FPAW from Trachinotus ovatus Attenuates Potassium-Oxonate-Induced Hyperuricemia in Mice via Xanthine Oxidase Inhibition and Gut Microbiota Modulation: Molecular Insights and In Vivo Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Animal Experiment Design
2.3. Analysis of Biochemical Parameters
2.4. Total DNA Extraction, PCR, and Sequencing of Fecal Samples
2.5. Measurement of the SCFA Content in Fecal Samples
2.6. Molecular Docking Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of FPAW on Physiological Indexes of Hyperuricemic Mice
3.2. Effect of FPAW on Biochemical Indexes of Hyperuricemic Mice
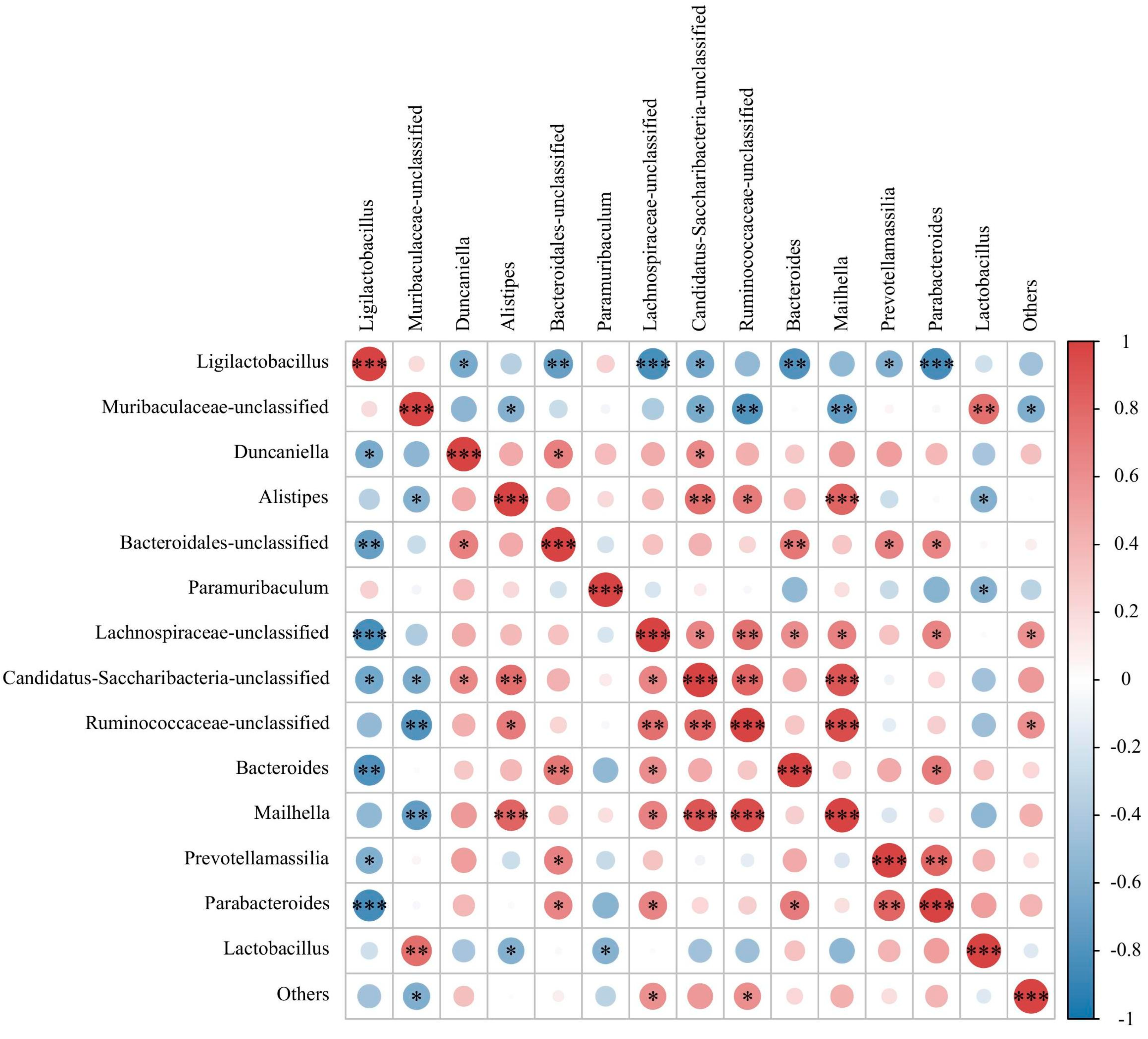
3.3. Effect of FPAW on the Modulation of the Gut Microbiota in Hyperuricemic Mice
3.4. Effect of FPAW on the Concentrations of SCFAs in Hyperuricemic Mice
3.5. Molecular Modeling Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, H.; Abdullah; Zhang, Y.; Deng, L.; Zhao, M.; Tang, J.; Zhang, H.; Feng, F.; Wang, J. Exploring the Potential of Novel Xanthine Oxidase Inhibitory Peptide (ACECD) Derived from Skipjack Tuna Hydrolysates Using Affinity-Ultrafiltration Coupled with HPLC–MALDI-TOF/TOF-MS. Food Chem. 2021, 347, 129068. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Chen, T.; Huang, B.; Liu, Y.; Chen, J. Untargeted Metabolomics Reveal the Therapeutic Effects of Ermiao Wan Categorized Formulas on Rats with Hyperuricemia. J. Ethnopharmacol. 2021, 281, 114545. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, H.; Xia, W.; Chang, X.; Wang, M.; An, L. Prevalence and Correlates of Hyperuricemia in the Middle-Aged and Older Adults in China. Sci. Rep. 2018, 8, 4314. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Bortolotti, M.; Polito, L.; Bolognesi, A. The Role of Xanthine Oxidoreductase and Uric Acid in Metabolic Syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2557–2565. [Google Scholar] [CrossRef]
- Song, D.; Zhao, X.; Wang, F.; Wang, G. A Brief Review of Urate Transporter 1 (URAT1) Inhibitors for the Treatment of Hyperuricemia and Gout: Current Therapeutic Options and Potential Applications. Eur. J. Pharmacol. 2021, 907, 174291. [Google Scholar] [CrossRef]
- Love, B.L.; Barrons, R.; Veverka, A.; Snider, K.M. Urate-Lowering Therapy for Gout: Focus on Febuxostat. Pharmacotherapy 2010, 30, 594–608. [Google Scholar] [CrossRef]
- Mugwagwa, A.N.; Fischer, R.; Zailan, I. HLA-B*5801: A Genetic Susceptibility to Allopurinol-Induced DRESS. Med. J. Aust. 2016, 204, 159–160. [Google Scholar] [CrossRef]
