Impact of Vitamin B1 and Vitamin B2 Supplementation on Anxiety, Stress, and Sleep Quality: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
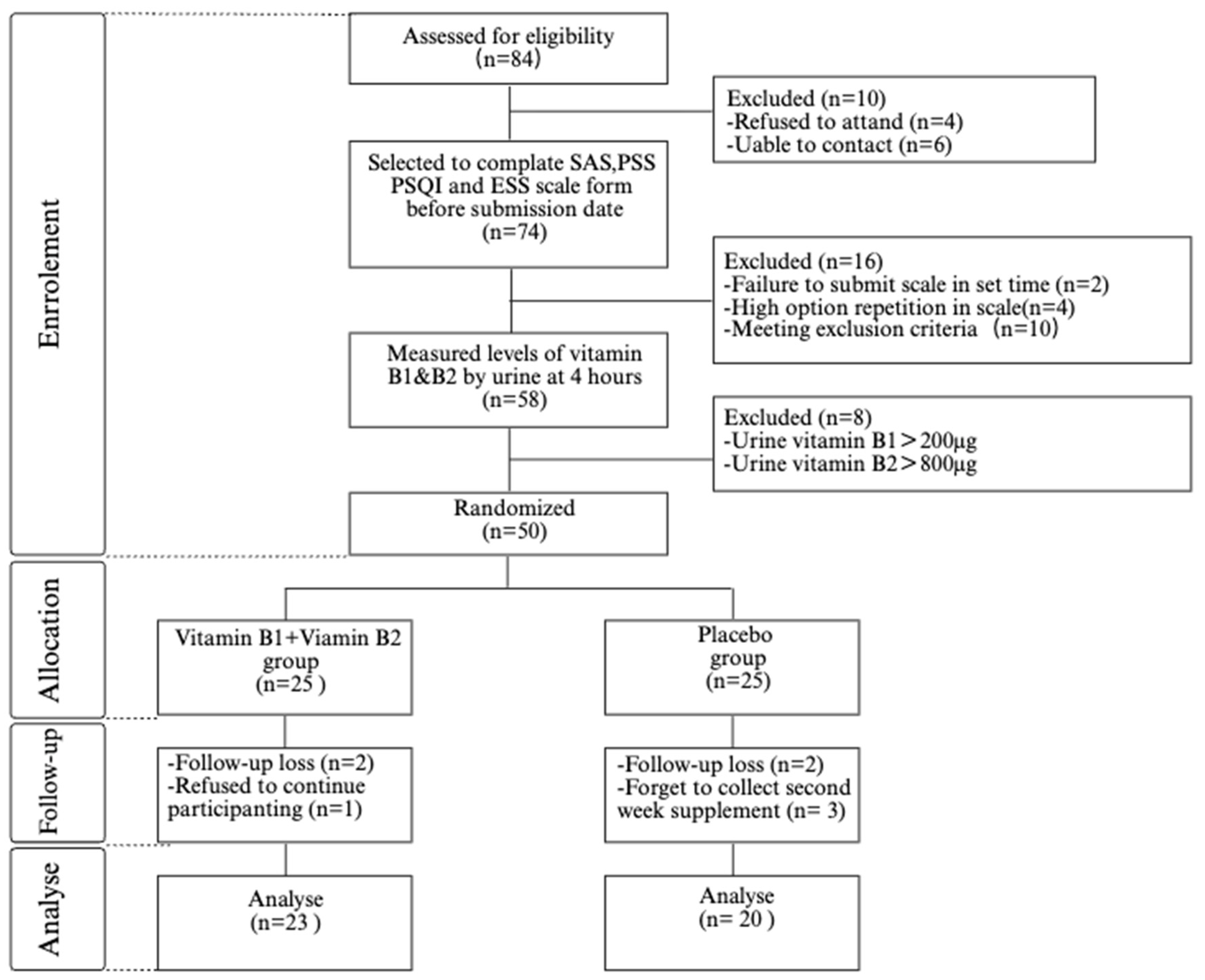
2.2. Participants
2.3. Randomization and Blinding
2.4. Intervention
2.5. Outcome Measures
2.6. Assessment of Vitamin B1 and Vitamin B2 Levels
2.7. Scale
- (1)
- Self-Rating Anxiety Scale (SAS)
- (2)
- Perceived Stress Scale (PSS)
- (3)
- Pittsburgh Sleep Quality Index (PSQI)
- (4)
- Epworth Sleepiness Scale (ESS)
2.8. Statistical Analysis
2.9. Ethical Considerations
3. Results
3.1. Participant Characteristics
3.2. Vitamin B1 and Vitamin B2 Levels
3.3. Changes in Anxiety Scores Between the Vitamin B1 and B2 Supplementation Group and the Placebo Group
3.4. Vitamin B1 and B2 Supplementation Group Showed Improvement in Stress Perception Scores Compared to the Placebo Group
3.5. Improvement in Sleep Quality Index in Vitamin B1 and B2 Supplementation Group Compared with Placebo Group
3.6. Improvement in Daytime Sleepiness Scores in the Vitamin B1 and B2 Supplementation Group Compared to the Placebo Group
4. Discussion
4.1. Summary of Main Findings
4.2. Examining the Findings in the Context of the Existing Literature
4.3. Limitations and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blampied, M.; Tylianakis, J.M.; Bell, C.; Gilbert, C.; Rucklidge, J.J. Efficacy and safety of a vitamin-mineral intervention for symptoms of anxiety and depression in adults: A randomised placebo-controlled trial “NoMAD”. J. Affect. Disord. 2023, 339, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, K.; Qi, J.; Wang, F.; Yang, R.; Wang, L.; Lyu, J.; Hu, J.; Wu, Y.; Cai, M. Exploring the moderated mediation of stress and media use: Social support’s impact on anxiety among older adults during the COVID-19 pandemic—Insights from a large-scale cross-sectional study in China. J. Affect. Disord. 2024, 367, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ellison, O.K.; Bullard, L.E.; Lee, G.K.; Vazou, S.; Pfeiffer, K.A.; Baez, S.E.; Pontifex, M.B. Examining efficacy and potential mechanisms of mindfulness-based cognitive therapy for anxiety and stress reduction among college students in a cluster-randomized controlled trial. Int. J. Clin. Health Psychol. 2024, 24, 100514. [Google Scholar] [CrossRef]
- Daviu, N.; Bruchas, M.R.; Moghaddam, B.; Sandi, C.; Beyeler, A. Neurobiological links between stress and anxiety. Neurobiol. Stress 2019, 11, 100191. [Google Scholar] [CrossRef]
- Goes, T.C.; Souza, T.H.A.; Marchioro, M.; Teixeira-Silva, F. Excitotoxic lesion of the medial prefrontal cortex in Wistar rats: Effects on trait and state anxiety. Brain Res. Bull. 2018, 142, 313–319. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gonzalez-Reigosa, F.; Martinez-Urrutia, A.; Natalicio, L.F.; Natalicio, D.S. The state-trait anxiety inventory. Rev. Interam. Psicol./Interam. J. Psychol. 1971, 5. [Google Scholar]
- Takagi, Y.; Sakai, Y.; Abe, Y.; Nishida, S.; Harrison, B.J.; Martínez-Zalacaín, I.; Soriano-Mas, C.; Narumoto, J.; Tanaka, S.C. A common brain network among state, trait, and pathological anxiety from whole-brain functional connectivity. Neuroimage 2018, 172, 506–516. [Google Scholar] [CrossRef]
