Protein Intake and Protein Quality Patterns in New Zealand Vegan Diets: An Observational Analysis Using Dynamic Time Warping
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Data Processing
2.3. Data Analysis
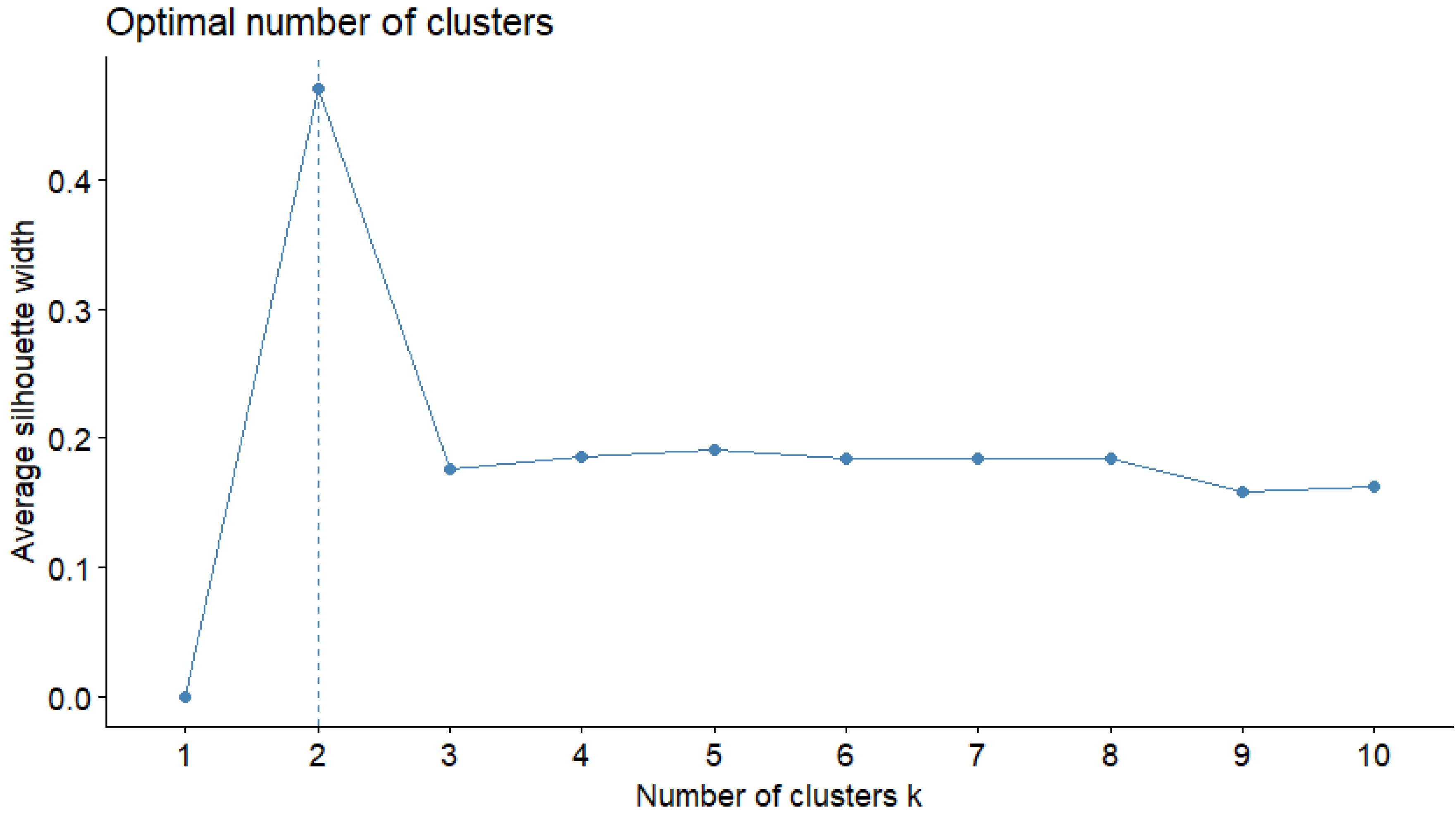
2.4. Cluster Analysis
2.4.1. Variation Across Clusters
2.4.2. Statistical Analysis
2.4.3. Temporal Variation in Each Cluster
3. Results
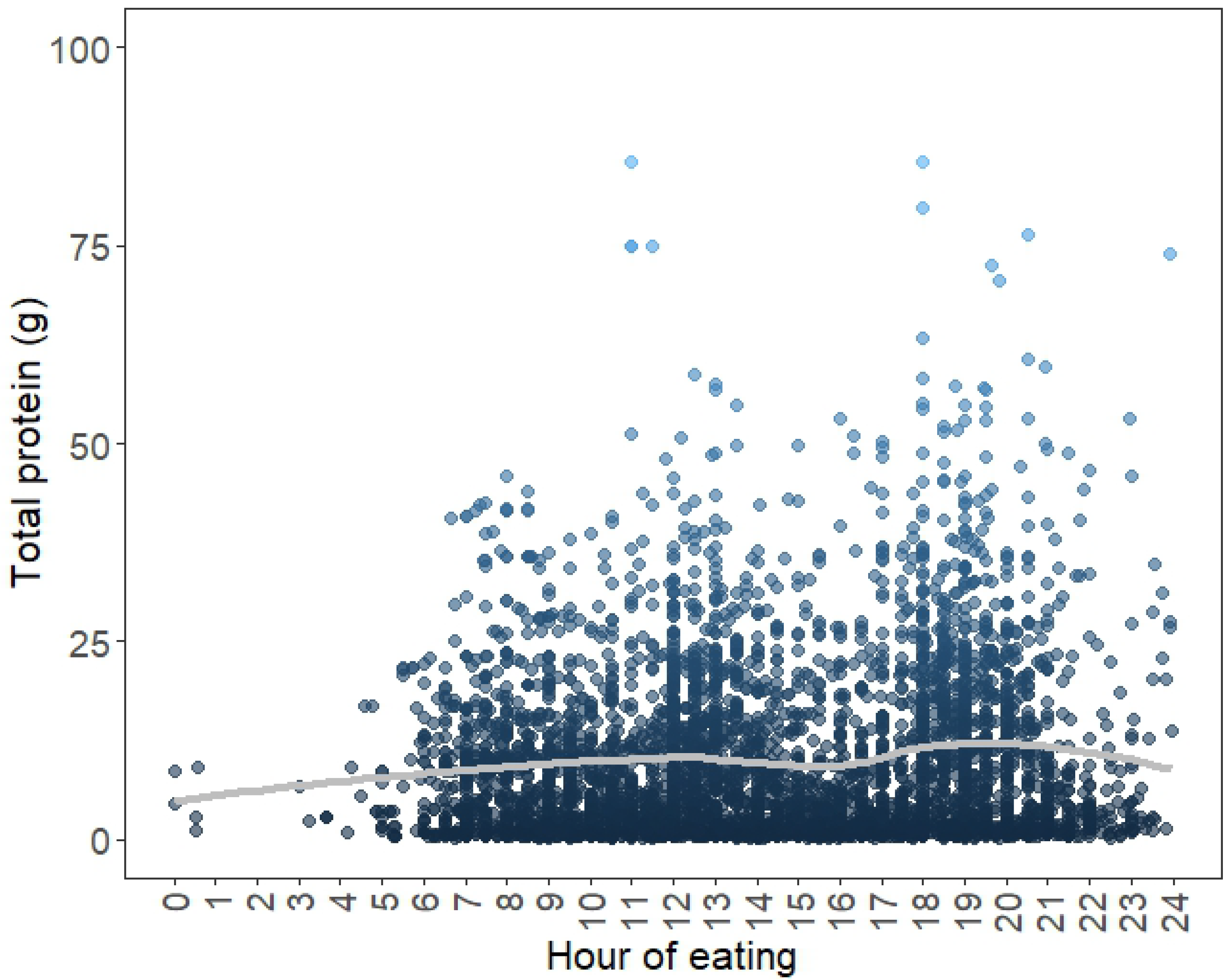
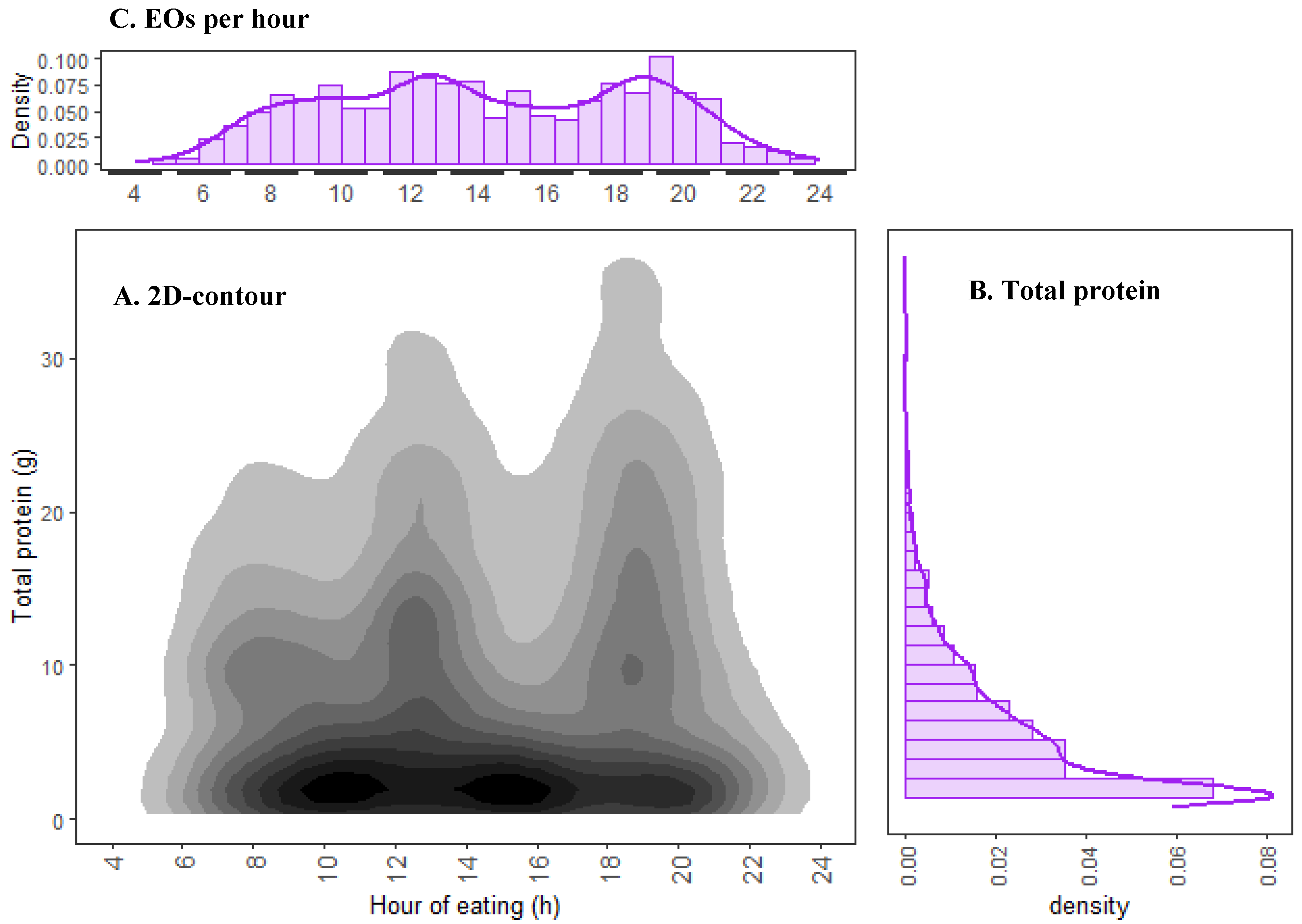
3.1. Total Protein Distribution of the Whole Cohort over 24 h
3.2. Cluster Analysis: Variation in Intake Patterns
3.3. Cluster Analysis: Contribution of Food Groups to Total Protein and IAA Intake
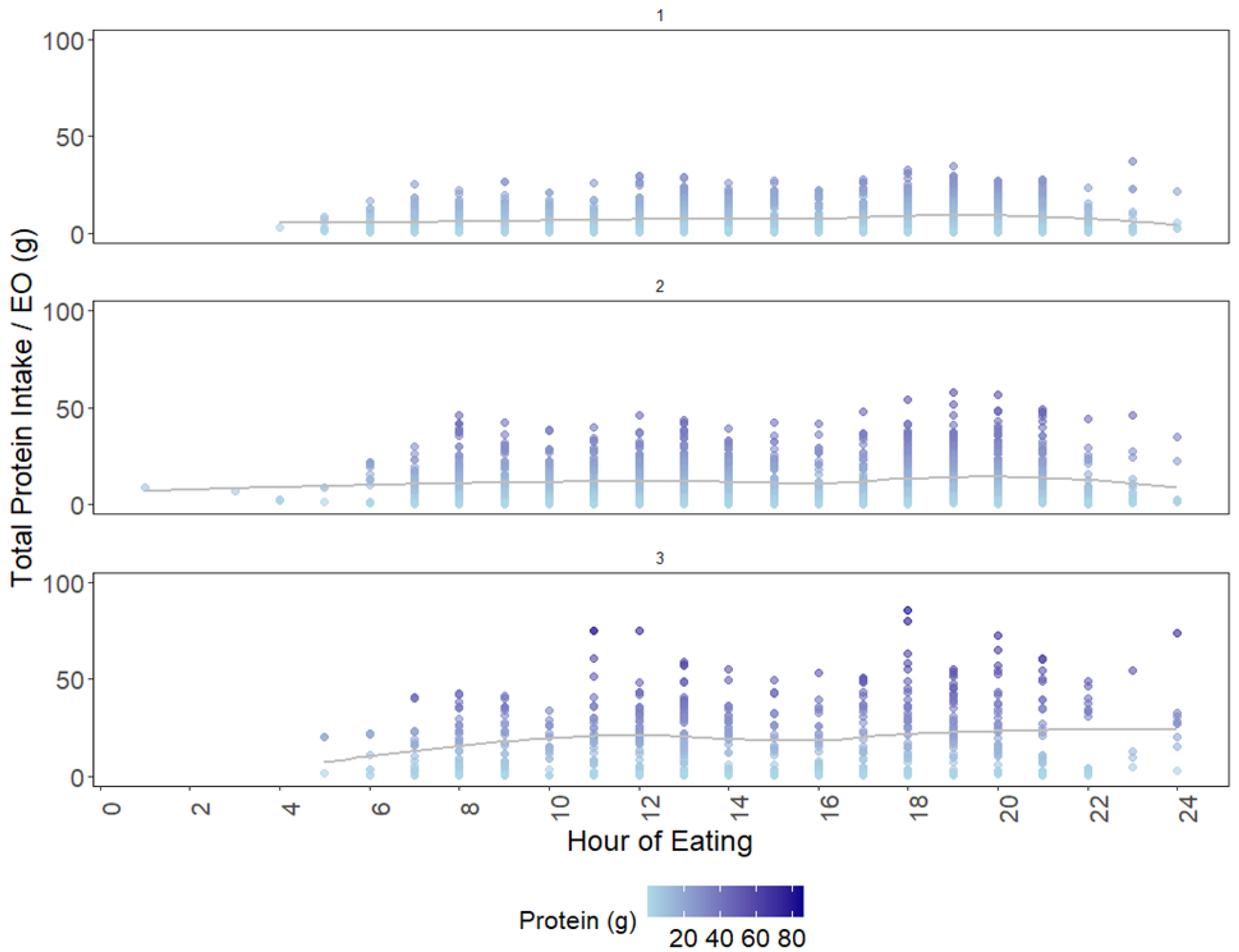
3.4. Cluster Analysis: Temporal Variation in TID-Adjusted Total Protein and IAAs
4. Discussion
4.1. Cluster Analysis
4.2. Temporal Distribution of Dietary Protein
4.3. Food Group Contribution to Dietary Protein
4.4. Strengths and Limitations
4.5. Implications for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| ASF | Animal-sourced food |
| BW | Body weight |
| DTW | Dynamic time warping |
| EAR | Estimated average requirement |
| EO | Eating occasion |
| FAO | Food and Agricultural Organization |
| HC | Hierarchical clustering |
| HDEC | Health and Disability Ethics Committee |
| IAA | Indispensable amino acid |
| KW | Kruskal–Wallis |
| MPB | Muscle protein breakdown |
| MDS | Multidimensional scaling |
| MPS | Muscle protein synthesis |
| NZ | New Zealand |
| PB | Plant based |
| PBB | Plant-based beverage |
| PBMA | Plant-based meat alternative |
| RDI | Recommended dietary intake |
| SD | Standard deviation |
| TID | True-ileal digestibility |
| USDA | United States Department of Agriculture |
Appendix A


| Food Group | Nutrient | % of Total Intake Across Clusters | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Fruits | Protein | 2.8 | 1.6 | 0.9 |
| Cystine | 3.7 | 2.1 | 1.2 | |
| Histidine | 5.2 | 2.9 | 1.7 | |
| Leucine | 2.6 | 1.4 | 0.8 | |
| Lysine | 3.6 | 1.9 | 0.9 | |
| Methionine | 4.2 | 2.4 | 1.3 | |
| Tryptophan | 2.4 | 1.3 | 0.8 | |
| Threonine | 3.2 | 1.8 | 1.0 | |
