Efficacy of Power Training on Sarcopenic Obesity in Community-Dwelling Older Women: A 32-Week Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
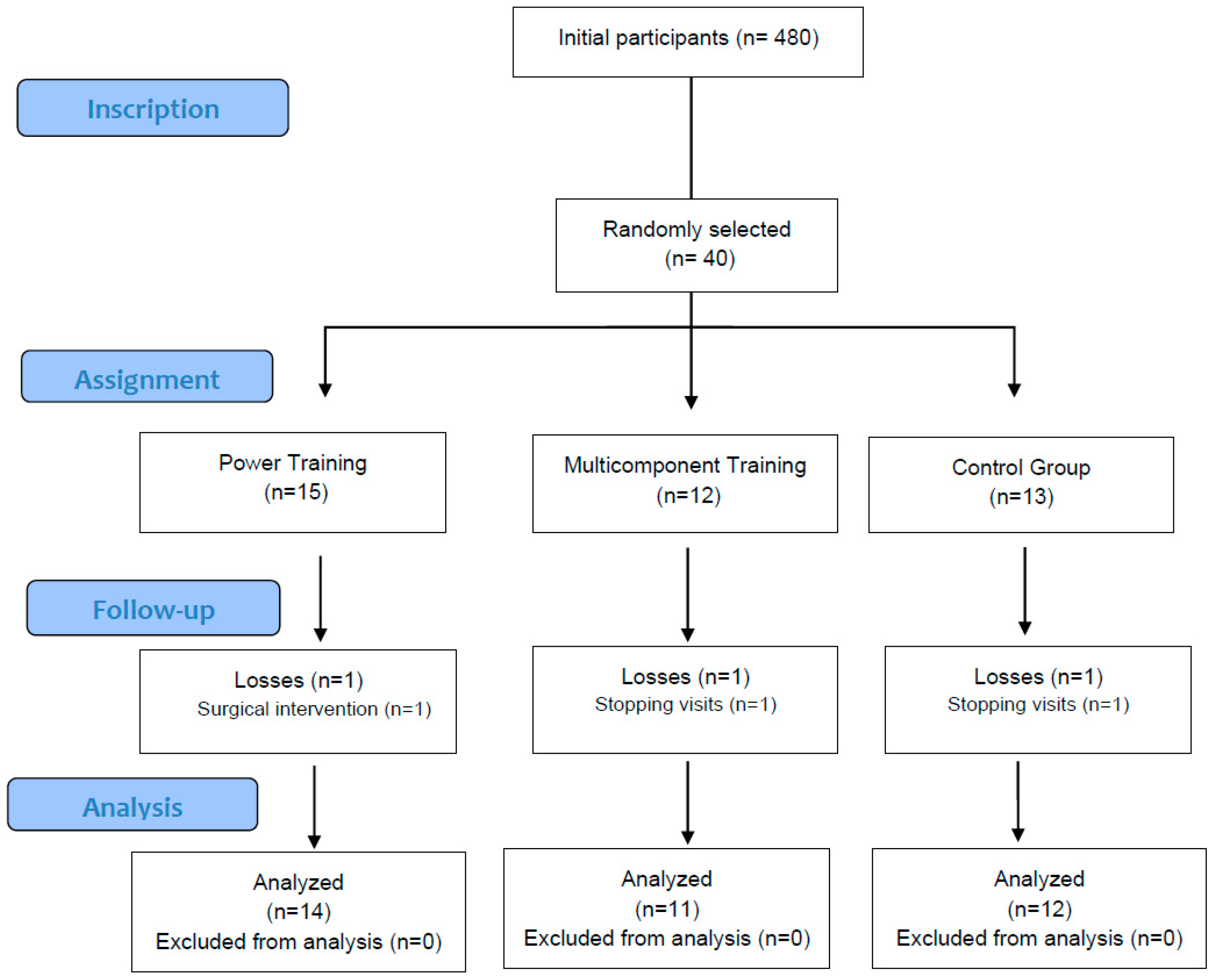
2.2. Participants
2.3. Randomization
2.4. Blinding
2.5. Assessments and Outcomes
2.5.1. Body Composition Variables
2.5.2. Strength and Functionality Variables
2.5.3. Nutritional Assessment
2.6. Interventions
2.6.1. Power Training (PT)
2.6.2. Multicomponent Training (MT)
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Baseline Results
3.2. Within-Group Analysis of Body Composition and Physical Function Post-Intervention
3.3. Comparative Group Analysis of Intervention Effects on Body Composition and Physical Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5STS | 5 times sit-to-stand test |
| ASM | Appendicular skeletal muscle mass |
| ASMI | Appendicular skeletal muscle mass index |
| BF% | Body fat percentage |
| BMI | Body mass index |
| CG | Control group |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| EWGSOP2 | European Working Group on Sarcopenia in Older People 2 |
| FFQ | Food Frequency Questionnaire |
| FITT-VP | Frequency, intensity, time, type, volume, progression |
| GS | Gait speed |
| HG | Hand grip strength |
| MT | Multicomponent training |
| PT | Power training |
| RT | Resistance training |
| RPE | Rating of perceived exertion |
| SO | Sarcopenic obesity |
| SPPB | Short Physical Performance Battery |
| TME2′ | 2-minute step test |
| TUG | Timed up and go test |
| VF | Visceral fat |
| WC | Waist circumference |
References
- Prado, C.M.; Batsis, J.A.; Donini, L.M.; Gonzalez, M.C.; Siervo, M. Sarcopenic obesity in older adults: A clinical overview. Nat. Rev. Endocrinol. 2024, 20, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin. Nutr. 2022, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Cawthon, P.M.; Arai, H.; Ávila-Funes, J.A.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyere, O.; Cederholm, T.; Chen, L.-K.; et al. The Conceptual Definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 2024, 53, afae052. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic obesity: Epidemiology, pathophysiology, cardiovascular disease, mortality, and management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Gao, Q.; Mei, F.; Shang, Y.; Hu, K.; Chen, F.; Zhao, L.; Ma, B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 4633–4641. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H. Exercise Therapy for People With Sarcopenic Obesity: Myokines and Adipokines as Effective Actors. Front. Endocrinol. 2022, 13, 811751. [Google Scholar] [CrossRef]
