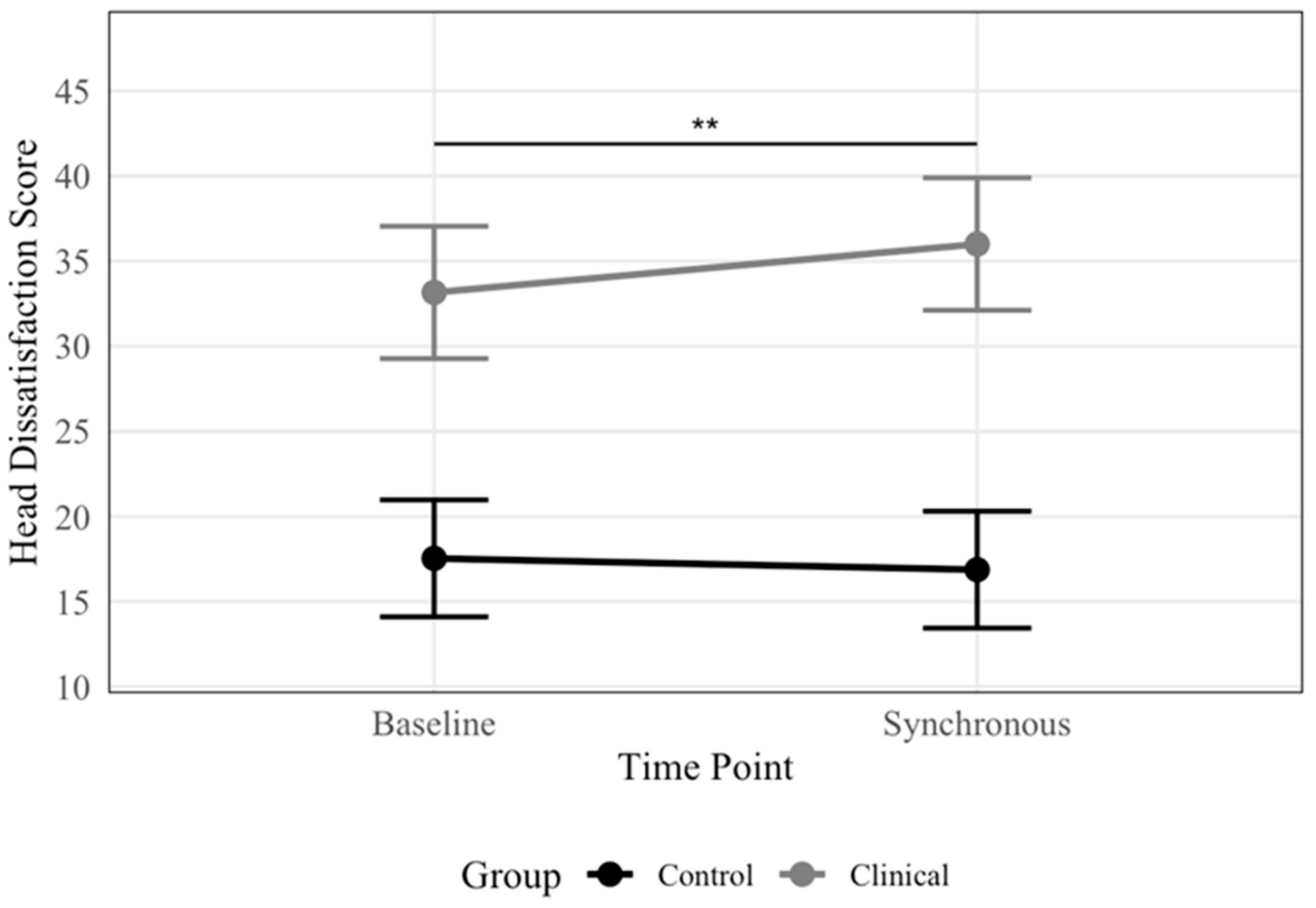
3.3.2. Head and Body Dissatisfaction
| Outcome Variable | Time | Total (N = 43) | Eating Disorder (n = 19) | Control (n = 24) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Observed Range | M | SD | Observed Range | M | SD | Observed Range | Possible Range | ||
| Subjective enfacement | |||||||||||
| Total | Sync | −0.22 | 1.31 | −2.67–1.67 | −0.38 | 1.24 | −2.67–1.67 | −0.09 | 1.37 | −2.67–1.67 | −3.00–3.00 |
| Async | −0.68 | 1.17 | −3.00–1.92 | −1.01 | 1.13 | 2.67–1.67 | −0.42 | 1.17 | −2.67–1.83 | ||
| Self-identification | Sync | −0.99 | 1.53 | −3.00–1.75 | −1.17 | 1.56 | −3.00–1.75 | −0.84 | 1.52 | −3.00–1.75 | −3.00–3.00 |
| Async | −1.53 | 1.35 | −3.00–1.50 | −1.57 | 1.24 | −3.00–1.25 | −1.50 | 1.46 | −3.00–1.50 | ||
| Similarity | Sync | −0.24 | 1.93 | −3.00–2.50 | −0.53 | 1.93 | −3.00–2.50 | −0.02 | 1.94 | −3.00–2.50 | −3.00–3.00 |
| Async | −0.77 | 1.80 | −3.00–2.50 | −1.21 | 1.72 | −3.00–2.50 | −0.42 | 1.82 | −3.00–2.00 | ||
| Affect | Sync | 0.57 | 1.62 | −3.00–3.00 | 0.55 | 1.85 | −3.00–3.00 | 0.58 | 1.45 | −2.00–3.00 | −3.00–3.00 |
| Async | 0.24 | 1.42 | −3.00–2.50 | −0.26 | 1.61 | −3.00–2.50 | 0.65 | 1.12 | −2.00–2.00 | ||
| Objective enfacement | Baseline | 60.96 | 16.28 | 27.73–95.54 | 61.42 | 19.29 | 41.88–90.00 | 60.59 | 13.87 | 27.73–95.54 | 0–100 |
| Sync | 54.08 | 16.91 | 17.00–96.02 | 53.66 | 18.07 | 27.80–88.00 | 54.41 | 16.31 | 17.00–96.02 | ||
| Async | 55.34 | 16.44 | 22.54–92.00 | 56.98 | 16.46 | 22.54–92.00 | 54.04 | 16.67 | 29.12–86.49 | ||
| Facial attractiveness | Baseline | 2.23 | 1.59 | 0–5 | 1.32 | 1.53 | 0–5 | 2.96 | 1.23 | 1–5 | 0–6 |
| Sync | 2.40 | 1.64 | 0–5 | 1.58 | 1.57 | 0–5 | 3.04 | 1.40 | 1–5 | ||
| Async | 2.26 | 1.72 | 0–5 | 1.53 | 1.78 | 0–5 | 2.83 | 1.46 | 0–5 | ||
| Facial adiposity | Baseline | 3.95 | 0.97 | 2–6 | 4.47 | 1.07 | 2–6 | 3.54 | 0.66 | 2–5 | 1–6 |
| Sync | 3.70 | 1.15 | 1–6 | 4.16 | 1.38 | 1–6 | 3.33 | 0.76 | 2–6 | ||
| Async | 3.65 | 1.17 | 0–6 | 4.05 | 1.54 | 0–6 | 3.33 | 0.64 | 2–5 | ||
| Head dissatisfaction | Baseline | 24.44 | 10.94 | 7–48 | 32.53 | 9.13 | 14–48 | 18.04 | 7.53 | 7–35 | 7–49 |
| Sync | 25.33 | 12.03 | 7–47 | 35.37 | 8.19 | 17–47 | 17.38 | 7.91 | 7–35 | ||
| Async | 26.16 | 12.28 | 7–48 | 36.26 | 9.37 | 17–48 | 18.17 | 7.47 | 7–32 | ||
| Body dissatisfaction | Baseline | 28.09 | 11.54 | 7–47 | 38.26 | 7.02 | 25–47 | 20.04 | 7.17 | 7–33 | 7–49 |
| Sync | 28.09 | 12.59 | 7–49 | 39.11 | 7.88 | 24–49 | 19.38 | 7.83 | 7–37 | ||
| Async | 28.72 | 12.46 | 7–49 | 39.42 | 7.83 | 25–49 | 20.25 | 8.13 | 7–41 | ||
| Outcome Variable | Fixed Effects | b | b 95% CI [LB, UB] | SE | df | p | Random Effects (Variance) | Conditional R2 a | Marginal R2 b | Semi-Partial R2 c | ICC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Residual | |||||||||||
| Subjective enfacement | ||||||||||||
| Total | Time: async | −0.33 | [−0.84, 0.18] | 0.26 | 41.00 | 0.212 | 0.72 | 0.81 | 0.51 | 0.08 | 0.01 | 0.47 |
| Group: ED | −0.18 | [−0.96, 0.60] | 0.40 | 63.08 | 0.655 | 0.00 | ||||||
| Age | −0.02 | [−0.06, 0.02] | 0.02 | 40.00 | 0.390 | 0.01 | ||||||
| Time: async × ED | −0.30 | [−1.07, 0.47] | 0.39 | 41.00 | 0.445 | 0.00 | ||||||
| Self-identification | Time: async | −0.66 | [−1.15, −0.17] | 0.25 | 41.00 | 0.012 | 1.39 | 0.75 | 0.67 | 0.05 | 0.03 | 0.65 |
| Group: ED | −0.21 | [−1.14, 0.72] | 0.48 | 54.35 | 0.665 | 0.00 | ||||||
| Age | −0.02 | [−0.07, 0.03] | 0.03 | 40.00 | 0.447 | 0.01 | ||||||
| Time: async × ED | 0.26 | [−0.48, 1.00] | 0.38 | 41.00 | 0.490 | 0.00 | ||||||
| Similarity | Time: async | −0.40 | [−1.12, 0.33] | 0.37 | 41.00 | 0.292 | 1.68 | 1.64 | 0.55 | 0.10 | 0.01 | 0.51 |