| Grains and pasta | Protein | 34.1 | 32.2 | 23.5 |
| Cystine | 41.0 | 38.1 | 27.9 | |
| Histidine | 31.9 | 29.7 | 21.2 | |
| Leucine | 34.7 | 32.8 | 23.8 | |
| Lysine | 24.1 | 22.2 | 16.4 | |
| Methionine | 36.3 | 34.9 | 26.3 | |
| Tryptophan | 37.0 | 35.5 | 28.7 | |
| Threonine | 30.0 | 28.4 | 21.1 | |
| Legumes and pulses | Protein | 35.5 | 41.7 | 57.0 |
| Cystine | 30.3 | 37.3 | 52.4 | |
| Histidine | 37.8 | 44.0 | 58.1 | |
| Leucine | 37.7 | 42.8 | 56.7 | |
| Lysine | 48.4 | 55.0 | 66.5 | |
| Methionine | 29.8 | 34.6 | 48.3 | |
| Tryptophan | 32.1 | 36.0 | 48.0 | |
| Threonine | 39.4 | 44.8 | 58.3 | |
| Nuts and seeds | Protein | 13.3 | 12.7 | 10.4 |
| Cystine | 16.4 | 14.9 | 12.8 | |
| Histidine | 17.5 | 16.9 | 14.1 | |
| Leucine | 17.3 | 16.4 | 13.5 | |
| Lysine | 14.0 | 12.7 | 10.1 | |
| Methionine | 19.8 | 19.1 | 17.1 | |
| Tryptophan | 18.3 | 18.1 | 15.1 | |
| Threonine | 16.0 | 14.9 | 12.2 | |
| Potatoes, kumara, taro | Protein | 2.0 | 1.8 | 0.6 |
| Cystine | 1.8 | 1.5 | 0.6 | |
| Histidine | 1.9 | 1.5 | 0.5 | |
| Leucine | 1.9 | 1.5 | 0.5 | |
| Lysine | 2.7 | 2.1 | 0.7 | |
| Methionine | 2.2 | 1.9 | 0.7 | |
| Tryptophan | 2.6 | 2.1 | 0.8 | |
| Threonine | 2.3 | 2.0 | 0.7 | |
| Vegetables | Protein | 6.1 | 5.2 | 3.2 |
| Cystine | 5.6 | 5.0 | 3.1 | |
| Histidine | 4.5 | 4.0 | 2.4 | |
| Leucine | 4.4 | 3.8 | 2.5 | |
| Lysine | 5.3 | 4.4 | 2.6 | |
| Methionine | 6.3 | 5.9 | 4.0 | |
| Tryptophan | 5.9 | 5.5 | 4.0 | |
| Threonine | 7.4 | 6.6 | 4.2 | |
| Others | Protein | 6.1 | 4.7 | 4.4 |
| Cystine | 1.3 | 1.1 | 2.1 | |
| Histidine | 1.3 | 1.1 | 2.0 | |
| Leucine | 1.5 | 1.3 | 2.3 | |
| Lysine | 1.9 | 1.7 | 2.8 | |
| Methionine | 1.4 | 1.3 | 2.3 | |
| Tryptophan | 1.7 | 1.5 | 2.7 | |
| Threonine | 1.7 | 1.5 | 2.6 | |
| Food Group | Nutrient | p-Values in Clusters | ||
|---|---|---|---|---|
| 1–2 | 2–3 | 1–3 | ||
| Grains and pasta | Protein | <0.001 | 1.00 | <0.001 |
| Cystine | <0.001 | 1.00 | 0.001 | |
| Histidine | <0.001 | 1.00 | 0.001 | |
| Leucine | <0.001 | 1.00 | <0.05 | |
| Lysine | <0.001 | 1.00 | <0.001 | |
| Methionine | <0.001 | 1.00 | <0.05 | |