- Peterson, M.D.; Rhea, M.R.; Sen, A.; Gordon, P.M. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Res. Rev. 2010, 9, 226–237. [Google Scholar] [CrossRef]
- Welch, A.A.; Hayhoe, R.P.G.; Cameron, D. The relationships between sarcopenic skeletal muscle loss during ageing and macronutrient metabolism, obesity and onset of diabetes. Proc. Nutr. Soc. 2020, 79, 158–169. [Google Scholar] [CrossRef]
- el Hadouchi, M.; Kiers, H.; de Vries, R.; Veenhof, C.; van Dieën, J. Effectiveness of power training compared to strength training in older adults: A systematic review and meta-analysis. Eur. Rev. Aging Phys. Act 2022, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zheng, W.; Zhou, X.; Liu, Q.; Zhao, X. Training modalities for elder sarcopenic obesity: A systematic review and network meta-analysis. Front. Nutr. 2025, 12, 1537291. [Google Scholar] [CrossRef] [PubMed]
- Debes, W.A.; Sadaqa, M.; Németh, Z.; Aldardour, A.; Prémusz, V.; Hock, M. Effect of Resistance Exercise on Body Composition and Functional Capacity in Older Women with Sarcopenic Obesity-A Systematic Review with Narrative Synthesis. J. Clin. Med. 2024, 13, 441. [Google Scholar] [CrossRef]
- Polo-Ferrero, L.; Navarro-López, V.; Fuentes, M.; Lacal, J.; Cancelas-Felgueras, M.D.; Santos-Blázquez, N.; Méndez-Sánchez, R.; Sánchez-González, J.L. Effect of Resistance Training on Older Adults with Sarcopenic Obesity: A Comprehensive Systematic Review and Meta-Analysis of Blood Biomarkers, Functionality, and Body Composition. Nurs. Rep. 2025, 15, 89. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Polo-Ferrero, L.; Sáez-Gutiérrez, S.; Dávila-Marcos, A.; Barbero-Iglesias, F.J.; Sánchez-Sánchez, M.C.; Puente-González, A.S.; Méndez-Sánchez, R. Effect of power training on function and body composition in older women with probable sarcopenia. A study protocol for a randomized controlled trial. PLoS ONE 2025, 20, e0313072. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking speed: The functional vital sign. J. Aging Phys. Act 2015, 23, 314–322. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. Act 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Jorge Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos. Ediciones Pirámide n.d. Available online: https://www.edicionespiramide.es/libro/ciencia-y-tecnica/tablas-de-composicion-de-alimentos-olga-moreiras-tuni-9788436846577/ (accessed on 1 October 2024).