| Group: ED | −0.19 | [−1.34, 0.97] | 0.59 | 61.34 | 0.754 | 0.00 | ||||||
| Age | −0.05 | [−0.11, 0.01] | 0.03 | 40.00 | 0.093 | 0.05 | ||||||
| Time: async × ED | 0.29 | [−1.38, 0.80] | 0.56 | 41.00 | 0.607 | 0.00 | ||||||
| Affect | Time: async | 0.06 | [−0.60, 0.73] | 0.34 | 41.00 | 0.854 | 0.90 | 1.38 | 0.43 | 0.07 | 0.00 | 0.40 |
| Group: ED | −0.15 | [−1.10, 0.81] | 0.49 | 66.66 | 0.766 | 0.00 | ||||||
| Age | 0.02 | [−0.03, 0.07] | 0.02 | 40.00 | 0.443 | 0.01 | ||||||
| Time: async × ED | −0.87 | [−1.88, 0.12] | 0.51 | 41.00 | 0.092 | 0.02 | ||||||
| Objective enfacement | Time: baseline | 6.18 | [2.02, 10.34] | 2.12 | 82.00 | 0.005 | 0.91 | 0.44 | 0.82 | 0.04 | 0.01 | 0.81 |
| Time: async | −0.37 | [−4.53, 3.78] | 2.12 | 82.00 | 0.861 | 0.00 | ||||||
| Group: ED | −0.35 | [−11.14, 10.45] | 5.51 | 50.31 | 0.950 | 0.00 | ||||||
| Age | −0.07 | [−0.68, 0.54] | 0.31 | 40.00 | 0.830 | 0.00 | ||||||
| Time: baseline × ED | 1.58 | [−4.68, 7.83] | 3.19 | 82.00 | 0.622 | 0.00 | ||||||
| Time: async × ED | 3.69 | [−2.56, 9.94] | 3.19 | 82.00 | 0.251 | 0.00 | ||||||
| Facial attractiveness | Time: baseline | −0.08 | [−0.45, 0.28] | 0.18 | 41.00 | 0.654 | 1.67 | 0.41 | 0.85 | 0.23 | 0.00 | 0.80 |
| Group: ED | −1.51 | [−2.43, −0.59] | 0.47 | 47.57 | 0.002 | 0.12 | ||||||
| Age | 0.01 | [−0.05, 0.06] | 0.03 | 40.00 | 0.781 | 0.00 | ||||||
| Time: baseline × ED | −0.18 | [−0.72, 0.36] | 0.28 | 41.00 | 0.521 | 0.00 | ||||||
| Facial adiposity | Time: baseline | 0.21 | [−0.10, 0.51] | 0.16 | 41.00 | 0.190 | 0.61 | 0.29 | 0.75 | 0.24 | 0.01 | 0.67 |
| Group: ED | 0.62 | [0.01, 1.22] | 0.31 | 53.26 | 0.051 | 0.05 | ||||||
| Age | 0.04 | [0.00, 0.07] | 0.02 | 40.00 | 0.047 | 0.08 | ||||||
| Time: baseline × ED | 0.11 | [−0.35, 0.57] | 0.24 | 41.00 | 0.650 | 0.00 | ||||||
| Head dissatisfaction | Time: baseline | 0.67 | [−0.92, 2.25] | 0.81 | 41.00 | 0.415 | 57.84 | 7.88 | 0.94 | 0.51 | 0.00 | 0.88 |
| Group: ED | 19.13 | [13.93, 24.33] | 2.66 | 44.44 | <0.001 | 0.40 | ||||||
| Age | −0.19 | [−0.49, 0.11] | 0.15 | 40.00 | 0.226 | 0.03 | ||||||
| Time: baseline × ED | −3.51 | [−5.90, −1.12] | 1.22 | 41.00 | 0.006 | 0.01 | ||||||
| Body dissatisfaction | Time: baseline | 0.67 | [−0.39, 1.72] | 0.54 | 41.00 | 0.223 | 52.38 | 3.49 | 0.98 | 0.62 | 0.00 | 0.94 |
| Group: ED | 19.14 | [15.85, 25.46] | 2.45 | 42.25 | <0.001 | 0.48 | ||||||
| Age | −0.15 | [−0.44, 0.13] | 0.14 | 40.00 | 0.292 | 0.03 | ||||||
| Time: baseline × ED | −1.51 | [−3.10, 0.08] | 0.81 | 41.00 | 0.070 | 0.00 | ||||||
3.4. Exploratory Insights from Free-Text Responses
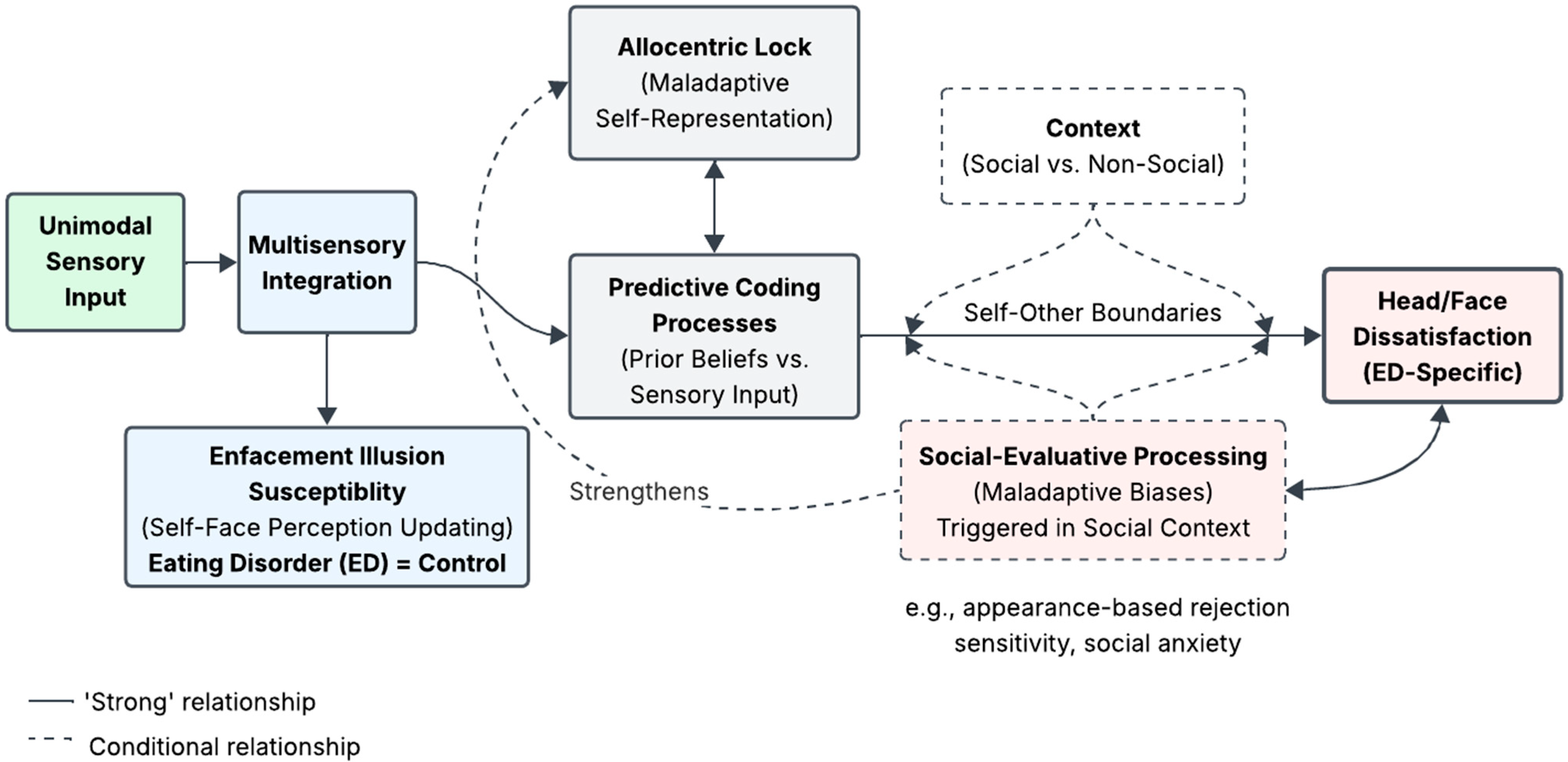
4. Discussion
4.1. Enfacement Illusion Susceptibility
4.2. Face and Body Image Changes Post-Enfacement
4.3. Strengths and Limitations
4.4. Proposed Dual-Process Model of Self-Face Perception in Eating Disorders
4.5. Future Research Directions and Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; text rev.; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Prnjak, K.; Jukic, I.; Mitchison, D.; Griffiths, S.; Hay, P. Body Image as a Multidimensional Concept: A Systematic Review of Body Image Facets in Eating Disorders and Muscle Dysmorphia. Body Image 2022, 42, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Glashouwer, K.A.; Van Der Veer, R.M.L.; Adipatria, F.; De Jong, P.J.; Vocks, S. The Role of Body Image Disturbance in the Onset, Maintenance, and Relapse of Anorexia Nervosa: A Systematic Review. Clin. Psychol. Rev. 2019, 74, 101771. [Google Scholar] [CrossRef] [PubMed]