| Tryptophan | <0.001 | 1.00 | <0.05 | |
| Threonine | <0.001 | 1.00 | 0.02 | |
| Legumes and pulses | Protein | <0.001 | <0.001 | <0.001 |
| Cystine | <0.001 | <0.001 | <0.001 | |
| Histidine | <0.001 | <0.001 | <0.001 | |
| Leucine | <0.001 | <0.001 | <0.001 | |
| Lysine | <0.001 | <0.001 | <0.001 | |
| Methionine | <0.001 | <0.001 | <0.001 | |
| Tryptophan | <0.001 | <0.001 | <0.001 | |
| Threonine | <0.001 | <0.001 | <0.001 | |
| Nuts and seeds | Protein | 0.06 | 0.30 | <0.01 |
| Cystine | 0.12 | 0.03 | 0.82 | |
| Histidine | 0.05 | <0.01 | 0.28 | |
| Leucine | 0.07 | 0.28 | <0.01 | |
| Lysine | 0.22 | 0.46 | 0.02 | |
| Methionine | 0.04 | 1.00 | 0.02 | |
| Tryptophan | 0.08 | 1.00 | 0.03 | |
| Threonine | 0.20 | 0.63 | 0.03 | |
| Vegetables | Protein | 0.14 | 0.39 | 0.01 |
| Cystine | 0.08 | 0.28 | <0.01 | |
| Histidine | 0.17 | 0.23 | <0.01 | |
| Leucine | 0.40 | 0.25 | 0.02 | |
| Lysine | 0.23 | 0.18 | <0.01 | |
| Methionine | 0.25 | 0.17 | <0.01 | |
| Tryptophan | 0.06 | 0.31 | <0.01 | |
| Threonine | 0.14 | 0.23 | <0.01 | |
| Fruit | Protein | ns | ns | ns |
| Cystine | 0.49 | 0.16 | 0.01 | |
| Histidine | ns | ns | ns | |
| Leucine | ns | ns | ns | |
| Lysine | 0.55 | 0.06 | <0.01 | |
| Methionine | 0.18 | 0.06 | <0.001 | |
| Tryptophan | 0.46 | 0.04 | <0.01 | |
| Threonine | 0.71 | 0.15 | 0.02 | |
| Others | Protein | 0.09 | 0.03 | <0.001 |
| Cystine | ns | ns | ns | |
| Histidine | 0.06 | 0.88 | 0.23 | |
| Leucine | ns | ns | ns | |
| Lysine | ns | ns | ns | |
| Methionine | 0.06 | 0.81 | 0.26 | |
| Tryptophan | ns | ns | ns | |
| Threonine | ns | ns | ns | |
References
- Layman, D.K. Impacts of protein quantity and distribution on body composition. Front. Nutr. 2024, 11, 1388986. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef] [PubMed]
- Moughan, P.J.; Deglaire, A.; Wolfe, R.R. Amino acid metabolism—An overview. In Feed Evaluation Science; Moughan, P.J., Hendriks, W.H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; pp. 219–242. [Google Scholar]