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.; Hassmén, P.; Lagerström, M. Perceived exertion related to heart rate and blood lactate during arm and leg exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 679–685. [Google Scholar] [CrossRef]
- Liao CDe Tsauo, J.Y.; Huang, S.W.; Ku, J.W.; Hsiao, D.J.; Liou, T.H. Effects of elastic band exercise on lean mass and physical capacity in older women with sarcopenic obesity: A randomized controlled trial. Sci. Rep. 2018, 8, 2317. [Google Scholar] [CrossRef]
- Liao, C.D.; Tsauo, J.Y.; Lin, L.F.; Huang, S.W.; Ku, J.W.; Chou, L.C.; Liou, T.H. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: A CONSORT-compliant prospective randomized controlled trial. Medicine 2017, 96, e7115. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Balachandran, A.; Krawczyk, S.N.; Potiaumpai, M.; Signorile, J.F. High-speed circuit training vs. hypertrophy training to improve physical function in sarcopenic obese adults: A randomized controlled trial. Exp. Gerontol. 2014, 60, 64–71. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Huang, S.W.; Ku, J.W.; Lin, L.F.; Liao CDe Chou, L.C.; Liou, T.H. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: A pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 556–563. [Google Scholar] [CrossRef]
- Leenders, M.; Verdijk, L.B.; Van Der Hoeven, L.; Van Kranenburg, J.; Nilwik, R.; Van Loon, L.J.C. Elderly men and women benefit equally from prolonged resistance-type exercise training. J. Gerontol. Biol. Sci. Med. Sci. 2013, 68, 769–779. [Google Scholar] [CrossRef]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Cunha, P.M.; Ribeiro, A.S.; Tomeleri, C.M.; Schoenfeld, B.J.; Silva, A.M.; Souza, M.F.; Nascimento, M.A.; Sardinha, L.B.; Cryno, E.S. The effects of resistance training volume on osteosarcopenic obesity in older women. J. Sports Sci. 2018, 36, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Banitalebi, E.; Ghahfarrokhi, M.M.; Dehghan, M. Effect of 12-weeks elastic band resistance training on MyomiRs and osteoporosis markers in elderly women with osteosarcopenic obesity: A randomized controlled trial. BMC Geriatr. 2021, 21, 682. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Kim, Y.Y.; Kim, J.W.; Park, H.Y. Effects of Circuit Training Program on Cardiovascular Risk Factors, Vascular Inflammatory Markers, and Insulin-like Growth Factor-1 in Elderly Obese Women with Sarcopenia. Rev. Cardiovasc. Med. 2022, 23, 134. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Chen, Z.T.; Weng, Z.X.; Lin, J.D.; Meng, Z.X. Myokines: Metabolic regulation in obesity and type 2 diabetes. Life Metab. 2024, 3, loae006. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, P.H.; Lin, L.F.; Liao, D.C.; Liou, T.H.; Huang, S.W. Effects of progressive elastic band resistance exercise for aged osteosarcopenic adiposity women. Exp. Gerontol. 2021, 147, 111272. [Google Scholar] [CrossRef]