- Mölbert, S.C.; Klein, L.; Thaler, A.; Mohler, B.J.; Brozzo, C.; Martus, P.; Karnath, H.-O.; Zipfel, S.; Giel, K.E. Depictive and Metric Body Size Estimation in Anorexia Nervosa and Bulimia Nervosa: A Systematic Review and Meta-Analysis. Clin. Psychol. Rev. 2017, 57, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ralph-Nearman, C.; Arevian, A.C.; Moseman, S.; Sinik, M.; Chappelle, S.; Feusner, J.D.; Khalsa, S.S. Visual Mapping of Body Image Disturbance in Anorexia Nervosa Reveals Objective Markers of Illness Severity. Sci. Rep. 2021, 11, 12262. [Google Scholar] [CrossRef]
- Boehm, I.; Finke, B.; Tam, F.I.; Fittig, E.; Scholz, M.; Gantchev, K.; Roessner, V.; Ehrlich, S. Effects of Perceptual Body Image Distortion and Early Weight Gain on Long-Term Outcome of Adolescent Anorexia Nervosa. Eur. Child Adolesc. Psychiatry 2016, 25, 1319–1326. [Google Scholar] [CrossRef]
- Gardner, R.M.; Brown, D.L. Body Size Estimation in Anorexia Nervosa: A Brief Review of Findings from 2003 through 2013. Psychiatry Res. 2014, 219, 407–410. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Cooper, Z.; Shafran, R. Cognitive Behaviour Therapy for Eating Disorders: A “Transdiagnostic” Theory and Treatment. Behav. Res. Ther. 2003, 41, 509–528. [Google Scholar] [CrossRef]
- Dalhoff, A.W.; Romero Frausto, H.; Romer, G.; Wessing, I. Perceptive Body Image Distortion in Adolescent Anorexia Nervosa: Changes After Treatment. Front. Psychiatry 2019, 10, 748. [Google Scholar] [CrossRef]
- Berlucchi, G.; Aglioti, S.M. The Body in the Brain Revisited. Exp. Brain Res. 2010, 200, 25–35. [Google Scholar] [CrossRef]
- Blanke, O.; Slater, M.; Serino, A. Behavioral, Neural, and Computational Principles of Bodily Self-Consciousness. Neuron 2015, 88, 145–166. [Google Scholar] [CrossRef]
- Pyasik, M.; Ciorli, T.; Pia, L. Full Body Illusion and Cognition: A Systematic Review of the Literature. Neurosci. Biobehav. Rev. 2022, 143, 104926. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.; Cohen, J. Rubber Hands ‘Feel’ Touch That Eyes See. Nature 1998, 391, 756. [Google Scholar] [CrossRef] [PubMed]
- Petkova, V.I.; Ehrsson, H.H. If I Were You: Perceptual Illusion of Body Swapping. PLoS ONE 2008, 3, e3832. [Google Scholar] [CrossRef]
- Normand, J.M.; Giannopoulos, E.; Spanlang, B.; Slater, M. Multisensory stimulation can induce an illusion of larger belly size in immersive virtual reality. PLoS ONE 2011, 6, e16128. [Google Scholar] [CrossRef]
- Serino, S.; Polli, N.; Riva, G. From avatars to body swapping: The use of virtual reality for assessing and treating body-size distortion in individuals with anorexia. J. Clin. Psychol. 2019, 75, 313–322. [Google Scholar] [CrossRef]
- Portingale, J.; Krug, I.; Liu, H.; Kiropoulos, L.; Butler, D. Your Body, My Experience: A Systematic Review of Embodiment Illusions as a Function of and Method to Improve Body Image Disturbance. Clin. Psychol. Sci. Pract. 2024, 31, 445–458. [Google Scholar] [CrossRef]
- Eshkevari, E.; Rieger, E.; Longo, M.R.; Haggard, P.; Treasure, J. Increased Plasticity of the Bodily Self in Eating Disorders. Psychol. Med. 2012, 42, 819–828. [Google Scholar] [CrossRef]
- Eshkevari, E.; Rieger, E.; Longo, M.R.; Haggard, P.; Treasure, J. Persistent Body Image Disturbance Following Recovery from Eating Disorders. Int. J. Eat. Disord. 2014, 47, 400–409. [Google Scholar] [CrossRef]
- Keizer, A.; Smeets, M.A.M.; Postma, A.; Van Elburg, A.; Dijkerman, H.C. Does the Experience of Ownership over a Rubber Hand Change Body Size Perception in Anorexia Nervosa Patients? Neuropsychologia 2014, 62, 26–37. [Google Scholar] [CrossRef]
- Keizer, A.; Van Elburg, A.; Helms, R.; Dijkerman, H.C. A Virtual Reality Full Body Illusion Improves Body Image Disturbance in Anorexia Nervosa. PLoS ONE 2016, 11, e0163921. [Google Scholar] [CrossRef]