- Pritsos, C.A. Cellular Distribution, Metabolism and Regulation of the Xanthine Oxidoreductase Enzyme System. Chem. Biol. Interact. 2000, 129, 195–208. [Google Scholar] [CrossRef]
- McManaman, J.L.; Bain, D.L. Structural and Conformational Analysis of the Oxidase to Dehydrogenase Conversion of Xanthine Oxidoreductase. J. Biol. Chem. 2002, 277, 21261–21268. [Google Scholar] [CrossRef]
- Harris, C.M.; Massey, V. The Reaction of Reduced Xanthine Dehydrogenase with Molecular Oxygen. Reaction Kinetics and Measurement of Superoxide Radical. J. Biol. Chem. 1997, 272, 8370–8379. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.-P.; Hu, Q.-H.; Lv, Y.-Z.; Zhang, X.; OuYang, Z.; Kong, L.-D. The Dual Actions of Sanmiao Wan as a Hypouricemic Agent: Down-Regulation of Hepatic XOD and Renal mURAT1 in Hyperuricemic Mice. J. Ethnopharmacol. 2010, 128, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, T.R.; George, G.N.; Bray, R.C. The Structure of the Inhibitory Complex of Alloxanthine (1H-Pyrazolo[3,4-d]Pyrimidine-4,6-Diol) with the Molybdenum Centre of Xanthine Oxidase from Electron-Paramagnetic-Resonance Spectroscopy. Biochem. J. 1984, 218, 961–968. [Google Scholar] [CrossRef]
- Skibo, E.B. Noncompetitive and Irreversible Inhibition of Xanthine Oxidase by Benzimidazole Analogues Acting at the Functional Flavin Adenine Dinucleotide Cofactor. Biochemistry 1986, 25, 4189–4194. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Lobo, S.; Goswami, T. What Is the Future of Noninvasive Routes for Protein- and Peptide-Based Drugs? Ther. Deliv. 2016, 7, 355–357. [Google Scholar] [CrossRef]
- Murota, I.; Taguchi, S.; Sato, N.; Park, E.Y.; Nakamura, Y.; Sato, K. Identification of Antihyperuricemic Peptides in the Proteolytic Digest of Shark Cartilage Water Extract Using in Vivo Activity-Guided Fractionation. J. Agric. Food Chem. 2014, 62, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Han, J.; Tang, S.; Bao, W.; Lu, C.; Zhou, J.; Ming, T.; Li, Y.; Su, X. Comparisons of Protective Effects between Two Sea Cucumber Hydrolysates against Diet Induced Hyperuricemia and Renal Inflammation in Mice. Food Funct. 2020, 11, 1074–1086. [Google Scholar] [CrossRef]
- He, W.; Su, G.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Zhao, M.; Liu, Y. In Vivo Anti-Hyperuricemic and Xanthine Oxidase Inhibitory Properties of Tuna Protein Hydrolysates and Its Isolated Fractions. Food Chem. 2019, 272, 453–461. [Google Scholar] [CrossRef]