- Bhutia, Y.D.; Ganapathy, V. Protein digestion and absorption. In Physiology of the Gastrointestinal Tract, 6th ed.; Said, H.M., Ed.; Academic Press: London, UK, 2018; pp. 1063–1086. [Google Scholar]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Groen, B.B.; Horstman, A.M.; Hamer, H.M.; De Haan, M.; Van Kranenburg, J.; Bierau, J.; Poeze, M.; Wodzig, W.K.; Rasmussen, B.B.; Van Loon, L.J. Post-prandial protein handling: You are what you just ate. PLoS ONE 2015, 10, e0141582. [Google Scholar] [CrossRef]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef]
- Woolf, P.J.; Fu, L.L.; Basu, A. vProtein: Identifying optimal amino acid complements from plant-based foods. PLoS ONE 2011, 6, e18836. [Google Scholar] [CrossRef]
- Adhikari, S.; Schop, M.; de Boer, I.J.; Huppertz, T. Protein quality in perspective: A review of protein quality metrics and their applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Loprinzi, P.D.; Murphy, C.H.; Phillips, S.M. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin. Nutr. 2016, 35, 1506–1511. [Google Scholar] [CrossRef]
- Greenwell, J.; Grant, M.; Young, L.; Mackay, S.; Bradbury, K.E. The prevalence of vegetarians, vegans and other dietary patterns that exclude some animal-source foods in a representative sample of New Zealand adults. Public Health Nutr. 2024, 27, e5. [Google Scholar] [CrossRef]
- Sandri, E.; Sguanci, M.; Cantín Larumbe, E.; Cerdá Olmedo, G.; Werner, L.U.; Piredda, M.; Mancin, S. Plant-based diets versus the Mediterranean dietary pattern and their socio-demographic determinants in the Spanish population: Influence on health and lifestyle habits. Nutrients 2024, 16, 1278. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.M.; Worsley, A.; Timperio, A.; McNaughton, S.A. Understanding meal patterns: Definitions, methodology and impact on nutrient intake and diet quality. Nutr. Res. Rev. 2015, 28, 1–21. [Google Scholar] [CrossRef]
- FAO; WHO. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Rome, Italy, 2007. [Google Scholar]
- Heerschop, S.N.; Kanellopoulos, A.; Biesbroek, S.; van ‘t Veer, P. Shifting towards optimized healthy and sustainable Dutch diets: Impact on protein quality. Eur. J. Nutr. 2023, 62, 2115–2128. [Google Scholar] [CrossRef]