- Endler, N.S.; Kocovski, N.L. State and trait anxiety revisited. J. Anxiety Disord. 2001, 15, 231–245. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Selye, H. A syndrome produced by diverse nocuous agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Hoehn-Saric, R. Neurotransmitters in anxiety. Arch. Gen. Psychiatry 1982, 39, 735–742. [Google Scholar] [CrossRef]
- Mora, F.; Segovia, G.; Del Arco, A.; de Blas, M.; Garrido, P. Stress, neurotransmitters, corticosterone and body–brain integration. Brain Res. 2012, 1476, 71–85. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Nutt, D.J. Role of GABA in anxiety and depression. Depress. Anxiety 2007, 24, 495–517. [Google Scholar] [CrossRef]
- Gottesmann, C. GABA mechanisms and sleep. Neuroscience 2002, 111, 231–239. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2024, 15, 15–34. [Google Scholar] [CrossRef]
- Hines, M.R.; Gomez-Contreras, P.C.; Liman, S.; Wilson, A.M.; Lu, K.J.; O’Neill, J.A.; Fisher, J.S.; Fredericks, D.C.; Wagner, B.A.; Buettner, G.R.; et al. A reciprocal relationship between mitochondria and lipid peroxidation determines the chondrocyte intracellular redox environment. Redox Biol. 2024, 75, 103306. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, J.; Chen, L.; Chen, W.; Yang, Y. Nutritional frailty and risk of depression and anxiety in middle-aged and older adults: A prospective cohort study of 176,987 UK Biobank participants. Clin. Nutr. ESPEN 2024, 63, 989. [Google Scholar] [CrossRef]
- Jansen, E.C.; Prather, A.; Leung, C.W. Associations between sleep duration and dietary quality: Results from a nationally-representative survey of US adults. Appetite 2020, 153, 104748. [Google Scholar] [CrossRef]
- Daneshzad, E.; Keshavarz, S.A.; Qorbani, M.; Larijani, B.; Azadbakht, L. Dietary total antioxidant capacity and its association with sleep, stress, anxiety, and depression score: A cross-sectional study among diabetic women. Clin. Nutr. ESPEN 2020, 37, 187–194. [Google Scholar] [CrossRef]
- Cheng, Z.; Zeng, Q.; Zhu, C.; Yang, G.; Zhong, L. Association between joint physical activity and sleep duration and hypertension in US adults: Cross-sectional NHANES study. Sleep Health 2024, 10, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Abbasi, K.; Feizi, A.; Kohan, S.; Amani, R. Effect of vitamin D supplementation on symptoms severity in vitamin D insufficient women with premenstrual syndrome: A randomized controlled trial. Clin. Nutr. ESPEN 2024, 59, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ke, Y.; Liu, X.; Zhang, Z.; Zhang, R.; Tian, F.; Zhi, L.; Zhao, G.; Lv, B.; Hua, S.; et al. Navigating the B vitamins: Dietary diversity, microbial synthesis, and human health. Cell Host Microbe 2024, 32, 12–18. [Google Scholar] [CrossRef]