- Ma, F.; Sun, S.; Ye, H.; Zhang, Z.; Chen, Q.; Yin, S.; Cao, Y.; Miao, J. Purification, Characterization and Anti-Hyperuricemic Mechanism of Novel Xanthine Oxidase Inhibitory Peptides from Tea (Camellia Sinensis L.) Protein. Food Biosci. 2024, 61, 104512. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Shao, T.; Shao, L.; Li, H.; Xie, Z.; He, Z.; Wen, C. Combined Signature of the Fecal Microbiome and Metabolome in Patients with Gout. Front. Microbiol. 2017, 8, 268. [Google Scholar] [CrossRef]
- Han, J.; Wang, X.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Wang, Z.J.; Su, X. Protective Effects of Tuna Meat Oligopeptides (TMOP) Supplementation on Hyperuricemia and Associated Renal Inflammation Mediated by Gut Microbiota. FASEB J. 2020, 34, 5061–5076. [Google Scholar] [CrossRef]
- Hou, M.; Xiang, H.; Hu, X.; Chen, S.; Wu, Y.; Xu, J.; Yang, X. Novel Potential XOD Inhibitory Peptides Derived from Trachinotus Ovatus: Isolation, Identification and Structure-Function Analysis. Food Biosci. 2022, 47, 101639. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; Zhang, Z.; Han, J.; Feng, Y.; Zhang, J.; Zhang, Z.; Li, Y.; Ming, T.; Lu, C.; et al. Modulation of Gut Microbiota and Serum Metabolome by Apostichopus Japonicus Derived Oligopeptide in High-Fructose Diet-Induced Hyperuricemia in Mice. Food Sci. Human. Wellness 2025, 14, 9250011. [Google Scholar] [CrossRef]
- Thanabalan, A.; Kiarie, E.G. Body Weight, Organ Development and Jejunal Histomorphology in Broiler Breeder Pullets Fed n-3 Fatty Acids Enriched Diets from Hatch through to 22 Weeks of Age. Poult. Sci. 2022, 101, 101514. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.L.; Saatkamp, C.J.; Fernandes, A.B.; Pinheiro, A.L.B.; Silveira, L. Estimating the Concentration of Urea and Creatinine in the Human Serum of Normal and Dialysis Patients through Raman Spectroscopy. Lasers Med. Sci. 2016, 31, 1415–1423. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, R.; Wei, Y.; Cai, M.; Ma, Y.; Gu, R.; Zhang, H.; Pan, X. Rice Peptide and Collagen Peptide Prevented Potassium Oxonate-Induced Hyperuricemia and Renal Damage. Food Biosci. 2021, 42, 101147. [Google Scholar] [CrossRef]