- Cardon-Thomas, D.K.; Riviere, T.; Tieges, Z.; Greig, C.A. Dietary protein in older adults: Adequate daily intake but potential for improved distribution. Nutrients 2017, 9, 184. [Google Scholar] [CrossRef]
- Layman, D.K.; Anthony, T.G.; Rasmussen, B.B.; Adams, S.H.; Lynch, C.J.; Brinkworth, G.D.; Davis, T.A. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am. J. Clin. Nutr. 2015, 101, 1330S–1338S. [Google Scholar] [CrossRef]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef]
- Moore, D.R.; Areta, J.; Coffey, V.G.; Stellingwerff, T.; Phillips, S.M.; Burke, L.M.; Cléroux, M.; Godin, J.-P.; Hawley, J.A. Daytime pattern of post-exercise protein intake affects whole-body protein turnover in resistance-trained males. Nutr. Metab. 2012, 9, 91. [Google Scholar] [CrossRef]
- Hiol, A.N.; von Hurst, P.R.; Conlon, C.A.; Mumme, K.D.; Beck, K.L. Protein intake, distribution, and sources in community-dwelling older adults living in Auckland, New Zealand. Nutr. Healthy Aging 2023, 8, 171–181. [Google Scholar] [CrossRef]
- Johnson, N.R.; Kotarsky, C.J.; Mahoney, S.J.; Sawyer, B.C.; Stone, K.A.; Byun, W.; Hackney, K.J.; Mitchell, S.; Stastny, S.N. Evenness of dietary protein intake is positively associated with lean mass and strength in healthy women. Nutr. Metab. Insights 2022, 15, 11786388221101829. [Google Scholar] [CrossRef]
- Eicher-Miller, H.A.; Khanna, N.; Boushey, C.J.; Gelfand, S.B.; Delp, E.J. Temporal dietary patterns derived among the adult participants of the National Health and Nutrition Examination Survey 1999-2004 are associated with diet quality. J. Acad. Nutr. Diet. 2016, 116, 283–291. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, C.; Gibney, E.R. Meal pattern analysis in nutritional science: Recent methods and findings. Adv. Nutr. 2021, 12, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Distribution but not amount of protein intake is associated with frailty: A cross-sectional investigation in the region of Nürnberg. Nutr. J. 2013, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.H.; Anderson, G.H. Snacking definitions: Impact on interpretation of the literature and dietary recommendations. Crit. Rev. Food Sci. Nutr. 2010, 50, 848–871. [Google Scholar] [CrossRef]