- Vasconcelos, K.S.S.; Dias, J.M.D.; Araújo, M.C.; Pinheiro, A.C.; Moreira, B.S.; Dias, R.C. Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: A randomized controlled trial. Braz. J. Phys. Ther. 2016, 20, 432–440. [Google Scholar] [CrossRef]
| PT (n = 15) | MT (n = 12) | CG (n = 13) | Shapiro–Wilk Test | p-Value | |
|---|---|---|---|---|---|
| Age | 78.1 ± 6 | 76.9 ± 6 | 74.5 ± 9.5 | 0.886 | 0.443 |
| Medication, no./day | 4.4 ± 2 | 5 ± 3.1 | 3.8 ± 3.09 | 0.012 * | 0.536 a |
| Height, m | 1.51 ± 0.08 | 1.51 ± 0.08 | 1.50 ± 0.06 | 0.985 | 0.878 |
| ASMI, kg/m2 | 5 ± 0.45 | 4.9 ± 0.34 | 4.9 ± 0.45 | 0.126 | 0.554 |
| BF%, % | 47.8 (7.7) | 46 (6) | 46.7 (7.4) | <0.001 * | 0.686 a |
| Weight, kg | 73.2 (21.8) | 75.1 (6.6) | 72.7 (14.9) | 0.021 * | 0.856 a |
| WC, cm | 110 ± 8.8 | 103.7 ± 5.3 | 104.9 ± 8.7 | 0.417 | 0.095 |
| VF | 15.3 ± 1.9 | 14.3 ± 1.2 | 14.2 ± 2.9 | 0.087 | 0.346 |
| ASM, kg | 10.7 (2.5) | 10.8 (1.3) | 10.4 (2.3) | 0.009 * | 0.648 a |
| BMI, kg/m2 | 33.6 (5.2) | 32.5 (3.1) | 32.6 (4.8) | 0.005 * | 0.678 a |
| 5STS, s | 18.2 (4.4) | 16.9 (3.5) | 17 (7) | 0.012 * | 0.436 a |
| HG, kg | 12.7 (3.7) | 14.5 (3.6) | 15.1 (6.5) | 0.010 * | 0.582 a |
| GS, m/s | 0.72 (0.15) | 0.74 (0.31) | 0.86 (0.53) | <0.001 * | 0.068 a |
| TUG, s | 10.7 (6.7) | 9.7 (1.4) | 9.1 (4.2) | <0.001 * | 0.384 a |
| SPPB | 8 (1) | 8 (1.8) | 9 (2) | 0.005 * | 0.354 a |
| TME2′, s | 81.1 ± 22.2 | 75.5 ± 12.9 | 71.1 ± 18.6 | 0.075 | 0.370 |
| Total energy (kcal/day) | 2112.2 ± 420.3 | 2157.0 ± 447.6 | 2098.7 ± 490.7 | 0.951 | 0.957 |
| Glycemic load | 108.1 ± 23.3 | 105.0 ± 31.5 | 114.3 ± 37.7 | 0.198 | 0.815 |
| Carbohydrates (g/day) | 232.8 ± 63.8 | 234.9 ± 61.4 | 224.3 ± 59.0 | 0.054 | 0.937 |
| Proteins (g/day) | 93.6 ± 17.1 | 94.8 ± 20.6 | 88.4 ± 19.7 | 0.937 | 0.774 |
| Fats (g/day) | 88.1 ± 19.3 | 92.8 ± 20.8 | 91.9 ± 27.9 | 0.676 | 0.861 |
| Monounsaturated fatty acids (g/day) | 40.3 ± 11.0 | 41.0 ± 6.8 | 41.6 ± 12.3 | 0.814 | 0.959 |
| Polyunsaturated fatty acids (g/day) | 15.9 ± 4.5 | 16.0 ± 8.6 | 14.5 ± 7.1 | 0.154 | 0.877 |
| Saturated fatty acids (g/day) | 23.7 ± 6.6 | 25.8 ± 8.6 | 24.8 ± 7.9 | 0.164 | 0.796 |
| PT (n = 14) | MT (n = 11) | CG (n = 12) | ||||
|---|---|---|---|---|---|---|
| p-Value | Cohen’s d | p-Value | Cohen’s d | p-Value | Cohen’s d | |
| ASMI | <0.001 * | ↑1.056 ** | 0.272 | ↓0.189 | 0.199 | ↓0.254 |
| BF% b | <0.001 * | ↓1.382 ** | 0.026 * | ↓0.785 | 0.515 | ↓0.146 |
| Weight b | 0.015 * | ↑0.617 | 0.033 * | ↑0.392 | 0.038 * | ↑0.367 |
| WC | 0.013 * | ↓0.886 ** | 0.098 | ↑0.418 | 0.054 | ↑0.506 |
| VF | 0.007 * | ↑0.961 ** | 0.034 * | ↑0.616 | 0.293 | ↑0.162 |