- Bakaryan, A.; Karapetyan, L.; Hakobyan, N.; Camaioni, E.; Mardanyan, S.; Antonyan, A. Adenosine Deaminase—A Target for New Piperazine Derivatives. Biophys. Chem. 2021, 277, 106658. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Anzai, N.; Enomoto, A.; Endou, H. Renal Urate Handling: Clinical Relevance of Recent Advances. Curr. Rheumatol. Rep. 2005, 7, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhang, Y.; Wang, B.; Walana, W.; Wei, J.; Gordon, J.R.; Li, F. CXCR1/CXCR2 Antagonist G31P Inhibits Nephritis in a Mouse Model of Uric Acid Nephropathy. Biomed. Pharmacother. 2018, 107, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Jayasekhar Babu, P.; Tirkey, A.; Mohan Rao, T.J.; Chanu, N.B.; Lalchhandama, K.; Singh, Y.D. Conventional and Nanotechnology Based Sensors for Creatinine (A Kidney Biomarker) Detection: A Consolidated Review. Anal. Biochem. 2022, 645, 114622. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Liu, K.; Li, W.; Yuan, Y.; Niu, R.; Zhou, L.; Xiao, Y.; Gao, H.; Yang, H.; Zhang, C.; et al. Blood Urea Nitrogen, Blood Urea Nitrogen to Creatinine Ratio and Incident Stroke: The Dongfeng-Tongji Cohort. Atherosclerosis 2021, 333, 1–8. [Google Scholar] [CrossRef]
- Qi, X.; Guan, K.; Liu, C.; Chen, H.; Ma, Y.; Wang, R. Whey Protein Peptides PEW and LLW Synergistically Ameliorate Hyperuricemia and Modulate Gut Microbiota in Potassium Oxonate and Hypoxanthine-Induced Hyperuricemic Rats. J. Dairy Sci. 2023, 106, 7367–7381. [Google Scholar] [CrossRef]
- Qi, X.; Ma, Y.; Guan, K.; Liu, C.; Wang, R.; Ma, Y.; Niu, T. Whey Protein Peptide PEW Attenuates Hyperuricemia and Associated Renal Inflammation in Potassium Oxonate and Hypoxanthine-Induced Rat. Food Biosci. 2023, 51, 102311. [Google Scholar] [CrossRef]
- Grassi, D.; Ferri, L.; Desideri, G.; Di Giosia, P.; Cheli, P.; Del Pinto, R.; Properzi, G.; Ferri, C. Chronic Hyperuricemia, Uric Acid Deposit and Cardiovascular Risk. Curr. Pharm. Des. 2013, 19, 2432–2438. [Google Scholar] [CrossRef]
- Mu, J.; Lin, Q.; Liang, Y. An Update on the Effects of Food-Derived Active Peptides on the Intestinal Microecology. Crit. Rev. Food Sci. Nutr. 2023, 63, 11625–11639. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.; St Clair, L.L.; O’Neill, K.L. The Immunostimulating Role of Lichen Polysaccharides: A Review. Phytother. Res. 2015, 29, 317–322. [Google Scholar] [CrossRef]
- Liu, B.; Qian, J.; Wang, Q.; Wang, F.; Ma, Z.; Qiao, Y. Butyrate Protects Rat Liver against Total Hepatic Ischemia Reperfusion Injury with Bowel Congestion. PLoS ONE 2014, 9, e106184. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading Players in the Maintenance of Gut Homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Amaya, Y.; Okamoto, K.; Nishino, T. Mutations Associated with Functional Disorder of Xanthine Oxidoreductase and Hereditary Xanthinuria in Humans. Int. J. Mol. Sci. 2012, 13, 15475–15495. [Google Scholar] [CrossRef]