- Kirk, D.; Kok, E.; Tufano, M.; Tekinerdogan, B.; Feskens, E.J.; Camps, G. Machine learning in nutrition research. Adv. Nutr. 2022, 13, 2573–2589. [Google Scholar] [CrossRef]
- Leech, R.M.; Timperio, A.; Livingstone, K.M.; Worsley, A.; McNaughton, S.A. Temporal eating patterns: Associations with nutrient intakes, diet quality, and measures of adiposity. Am. J. Clin. Nutr. 2017, 106, 1121–1130. [Google Scholar] [CrossRef]
- Soh, B.X.P.; Vignes, M.; Smith, N.W.; von Hurst, P.R.; McNabb, W.C. Evaluation of protein intake and protein quality in New Zealand vegans. PLoS ONE 2025, 20, e0314889. [Google Scholar] [CrossRef]
- The New Zealand Institute for Plant and Food Research Limited and the Ministry of Health (New Zealand). New Zealand Food Composition Database. New Zealand FOODfiles™ 2018 Manual. 2018. Available online: https://www.foodcomposition.co.nz/ (accessed on 1 April 2024).
- Moughan, P.J.; Lim, W.X.J. Digestible indispensable amino acid score (DIAAS): 10 years on. Front. Nutr. 2024, 11, 1389719. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation; FAO: Auckland, New Zealand, 2011. [Google Scholar]
- USDA. FoodData Central [Internet]. Available online: https://fdc.nal.usda.gov/download-datasets.html (accessed on 27 November 2023).
- Gilani, S.; Tomé, D.; Moughan, P.; Burlingame, B. Report of a Sub-Committee of the 2011 FAO Consultation on “Protein Quality Evaluation in Human Nutrition”. 2012. Available online: https://www.fao.org/ag/humannutrition/36216-04a2f02ec02eafd4f457dd2c9851b4c45.pdf (accessed on 1 April 2024).
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef]
- Moughan, P.; Gilani, S.; Rutherfurd, S.; Tomé, D. True Ileal Amino Acid Digestibility Coefficients for Application in the Calculation of Digestible Indispensable Amino Acid Score (DIAAS) in Human Nutrition; FAO: Rome, Italy, 2012. [Google Scholar]
- Aghabozorgi, S.; Shirkhorshidi, A.S.; Wah, T.Y. Time-series clustering—A decade review. Inf. Syst. 2015, 53, 16–38. [Google Scholar] [CrossRef]