- Preston, C.; Ehrsson, H.H. Implicit and Explicit Changes in Body Satisfaction Evoked by Body Size Illusions: Implications for Eating Disorder Vulnerability in Women. PLoS ONE 2018, 13, e0199426. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.; Preston, C. Investigating the Components of Body Image Disturbance Within Eating Disorders. Front. Psychiatry 2019, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Porciello, G.; Bufalari, I.; Minio-Paluello, I.; Di Pace, E.; Aglioti, S.M. The ‘Enfacement’ Illusion: A Window on the Plasticity of the Self. Cortex 2018, 104, 261–275. [Google Scholar] [CrossRef]
- Tsakiris, M. Looking for Myself: Current Multisensory Input Alters Self-Face Recognition. PLoS ONE 2008, 3, e4040. [Google Scholar] [CrossRef]
- Tajadura-Jiménez, A.; Longo, M.R.; Coleman, R.; Tsakiris, M. The Person in the Mirror: Using the Enfacement Illusion to Investigate the Experiential Structure of Self-Identification. Conscious. Cogn. 2012, 21, 1725–1738. [Google Scholar] [CrossRef]
- Hugenberg, K.; Wilson, J.P. Faces Are Central to Social Cognition; The Oxford Handbook of Social Cognition: Oxford, UK, 2013. [Google Scholar]
- Zebrowitz, L.A.; Montepare, J.M. Social Psychological Face Perception: Why Appearance Matters. Soc. Personal. Psychol. Compass 2008, 2, 1497–1517. [Google Scholar] [CrossRef]
- De Jager, S.; Coetzee, N.; Coetzee, V. Facial Adiposity, Attractiveness, and Health: A Review. Front. Psychol. 2018, 9, 2562. [Google Scholar] [CrossRef]
- Beos, N.; Kemps, E.; Prichard, I. Photo Manipulation as a Predictor of Facial Dissatisfaction and Cosmetic Procedure Attitudes. Body Image 2021, 39, 194–201. [Google Scholar] [CrossRef]
- Yang, J.; Fardouly, J.; Wang, Y.; Shi, W. Selfie-Viewing and Facial Dissatisfaction among Emerging Adults: A Moderated Mediation Model of Appearance Comparisons and Self-Objectification. Int. J. Environ. Res. Public Health 2020, 17, 672. [Google Scholar] [CrossRef]
- Croce, S.R.; Malcolm, A.C.; Ralph-Nearman, C.; Phillipou, A. The Role of Identity in Anorexia Nervosa: A Narrative Review. New Ideas Psychol. 2024, 72, 101060. [Google Scholar] [CrossRef]
- Verschueren, M.; Luyckx, K.; Kaufman, E.A.; Vansteenkiste, M.; Moons, P.; Sleuwaegen, E.; Berens, A.; Schoevaerts, K.; Claes, L. Identity Processes and Statuses in Patients with and without Eating Disorders. Eur. Eat. Disord. Rev. 2017, 25, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Cardi, V.; Tchanturia, K.; Treasure, J. Premorbid and Illness-Related Social Difficulties in Eating Disorders: An Overview of the Literature and Treatment Developments. Curr. Neuropharmacol. 2018, 16, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Dane, A.; Bhatia, K. The Social Media Diet: A Scoping Review to Investigate the Association between Social Media, Body Image and Eating Disorders amongst Young People. PLoS Glob. Public Health 2023, 3, e0001091. [Google Scholar] [CrossRef] [PubMed]
- Hirot, F.; Lesage, M.; Pedron, L.; Meyer, I.; Thomas, P.; Cottencin, O.; Guardia, D. Impaired Processing of Self-Face Recognition in Anorexia Nervosa. Eat. Weight Disord.—Stud. Anorex. Bulim. Obes. 2016, 21, 31–40. [Google Scholar] [CrossRef]
- Portingale, J.; Krug, I.; Butler, D. Beyond Body Image: Self-face Recognition and Negative Self-face Evaluations in Women at High Risk for an Eating Disorder. Eur. Eat. Disord. Rev. 2025, 33, 374–389. [Google Scholar] [CrossRef]
- Hrabosky, J.I.; Cash, T.F.; Veale, D.; Neziroglu, F.; Soll, E.A.; Garner, D.M.; Strachan-Kinser, M.; Bakke, B.; Clauss, L.J.; Phillips, K.A. Multidimensional Body Image Comparisons among Patients with Eating Disorders, Body Dysmorphic Disorder, and Clinical Controls: A Multisite Study. Body Image 2009, 6, 155–163. [Google Scholar] [CrossRef]