| ASM b | 0.001 * | ↓0.925 ** | 0.477 | ↓0.151 | 0.093 | ↑0.243 |
| BMI b | 0.013 * | ↑0.722 | 0.100 | ↑0.544 | 0.038 * | ↑0.588 |
| 5STS b | <0.001 * | ↓1.379 ** | 0.424 | ↑0.040 | 0.006 * | ↑0.939 ** |
| HG b | 0.048 * | ↓0.451 | 0.042 * | ↓0.466 | 0.528 | ↓0.018 |
| GS b | <0.001 * | ↓0.998 ** | 0.260 | ↓0.129 | 0.906 | ↑0.016 |
| TUG b | 0.013 * | ↓0.734 | 0.722 | ↓0.108 | 0.086 | ↓0.479 |
| SPPB b | <0.001 * | ↓1.921 ** | 0.039 * | ↓0.546 | 0.713 | ↓0.122 |
| TME2′ | <0.001 * | ↓1.055 ** | 0.026 * | ↓0.665 | 0.155 | ↓0.413 |
| PT (n = 14) | MT (n = 11) | CG (n = 12) | p-Value | |
|---|---|---|---|---|
| ASMI, kg/m2 | 0.36 ± 0.34 | 0.1 ± 0.36 | −0.3 ± 1.19 | 0.039 * |
| BF%, % b | −6.9 ± 6 | −3.9 ± 7 | 0.7 ± 4.7 | 0.002 * |
| Weight, kg b | −5.56 ± 6.64 | −2.31 ± 7.39 | −1.06 ± 4.17 | 0.102 |
| WC, cm | −12.4 ± 14 | −6 ± 14.2 | −3.6 ± 7.1 | 0.179 |
| VF | −3.4 ± 3.6 | −2.3 ± 3.7 | 0.3 ± 2.1 | 0.044 * |
| ASM, kg b | 0.9 ± 0.9 | 0.3 ± 1.6 | −0.8 ± 2.9 | 0.021 * |
| BMI, kg/m2 b | −2.4 ± 2.9 | −1 ± 3.2 | −0.5 ± 1.9 | 0.399 |
| 5STS, s b | −3.8 ± 2.6 ** | −0.2 ± 3.7 | 1.3 ± 1.3 | <0.001 * |
| HG b | 2 ± 4.3 | 1.9 ± 3.8 | 0.1 ± 4.7 | 0.356 |
| GS, m/s b | 0.13 ± 0.1 | 0.03 ± 0.22 | 0.00 ± 0.10 | 0.018 * |
| TUG, s b | −2 ± 2.5 ** | 0.2 ± 1.6 | 1 ± 1.9 | 0.005 * |
| SPPB b | 2.5 ± 1.2 | 1.3 ± 2.2 | 0.2 ± 1.3 | 0.002 * |
| TME2′ b | 10.7 ± 21.1 | 11.5 ± 17.2 | 6.3 ± 14.1 | 0.327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo-Ferrero, L.; Martin, M.J.; Puente-González, A.S.; Barbero-Iglesias, F.J.; González-Manzano, S.; Méndez-Sánchez, R. Efficacy of Power Training on Sarcopenic Obesity in Community-Dwelling Older Women: A 32-Week Randomized Clinical Trial. Nutrients 2025, 17, 1822. https://doi.org/10.3390/nu17111822
Polo-Ferrero L, Martin MJ, Puente-González AS, Barbero-Iglesias FJ, González-Manzano S, Méndez-Sánchez R. Efficacy of Power Training on Sarcopenic Obesity in Community-Dwelling Older Women: A 32-Week Randomized Clinical Trial. Nutrients. 2025; 17(11):1822. https://doi.org/10.3390/nu17111822
Chicago/Turabian StylePolo-Ferrero, Luis, Maria J. Martin, Ana Silvia Puente-González, Fausto J. Barbero-Iglesias, Susana González-Manzano, and Roberto Méndez-Sánchez. 2025. "Efficacy of Power Training on Sarcopenic Obesity in Community-Dwelling Older Women: A 32-Week Randomized Clinical Trial" Nutrients 17, no. 11: 1822. https://doi.org/10.3390/nu17111822
APA StylePolo-Ferrero, L., Martin, M. J., Puente-González, A. S., Barbero-Iglesias, F. J., González-Manzano, S., & Méndez-Sánchez, R. (2025). Efficacy of Power Training on Sarcopenic Obesity in Community-Dwelling Older Women: A 32-Week Randomized Clinical Trial. Nutrients, 17(11), 1822. https://doi.org/10.3390/nu17111822