- Ghaleiha, A.; Davari, H.; Jahangard, L.; Haghighi, M.; Ahmadpanah, M.; Seifrabie, M.A.; Bajoghli, H.; Holsboer-Trachsler, E.; Brand, S. Adjuvant thiamine improved standard treatment in patients with major depressive disorder: Results from a randomized, double-blind, and placebo-controlled clinical trial. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Mahdavifar, B.; Hosseinzadeh, M.; Salehi-Abargouei, A.; Mirzaei, M.; Vafa, M. Dietary intake of B vitamins and their association with depression, anxiety, and stress symptoms: A cross-sectional, population-based survey. J. Affect. Disord. 2021, 288, 92–98. [Google Scholar] [CrossRef]
- Kharitonova, T.; Shvarts, Y.G.; Verbovoy, A.F.; Orlova, N.S.; Puzyreva, V.P.; Strokov, I.A. Efficacy and safety of the combined metabolic medication, containing inosine, nicotinamide, riboflavin and succinic acid, for the treatment of diabetic neuropathy: A multicenter randomized, double-blind, placebo-controlled parallel group clinical trial (CYLINDER). BMJ Open Diabetes Res. Care 2022, 10, e002785. [Google Scholar] [CrossRef]
- Zandifar, A.; Mousavi, S.; Schmidt, N.B.; Badrfam, R.; Seif, E.; Qorbani, M.; Mehrabani Natanzi, M. Efficacy of vitamins B1 and B6 as an adjunctive therapy to lithium in bipolar-I disorder: A double-blind, randomized, placebo-controlled, clinical trial. J. Affect. Disord. 2024, 345, 103–111. [Google Scholar] [CrossRef]
- Teixeira de Santana, P.; Marqueze, E.C. Effect of foods rich in tryptophan, melatonin and complex vitamins a, b, c, d and e associated with administration of melatonin on sleep quality of working women overweight night days. Sleep Med. 2024, 115, S53. [Google Scholar] [CrossRef]
- Kumar, P.N.S.; Menon, V.; Andrade, C. A randomized, double-blind, placebo-controlled, 12-week trial of vitamin D augmentation in major depressive disorder associated with vitamin D deficiency. J. Affect. Disord. 2022, 314, 143–149. [Google Scholar] [CrossRef]
- Sim, M.; Hong, S.; Jung, S.; Kim, J.-S.; Goo, Y.-T.; Chun, W.Y.; Shin, D.-M. Vitamin C supplementation promotes mental vitality in healthy young adults: Results from a cross-sectional analysis and a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2022, 61, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Field, D.T.; Cracknell, R.O.; Eastwood, J.R.; Scarfe, P.; Williams, C.M.; Zheng, Y.; Tavassoli, T. High-dose Vitamin B6 supplementation reduces anxiety and strengthens visual surround suppression. Hum. Psychopharmacol. Clin. Exp. 2022, 37, e2852. [Google Scholar] [CrossRef] [PubMed]
- Borges-Vieira, J.G.; Cardoso, C.K.S. Efficacy of B-vitamins and vitamin D therapy in improving depressive and anxiety disorders: A systematic review of randomized controlled trials. Nutr. Neurosci. 2023, 26, 187–207. [Google Scholar] [CrossRef]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lee, H.-F.; Tsai, C.-H.; Hsu, Y.-Y.; Fang, C.-J.; Chen, C.-J.; Hung, Y.-H.; Hu, F.-W. Effect of Vitamin B2 supplementation on migraine prophylaxis: A systematic review and meta-analysis. Nutr. Neurosci. 2022, 25, 1801–1812. [Google Scholar] [CrossRef]
- Li, H.; Krall, J.R.; Frankenfeld, C.; Slavin, M. Nutritional intake of riboflavin (vitamin B2) and migraine: A cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES) 2001–2004. Nutr. Neurosci. 2023, 26, 1068–1077. [Google Scholar] [CrossRef]