- Sforza, A.; Bufalari, I.; Haggard, P.; Aglioti, S.M. My Face in Yours: Visuo-Tactile Facial Stimulation Influences Sense of Identity. Soc. Neurosci. 2010, 5, 148–162. [Google Scholar] [CrossRef]
- Portingale, J.; Krug, I.; Butler, D. Enfacement Illusions: Filling a Knowledge Gap in Eating Disorder Risk Assessment, Prevention, and Intervention? Int. J. Eat. Disord. 2024, 57, 1805–1810. [Google Scholar] [CrossRef]
- Portingale, J.; Krug, I.; Rheenen, T.E.V.; Kiropoulos, L.; Bartholomeusz, C.F.; Nasser, H.; Butler, D. Divergent Effects of the Enfacement Illusion on Face and Body Image Disturbance Across Female Eating Disorder Risk Groups. Preprints 2025. [CrossRef]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The Eating Attitudes Test: Psychometric Features and Clinical Correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef]
- Scarpina, F.; Serino, S.; Keizer, A.; Chirico, A.; Scacchi, M.; Castelnuovo, G.; Mauro, A.; Riva, G. The Effect of a Virtual-Reality Full-Body Illusion on Body Representation in Obesity. J. Clin. Med. 2019, 8, 1330. [Google Scholar] [CrossRef] [PubMed]
- Portingale, J.; Butler, D.; Krug, I. Novel Online Enfacement Illusion for Investigating Self-Perception in Mental Disorders: An Experimental Study Protocol. J. Eat. Disord. 2024, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Cooper, Z.; Fairburn, C. The Eating Disorder Examination: A Semi-Structured Interview for the Assessment of the Specific Psychopathology of Eating Disorders. Int. J. Eat. Disord. 1987, 6, 1–8. [Google Scholar] [CrossRef]
- Panagiotopoulou, E.; Crucianelli, L.; Lemma, A.; Fotopoulou, A. Identifying with the Beautiful: Facial Attractiveness Effects on Unisensory and Multisensory Self–Other Distinction. Q. J. Exp. Psychol. 2022, 75, 1314–1329. [Google Scholar] [CrossRef]
- Abrosoft. FantaMorph. 2020. Available online: https://www.fantamorph.com (accessed on 13 March 2020).
- Panagiotopoulou, E.; Filippetti, M.L.; Tsakiris, M.; Fotopoulou, A. Affective Touch Enhances Self-Face Recognition During Multisensory Integration. Sci. Rep. 2017, 7, 12883. [Google Scholar] [CrossRef]
- Coetzee, V.; Faerber, S.J.; Greeff, J.M.; Lefevre, C.E.; Re, D.E.; Perrett, D.I. African Perceptions of Female Attractiveness. PLoS ONE 2012, 7, e48116. [Google Scholar] [CrossRef]
- Tinlin, R.M.; Watkins, C.D.; Welling, L.L.M.; DeBruine, L.M.; Al-Dujaili, E.A.S.; Jones, B.C. Perceived Facial Adiposity Conveys Information about Women’s Health. Br. J. Psychol. 2013, 104, 235–248. [Google Scholar] [CrossRef]
- Kramer, R.S.; Ritchie, K.L.; Flack, T.R.; Mireku, M.O.; Jones, A.L. The psychometrics of rating facial attractiveness using different response scales. Perception 2024, 53, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Slade, P.D.; Dewey, M.E.; Newton, T.; Brodie, D.; Kiemle, G. Development and Preliminary Validation of the Body Satisfaction Scale (BSS). Psychol. Health 1990, 4, 213–220. [Google Scholar] [CrossRef]
- Rohde, P.; Stice, E.; Shaw, H.; Gau, J.M.; Ohls, O.C. Age Effects in Eating Disorder Baseline Risk Factors and Prevention Intervention Effects. Int. J. Eat. Disord. 2017, 50, 1273–1280. [Google Scholar] [CrossRef]
- Potterton, R.; Richards, K.; Allen, K.; Schmidt, U. Eating Disorders During Emerging Adulthood: A Systematic Scoping Review. Front. Psychol. 2020, 10, 3062. [Google Scholar] [CrossRef] [PubMed]