| Enzyme | Ligands | Energy (Kcal/mol) | Hydrogen Bond | Hydrophobic | |||
|---|---|---|---|---|---|---|---|
| Classical | Non-Classical | Pi-Hydrophobic | Alkyl-Hydrophobic | Mixed Hydrophobic | |||
| XOD | allopurinol | −6.5 | Arg880 (3.22, 2.90, 3.15 Å); Thr1010 (3.19, 3.09 Å) | Phe1009 (4.70 Å); Phe914 (3.25 Å) | Ala1078 (4.83 Å) Ala1079 (3.80 Å) | ||
| FPAW | −5.8 | Ser876 (2.75 Å) | Glu802 (3.40 Å) | Phe1009 (4.73 Å); Phe914 (4.40 Å) | Pro1076 (4.42 Å) Lys771 (5.43 Å); Leu648 (5.49 Å) Leu873 (5.11 Å) | Val1011 (5.43, 3.74, 3.74, 3.89 Å) | |
| ADA | allopurinol | −5.2 | Asp296 (2.08 Å); Gly184 (2.96 Å) | His17 (3.57 Å) | |||
| FPAW | −9.2 | Gly184 (3.10 Å); Glu217 (2.07 Å) | Gly184 (3.64 Å) | Val218 (4.94 Å); Leu58 (5.06 Å); His17 (3.63 Å); Phe65 (3.83 Å); Leu62 (3.92 Å) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; Sun-Waterhouse, D.; Hu, X.; Hou, M.; Chen, S.; Wu, Y.; Zhao, Y.; Wang, Y. FPAW from Trachinotus ovatus Attenuates Potassium-Oxonate-Induced Hyperuricemia in Mice via Xanthine Oxidase Inhibition and Gut Microbiota Modulation: Molecular Insights and In Vivo Efficacy. Nutrients 2025, 17, 1831. https://doi.org/10.3390/nu17111831
Xiang H, Sun-Waterhouse D, Hu X, Hou M, Chen S, Wu Y, Zhao Y, Wang Y. FPAW from Trachinotus ovatus Attenuates Potassium-Oxonate-Induced Hyperuricemia in Mice via Xanthine Oxidase Inhibition and Gut Microbiota Modulation: Molecular Insights and In Vivo Efficacy. Nutrients. 2025; 17(11):1831. https://doi.org/10.3390/nu17111831
Chicago/Turabian StyleXiang, Huan, Dongxiao Sun-Waterhouse, Xiao Hu, Mengfan Hou, Shengjun Chen, Yanyan Wu, Yongqiang Zhao, and Yueqi Wang. 2025. "FPAW from Trachinotus ovatus Attenuates Potassium-Oxonate-Induced Hyperuricemia in Mice via Xanthine Oxidase Inhibition and Gut Microbiota Modulation: Molecular Insights and In Vivo Efficacy" Nutrients 17, no. 11: 1831. https://doi.org/10.3390/nu17111831
APA StyleXiang, H., Sun-Waterhouse, D., Hu, X., Hou, M., Chen, S., Wu, Y., Zhao, Y., & Wang, Y. (2025). FPAW from Trachinotus ovatus Attenuates Potassium-Oxonate-Induced Hyperuricemia in Mice via Xanthine Oxidase Inhibition and Gut Microbiota Modulation: Molecular Insights and In Vivo Efficacy. Nutrients, 17(11), 1831. https://doi.org/10.3390/nu17111831