- Dean, O.M.; Turner, A.; Malhi, G.S.; Ng, C.; Cotton, S.M.; Dodd, S.; Sarris, J.; Samuni, Y.; Tanious, M.; Dowling, N. Design and rationale of a 16-week adjunctive randomized placebo-controlled trial of mitochondrial agents for the treatment of bipolar depression. Braz. J. Psychiatry 2014, 37, 3–12. [Google Scholar] [CrossRef]
- Hara, A.; Takazawa, C.; Tsujiguchi, H.; Zhao, J.; Nakamura, M.; Kasahara, T.; Shimizu, Y.; Nakamura, H. Effect of vitamin B1 supplementation on bone turnover markers in adults: An exploratory single-arm pilot study. J. Nutr. Sci. 2025, 14, e34. [Google Scholar] [CrossRef]
- Calderon-Ospina, C.-A.; Nava-Mesa, M.O.; Paez-Hurtado, A.M. Update on safety profiles of vitamins B1, B6, and B12: A narrative review. Ther. Clin. Risk Manag. 2020, 16, 1275–1288. [Google Scholar] [CrossRef]
- Shaoxia, L.; Fen, L.; Binghui, Z.; Ming, C.; Jing, Y. Determination of thiamine in urine. Chin. J. Health Lab. Technol. 2011, 21, 1631. [Google Scholar]
- Rivlin, R.S.; Pinto, J.T. Riboflavin (vitamin B2). In Handbook of Vitamins; CRC Press: Boca Raton, FL, USA, 2007; Volume 3. [Google Scholar]
- Mohamed, A.M.; Mohamed, H.A.; Abdel-Latif, N.M.; Mohamed, M.R. Spectrofluorimetric determination of some water-soluble vitamins. J. AOAC Int. 2011, 94, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W. A rating instrument for anxiety disorders. Psychosom. J. Consult. Liaison Psychiatry 1971, 12, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. Perceived stress scale. Meas. Stress A Guide Health Soc. Sci. 1994, 10, 1–2. [Google Scholar]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Mitra, M.; Mitra, S.; Nandi, D.K. Vitamins, micronutrients, antioxidants, and nutraceuticals in neuroprotection: An overview. In A Review on Diverse Neurological Disorders; Elsevier: Amsterdam, The Netherlands, 2024; pp. 585–601. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Veasey, R.; Watson, A.; Dodd, F.; Jones, E.; Maggini, S.; Haskell, C.F. Effects of high-dose B vitamin complex with vitamin C and minerals on subjective mood and performance in healthy males. Psychopharmacology 2010, 211, 55–68. [Google Scholar] [CrossRef]
- Lakhan, S.; Finesmith, R. Nutritional supplements and sleep: An overview. In Handbook of Nutrition, Diet and Sleep; Springer: Berlin/Heidelberg, Germany, 2013; pp. 400–414. [Google Scholar]
- Matsunaga, T.; Nishikawa, K.; Adachi, T.; Yasuda, K. Associations between dietary consumption and sleep quality in young Japanese males. Sleep Breath. 2021, 25, 199–206. [Google Scholar] [CrossRef]
- Tehrani, S.; Mahmoudnejad, N.; Asour, A.A.; Keyvanfar, A.; Chalmiani, E.M. Efficacy of Thiamine (Vitamin B1) on Post-Acute COVID-19 Syndrome: An Open-Label, Randomized, Controlled Trial. Arch. Clin. Infect. Dis. 2024, 19, e144280. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Oh, H.; Kim, M.S. Mixtures modeling identifies vitamin B1 and B3 intakes associated with depression. J. Affect. Disord. 2022, 301, 68–80. [Google Scholar] [CrossRef]
- Herbison, C.E.; Hickling, S.; Allen, K.L.; O’Sullivan, T.A.; Robinson, M.; Bremner, A.P.; Huang, R.C.; Beilin, L.J.; Mori, T.A.; Oddy, W.H. Low intake of B-vitamins is associated with poor adolescent mental health and behaviour. Prev. Med. 2012, 55, 634–638. [Google Scholar] [CrossRef]