- Turbyne, C.; Goedhart, A.; De Koning, P.; Schirmbeck, F.; Denys, D. Systematic Review and Meta-Analysis of Virtual Reality in Mental Healthcare: Effects of Full Body Illusions on Body Image Disturbance. Front. Virtual Real. 2021, 2, 657638. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A General and Simple Method for Obtaining R2 from Generalized Linear Mixed-effects Models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Rights, J.D.; Sterba, S.K. Quantifying Explained Variance in Multilevel Models: An Integrative Framework for Defining R-Squared Measures. Psychol. Methods 2019, 24, 309–338. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Aguinis, H.; Gottfredson, R.K.; Culpepper, S.A. Best-Practice Recommendations for Estimating Cross-Level Interaction Effects Using Multilevel Modeling. J. Manag. 2013, 39, 1490–1528. [Google Scholar] [CrossRef]
- Snijders, T.A. Power and sample size in multilevel modeling. Encycl. Stat. Behav. Sci. 2005, 3, 1573. [Google Scholar]
- Levine, M.; Ensom, M.H.H. Post Hoc Power Analysis: An Idea Whose Time Has Passed? Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 405–409. [Google Scholar] [CrossRef]
- Nasr, S.; Tootell, R.B.H. Role of Fusiform and Anterior Temporal Cortical Areas in Facial Recognition. NeuroImage 2012, 63, 1743–1753. [Google Scholar] [CrossRef]
- Pann, A.; Bonnard, M.; Felician, O.; Romaiguère, P. The Extrastriate Body Area and Identity Processing: An fMRI Guided TMS Study. Physiol. Rep. 2021, 9, e14711. [Google Scholar] [CrossRef] [PubMed]
- Apps, M.A.J.; Tsakiris, M. The Free-Energy Self: A Predictive Coding Account of Self-Recognition. Neurosci. Biobehav. Rev. 2014, 41, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. The Free-Energy Principle: A Unified Brain Theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Brizzi, G.; Sansoni, M.; Di Lernia, D.; Frisone, F.; Tuena, C.; Riva, G. The Multisensory Mind: A Systematic Review of Multisensory Integration Processing in Anorexia and Bulimia Nervosa. J. Eat. Disord. 2023, 11, 204. [Google Scholar] [CrossRef]
- Toh, W.L.; Grace, S.A.; Rossell, S.L.; Castle, D.J.; Phillipou, A. Body Parts of Clinical Concern in Anorexia Nervosa versus Body Dysmorphic Disorder: A Cross-Diagnostic Comparison. Australas. Psychiatry 2020, 28, 134–139. [Google Scholar] [CrossRef]
- Sfärlea, A.; Radix, A.K.; Schulte-Körne, G.; Legenbauer, T.; Platt, B. Attention Biases for Eating Disorder-Related Stimuli Versus Social Stimuli in Adolescents with Anorexia Nervosa—An Eye-Tracking Study. Res. Child Adolesc. Psychopathol. 2023, 51, 541–555. [Google Scholar] [CrossRef]
- Germine, L.T.; Duchaine, B.; Nakayama, K. Where Cognitive Development and Aging Meet: Face Learning Ability Peaks after Age 30. Cognition 2011, 118, 201–210. [Google Scholar] [CrossRef]
- Riva, G. Neuroscience and Eating Disorders: The Allocentric Lock Hypothesis. Med. Hypotheses 2012, 78, 254–257. [Google Scholar] [CrossRef]
- Riva, G.; Dakanalis, A. Altered Processing and Integration of Multisensory Bodily Representations and Signals in Eating Disorders: A Possible Path Toward the Understanding of Their Underlying Causes. Front. Hum. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Fenigstein, A.; Scheier, M.F.; Buss, A.H. Public and private self-consciousness: Assessment and theory. J. Consult. Clin. Psychol. 1975, 43, 522. [Google Scholar] [CrossRef]
- Sacchetti, S.; Mirams, L.; McGlone, F.; Cazzato, V. Self-focused attention enhances tactile sensitivity in women at risk from eating disorders. Sci. Rep. 2020, 10, 11614. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Henriksson, L.; Malinen, S.; Parkkonen, L. Centrality of social interaction in human brain function. Neuron 2015, 88, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Phillipou, A.; Abel, L.A.; Castle, D.J.; Hughes, M.E.; Gurvich, C.; Nibbs, R.G.; Rossell, S.L. Self Perception and Facial Emotion Perception of Others in Anorexia Nervosa. Front. Psychol. 2015, 6, 1181. [Google Scholar] [CrossRef]