- Yasuda, J.; Asako, M.; Arimitsu, T.; Fujita, S. Association of protein intake in three meals with muscle mass in healthy young subjects: A cross-sectional study. Nutrients 2019, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, S.E.; Agergaard, J. Evenness of dietary protein distribution is associated with higher muscle mass but not muscle strength or protein turnover in healthy adults: A systematic review. Eur. J. Nutr. 2021, 60, 3185–3202. [Google Scholar] [CrossRef]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Katsanos, C.S.; Zhang, X.-J.; Wolfe, R.R. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp. Gerontol. 2006, 41, 215–219. [Google Scholar] [CrossRef]
- Tormene, P.; Giorgino, T.; Quaglini, S.; Stefanelli, M. Matching incomplete time series with dynamic time warping: An algorithm and an application to post-stroke rehabilitation. Artif. Intell. Med. 2008, 45, 11–34. [Google Scholar] [CrossRef]
- Li, K.; Sward, K.; Deng, H.; Morrison, J.; Habre, R.; Franklin, M.; Chiang, Y.-Y.; Ambite, J.L.; Wilson, J.P.; Eckel, S.P. Using dynamic time warping self-organizing maps to characterize diurnal patterns in environmental exposures. Sci. Rep. 2021, 11, 24052. [Google Scholar] [CrossRef]
- Khanna, N.; Eicher-Miller, H.A.; Boushey, C.J.; Gelfand, S.B.; Delp, E.J. Temporal dietary patterns using kernel k-means clustering. In Proceedings of the 2011 IEEE International Symposium on Multimedia, Dana Point, CA, USA, 5–7 December 2011; pp. 375–380. [Google Scholar]
- Liao, T.W. Clustering of time series data—A survey. Pattern Recognit. 2005, 38, 1857–1874. [Google Scholar] [CrossRef]
- Galili, T. dendextend: An R pacakge for visualizing, adjusting, and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]
- Burney, S.A.; Tariq, H. K-means cluster analysis for image segmentation. Int. J. Comput. Appl. 2014, 96, 1–8. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualise the Results of Multivariate Data Analyses. R Package Version 1.0.7. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 1 April 2024).