- Wang, F.; Bíró, É. Determinants of sleep quality in college students: A literature review. Explore 2021, 17, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Lund, H.G.; Reider, B.D.; Whiting, A.B.; Prichard, J.R. Sleep patterns and predictors of disturbed sleep in a large population of college students. J. Adolesc. Health 2010, 46, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Noreen, H.; Rehman, S.; Kamal, M.A.; da Rocha, J.B. Association of oxidative stress with neurological disorders. Curr. Neuropharmacol. 2022, 20, 1046–1072. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxid. Med. Cell. Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Thurnham, D.I. Thiamin: Physiology. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 274–279. [Google Scholar] [CrossRef]
- Bozic, I.; Lavrnja, I. Thiamine and benfotiamine: Focus on their therapeutic potential. Heliyon 2023, 9, e21839. [Google Scholar] [CrossRef]
- Ibrahim, M.; Khan, S.; Pathak, S.; Mazhar, M.; Singh, H. Vitamin B-Complex and its Relationship with the Health of Vegetarian People. Nat. Resour. Hum. Health 2023, 3, 342–354. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruss, A.; Georgieva, R.; Baumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Henriques, B.J.; Gomes, C.M. Riboflavin (vitamin B2) and mitochondrial energy. In Molecular Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–244. [Google Scholar] [CrossRef]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B(2)) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Sun, L.; Zhang, H.; Liu, C.; Zhang, C.; Yu, L. B-vitamin supplementation ameliorates anxiety- and depression-like behavior induced by gestational urban PM(2.5) exposure through suppressing neuroinflammation in mice offspring. Environ. Pollut. 2020, 266, 115146. [Google Scholar] [CrossRef]
- Thurnham, D.I.; Cathcart, A.E.; Livingstone, M.B. A retrospective investigation of thiamin and energy intakes following an outbreak of beriberi in The Gambia. Nutrients 2011, 3, 135–151. [Google Scholar] [CrossRef]
| Variables | Vitamin B1 and B2 Group | Placebo Group |
|---|---|---|
| Sociodemographic | ||
| N | 23 | 20 |
| Age, y | 19.5 ± 1.2 | 19.7 ± 1.5 |
| Sex, n (%) | ||
| Female | 12 (52.2%) | 12 (60%) |
| Male | 11 (47.8%) | 8 (40%) |
| Native place, n (%) | ||
| Northern China | 5 (21.8%) | 2 (10%) |
| South China | 18 (78.2%) | 18 (90%) |
| ≤College junior education, n (%) | 12 (52.2%) | 10 (50%) |
| Lifestyle | ||
| BMI, kg/m2 | 22.9 ± 2.5 | 21.4 ± 3.2 |
| Daily physical activity practician (%) | ||
| Mild level | 21 (91.3%) | 17 (85%) |
| Middle level | 1 (4.3%) | 2 (10%) |
| Severe level | 1 (4.3%) | 1 (5%) |
| Smoking, yes (%) | 3 (13%) | 0 (0%) |
| Drinking, yes (%) | 2 (8.6%) | 1 (5%) |
| Nutritional status | ||
| Vitamin B1 in urine,ug | 158.8 ± 108.9 | 113 ± 68.9 |
| Vitamin B2 in urine,ug | 308 ± 198.3 | 309.4 ± 190.4 |
| Dietary consumption | ||
| Dietary consumption per day, yuan | 130 ± 39.9 | 108 ± 33 |
| Consumption per meal, yuan | ||
| Breakfast | 6.6 ± 8.8 | 7.9 ± 10 |