- Rowlands, K.; Wilson, E.; Simic, M.; Harrison, A.; Cardi, V. A Critical Review of Studies Assessing Interpretation Bias Towards Social Stimuli in People with Eating Disorders and the Development and Pilot Testing of Novel Stimuli for a Cognitive Bias Modification Training. Front. Psychol. 2020, 11, 538527. [Google Scholar] [CrossRef]
- Provenzano, L.; Porciello, G.; Ciccarone, S.; Lenggenhager, B.; Tieri, G.; Marucci, M.; Dazzi, F.; Loriedo, C.; Bufalari, I. Characterizing Body Image Distortion and Bodily Self-Plasticity in Anorexia Nervosa via Visuo-Tactile Stimulation in Virtual Reality. J. Clin. Med. 2019, 9, 98. [Google Scholar] [CrossRef]
- Serino, S.; Chirico, A.; Pedroli, E.; Polli, N.; Cacciatore, C.; Riva, G. Two-Phases Innovative Treatment for Anorexia Nervosa: The Potential of Virtual Reality Body-Swap. Annu. Rev. CyberTher. Telemed. 2017, 15, 111–115. [Google Scholar]
- Zopf, R.; Contini, E.; Fowler, C.; Mondraty, N.; Williams, M.A. Body Distortions in Anorexia Nervosa: Evidence for Changed Processing of Multisensory Bodily Signals. Psychiatry Res. 2016, 245, 473–481. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Cascino, G. A Systematic Review of Network Analysis Studies in Eating Disorders: Is Time to Broaden the Core Psychopathology to Non Specific Symptoms. Eur. Eat. Disord. Rev. 2021, 29, 531–547. [Google Scholar] [CrossRef]
- Mesaros, A.; Cornea, D.; Cioara, L.; Dudea, D.; Mesaros, M.; Badea, M. Facial Attractiveness Assessment using Illustrated Questionnairers. Clujul Med. 2015, 88, 73–78. [Google Scholar] [CrossRef]
- Fardouly, J.; Pinkus, R.T.; Vartanian, L.R. The Impact of Appearance Comparisons Made through Social Media, Traditional Media, and in Person in Women’s Everyday Lives. Body Image 2017, 20, 31–39. [Google Scholar] [CrossRef]
- Frederick, D.A.; Kelly, M.C.; Latner, J.D.; Sandhu, G.; Tsong, Y. Body Image and Face Image in Asian American and White Women: Examining Associations with Surveillance, Construal of Self, Perfectionism, and Sociocultural Pressures. Body Image 2016, 16, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.; Waterhouse, M.; Mamat, N.H.B.; Xu, X.; Cochrane, J.; McCabe, M.; Ricciardelli, L. Which Body Features Are Associated with Female Adolescents’ Body Dissatisfaction? A Cross-Cultural Study in Australia, China and Malaysia. Body Image 2013, 10, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Portingale, J.; Krug, I.; Butler, D. Whose Body Is It Anyway? Cultural Reflections on Embodiment Illusion Research in Eating Disorders and Body Dysmorphic Disorder. Front. Psychiatry 2024, 15, 1433596. [Google Scholar] [CrossRef]
- Westwood, H.; Kerr-Gaffney, J.; Stahl, D.; Tchanturia, K. Alexithymia in Eating Disorders: Systematic Review and Meta-Analyses of Studies Using the Toronto Alexithymia Scale. J. Psychosom. Res. 2017, 99, 66–81. [Google Scholar] [CrossRef]
- Huke, V.; Turk, J.; Saeidi, S.; Kent, A.; Morgan, J.F. Autism Spectrum Disorders in Eating Disorder Populations: A Systematic Review. Eur. Eat. Disord. Rev. 2013, 21, 345–351. [Google Scholar] [CrossRef]
- Crespi, B.; Dinsdale, N. Autism and Psychosis as Diametrical Disorders of Embodiment. Evol. Med. Public Health 2019, 2019, 121–138. [Google Scholar] [CrossRef]
- Malighetti, C.; Chirico, A.; Serino, S.; Cavedoni, S.; Matamala-Gomez, M.; Stramba-Badiale, C.; Mancuso, V.; Corno, G.; Polli, N.; Cacciatore, C.; et al. Manipulating body size distortions and negative body-related memories in patients with Anorexia Nervosa: A virtual reality based pilot study. Annu. Rev. CyberTher. Telemed. 2020, 18, 177–181. Available online: https://hdl.handle.net/10281/316374 (accessed on 4 April 2025).