- University of Otago and Ministry of Health. A Focus on Nutrition: Key findings of the 2008/2009 New Zealand Adult Nutrition Survey; Ministry of Health: Wellington, New Zealand, 2011.
- NHMRC. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes Version 1.2; NHMRC: Canberra, Australia, 2017.
- Layman, D.K. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr. Metab. 2009, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Arnal, M.-A.; Mosoni, L.; Boirie, Y.; Houlier, M.-L.; Morin, L.; Verdier, E.; Ritz, P.; Antoine, J.-M.; Prugnaud, J.; Beaufrère, B. Protein pulse feeding improves protein retention in elderly women. Am. J. Clin. Nutr. 1999, 69, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Borgonjen-Van den Berg, K.J.; van Loon, L.J.; de Groot, L.C. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: Scope for improvement. Eur. J. Nutr. 2012, 51, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bohé, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose-response study. J. Physiol. 2003, 552, 315–324. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 1–22. [Google Scholar] [CrossRef]
- Wilson, G.J.; Layman, D.K.; Moulton, C.J.; Norton, L.E.; Anthony, T.G.; Proud, C.G.; Rupassara, S.I.; Garlick, P.J. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am. J. Physiol.-Endocrinol. Metab. 2011, 301, E1236–E1242. [Google Scholar] [CrossRef]
- Rolands, M.R.; Hackl, L.S.; Bochud, M.; Lê, K.A. Protein adequacy, plant protein proportion and main plant protein sources consumed across vegan, vegetarian, pesco-vegetarian and semi-vegetarian diets: A systematic review. J. Nutr. 2025, 155, 153–167. [Google Scholar] [CrossRef]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet. 2013, 113, 1610–1619. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based milks: A review of the science underpinning their design, fabrication, and performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.; Crombag, J.J.; Senden, J.M.; Waterval, W.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia: Protein, amino acid metabolism and therapy. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86. [Google Scholar] [CrossRef]
- Giorgino, T. Computing and visualizing dynamic time warping alignments in R: The dtw package. J. Stat. Softw. 2009, 31, 1–24. [Google Scholar] [CrossRef]





| 2.1 | 2.2 | 3.1 | 4.2 | |
|---|---|---|---|---|
| 2.2 | 2.34 | |||
| 3.1 | 5.45 | 5.70 | ||
| 4.2 | 25.0 | 21.9 | 14.0 | |
| 5.3 | 8.07 | 7.99 | 8.44 | 19.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soh, B.X.P.; Vignes, M.; Smith, N.W.; von Hurst, P.R.; McNabb, W.C. Protein Intake and Protein Quality Patterns in New Zealand Vegan Diets: An Observational Analysis Using Dynamic Time Warping. Nutrients 2025, 17, 1806. https://doi.org/10.3390/nu17111806
Soh BXP, Vignes M, Smith NW, von Hurst PR, McNabb WC. Protein Intake and Protein Quality Patterns in New Zealand Vegan Diets: An Observational Analysis Using Dynamic Time Warping. Nutrients. 2025; 17(11):1806. https://doi.org/10.3390/nu17111806
Chicago/Turabian StyleSoh, Bi Xue Patricia, Matthieu Vignes, Nick W. Smith, Pamela R. von Hurst, and Warren C. McNabb. 2025. "Protein Intake and Protein Quality Patterns in New Zealand Vegan Diets: An Observational Analysis Using Dynamic Time Warping" Nutrients 17, no. 11: 1806. https://doi.org/10.3390/nu17111806
APA StyleSoh, B. X. P., Vignes, M., Smith, N. W., von Hurst, P. R., & McNabb, W. C. (2025). Protein Intake and Protein Quality Patterns in New Zealand Vegan Diets: An Observational Analysis Using Dynamic Time Warping. Nutrients, 17(11), 1806. https://doi.org/10.3390/nu17111806