| Lunch | 37.3 ± 12.6 | 39.3 ± 11.4 |
| Dinner | 38.7 ± 15.5 | 42 ± 15.1 |
| Midnight snack | 5.4 ± 11.4 | 4.4 ± 8.6 |
| Drink and snack | 18.2 ± 12.6 | 17.4 ± 10.6 |
| Vitamin B1 and B2 Group | Placebo Group | p-Value a | Adjusted p-Value d | |
|---|---|---|---|---|
| Baseline (ng) | 158.8 ± 108.9 | 167.6 ± 125.0 | 0.609 | 0.039 c |
| After 4 weeks | 1333.1 ± 1204.5 | 408.3 ± 299.7 | ||
| Mean difference (95% CI) | −808.0 (−1075.7, −540.3) | −240.5 (−461.2, −19.8) | ||
| p-value b | <0.001 c | 0.036 c |
| Vitamin B1 and B2 Group | Placebo Group | p-Value a | Adjusted p-Value d | |
|---|---|---|---|---|
| Baseline (ng) | 308.0 ± 198.3 | 309.4 ± 109.4 | 0.980 | 0.004 c |
| After 4 weeks | 6123.2 ± 4847.2 | 372.0 ± 209.7 | ||
| Mean difference(95% CI) | −5831.8 (−8283.4, −3380.6) | −39.0 (−167.1, 83.6) | ||
| p-value b | <0.001 c | 0.509 |
| Vitamin B1 and B2 Group | Placebo Group | p-Value a | Adjusted p-Value d | ||
|---|---|---|---|---|---|
| Mean score of SAS | Baseline | 45.8 ± 10.2 | 44.1 ± 11.6 | 0.67 | 0.36 |
| After 4 weeks | 44.0 ± 6.0 | 45.1 ± 13.2 | |||
| Mean difference (95% CI) | 1.8 (−1.7, 5.4) | −1.1 (−7.1, 5.1) | |||
| p-value b | 0.294 | 0.730 | |||
| Mean score of PSS | Baseline | 21.5 ± 4.1 | 20.3 ± 4.3 | 0.37 | <0.005 c |
| After 4 weeks | 15.5 ± 4.5 | 19.8 ± 5.5 | |||
| Mean difference (95% CI) | 5.9 (4.4, 7.4) | 0.5 (−2.4, 3.4) | |||
| p-value b | <0.001 c | 0.725 |
| Vitamin B1 and B2 Group | Placebo Group | p-Value a | Adjusted p-Value d | ||
|---|---|---|---|---|---|
| Mean score of PSQI | Baseline | 8.0 ± 3.2 | 5.7 ± 2.7 | 0.019 c | 0.018 c |
| After 4 weeks | 6.3 ± 2.0 | 7.4 ± 2.9 | |||
| Mean difference(95% CI) | 1.6 (0.49, 2.9) | −1.6 (−3.1, −0.1) | |||
| p-value b | 0.008 c | 0.037 c | |||
| Mean score of ESS | Baseline | 13.0 ± 3.4 | 10.6 ± 4.2 | 0.04 c | 0.001 c |
| After 4 weeks | 9.1 ± 3.9 | 10.3 ± 4.7 | |||
| Mean difference(95% CI) | 3.9 (2.8, 5) | 0.3 (−1, 1.7) | |||
| p-value b | <0.001 c | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Wu, M.; Su, B.; Lin, H.; Li, Q.; Zhong, T.; Xiao, Y.; Yu, X. Impact of Vitamin B1 and Vitamin B2 Supplementation on Anxiety, Stress, and Sleep Quality: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2025, 17, 1821. https://doi.org/10.3390/nu17111821
Tao Y, Wu M, Su B, Lin H, Li Q, Zhong T, Xiao Y, Yu X. Impact of Vitamin B1 and Vitamin B2 Supplementation on Anxiety, Stress, and Sleep Quality: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2025; 17(11):1821. https://doi.org/10.3390/nu17111821
Chicago/Turabian StyleTao, Yingxuan, Murong Wu, Boyao Su, Heng Lin, Qianzi Li, Tian Zhong, Ying Xiao, and Xi Yu. 2025. "Impact of Vitamin B1 and Vitamin B2 Supplementation on Anxiety, Stress, and Sleep Quality: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 17, no. 11: 1821. https://doi.org/10.3390/nu17111821
APA StyleTao, Y., Wu, M., Su, B., Lin, H., Li, Q., Zhong, T., Xiao, Y., & Yu, X. (2025). Impact of Vitamin B1 and Vitamin B2 Supplementation on Anxiety, Stress, and Sleep Quality: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 17(11), 1821. https://doi.org/10.3390/nu17111821