| Variable | Group | ||||
|---|---|---|---|---|---|
| ED (n = 19) | Control (n = 24) | Total (N = 43) | t/χ2 | p | |
| Age (M ± SD) | 27.47 ± 10.70 | 21.50 ± 4.77 | 24.14 ± 8.40 | −2.45 | 0.019 |
| BMI (M ± SD) | 21.15 ± 7.00 | 22.60 ± 3.55 | 21.96 ± 5.34 | 0.89 | 0.381 |
| Primary language spoken at home (n, %) | 2.55 | 0.110 | |||
| English | 19 (100.0%) | 21 (87.5%) | 40 (93.0%) | ||
| Other | 0 (0.0%) | 3 (12.5%) | 3 (7.0%) | ||
| Highest education completed (n, %) | 11.18 | 0.048 | |||
| Year 12 or below | 7 (36.8%) | 15 (62.5%) | 22 (51.2%) | ||
| Certificate/diploma | 4 (21.0%) | 0 (0.0%) | 4 (9.4%) | ||
| Bachelor’s degree | 5 (26.3%) | 4 (16.7%) | 9 (20.9%) | ||
| Postgraduate degree (e.g., Honours, Masters, PhD) | 3 (15.8%) | 5 (20.8%) | 8 (18.6%) | ||
| Sexual orientation (n, %) | 4.13 | 0.388 | |||
| Heterosexual | 11 (57.9%) | 16 (66.7%) | 27 (62.8%) | ||
| Lesbian/gay | 1 (5.3%) | 0 (0.0%) | 1 (2.3%) | ||
| Bisexual | 4 (21.1%) | 6 (25.0%) | 10 (23.3%) | ||
| Asexual | 2 (10.5%) | 0 (0.0%) | 2 (4.7%) | ||
| Other | 1 (5.3%) | 2 (8.3%) | 3 (7.0%) | ||
| Marital status (n, %) | 3.51 | 0.320 | |||
| Single | 11 (57.9%) | 12 (50.0%) | 23 (53.5%) | ||
| Relationship (including open relationship) | 4 (21.1%) | 8 (33.3%) | 12 (27.9%) | ||
| Married | 2 (10.5%) | 0 (0.0%) | 2 (4.7%) | ||
| De facto | 2 (10.5%) | 4 (16.7%) | 6 (14.0%) | ||
| ED diagnosis type (yes, %) a | - | - | |||
| Anorexia nervosa (restricting) | 6 (31.6%) | - | - | ||
| Anorexia nervosa (binge-purge) | 2 (10.5%) | - | - | ||
| Bulimia nervosa | 2 (10.5%) | - | - | ||
| OSFED b | 9 (47.4%) | - | - | ||
| ED diagnosis duration (years) (M ± SD) | 5.31 ± 8.13 | - | - | - | - |
| Average age of ED onset (years) (M ± SD) | 22.16 ± 9.72 | - | - | - | - |
| EDE interview score (M ± SD) | - | - | |||
| Global severity | 4.02 ± 0.99 | - | - | ||
| Restraint | 3.99 ± 1.26 | - | - | ||
| Eating concern | 3.12 ± 1.13 | - | - | ||
| Weight concern | 4.87 ± 0.94 | - | - | ||
| Shape concern | 4.56 ± 1.12 | - | - | ||
| EAT-26 score (M ± SD) | 46.58 ± 13.05 | 2.79 ± 1.56 | 22.14 ± 23.63 | −14.54 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portingale, J.; Butler, D.; Krug, I. Does Identifying with Another Face Alter Body Image Disturbance in Women with an Eating Disorder? An Enfacement Illusion Study. Nutrients 2025, 17, 1861. https://doi.org/10.3390/nu17111861
Portingale J, Butler D, Krug I. Does Identifying with Another Face Alter Body Image Disturbance in Women with an Eating Disorder? An Enfacement Illusion Study. Nutrients. 2025; 17(11):1861. https://doi.org/10.3390/nu17111861
Chicago/Turabian StylePortingale, Jade, David Butler, and Isabel Krug. 2025. "Does Identifying with Another Face Alter Body Image Disturbance in Women with an Eating Disorder? An Enfacement Illusion Study" Nutrients 17, no. 11: 1861. https://doi.org/10.3390/nu17111861
APA StylePortingale, J., Butler, D., & Krug, I. (2025). Does Identifying with Another Face Alter Body Image Disturbance in Women with an Eating Disorder? An Enfacement Illusion Study. Nutrients, 17(11), 1861. https://doi.org/10.3390/nu17111861







