The Lack of a Glucose Peak During the Oral Glucose Tolerance Test in Pregnancy: What Does It Portend for Perinatal Outcomes?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistics
3. Results
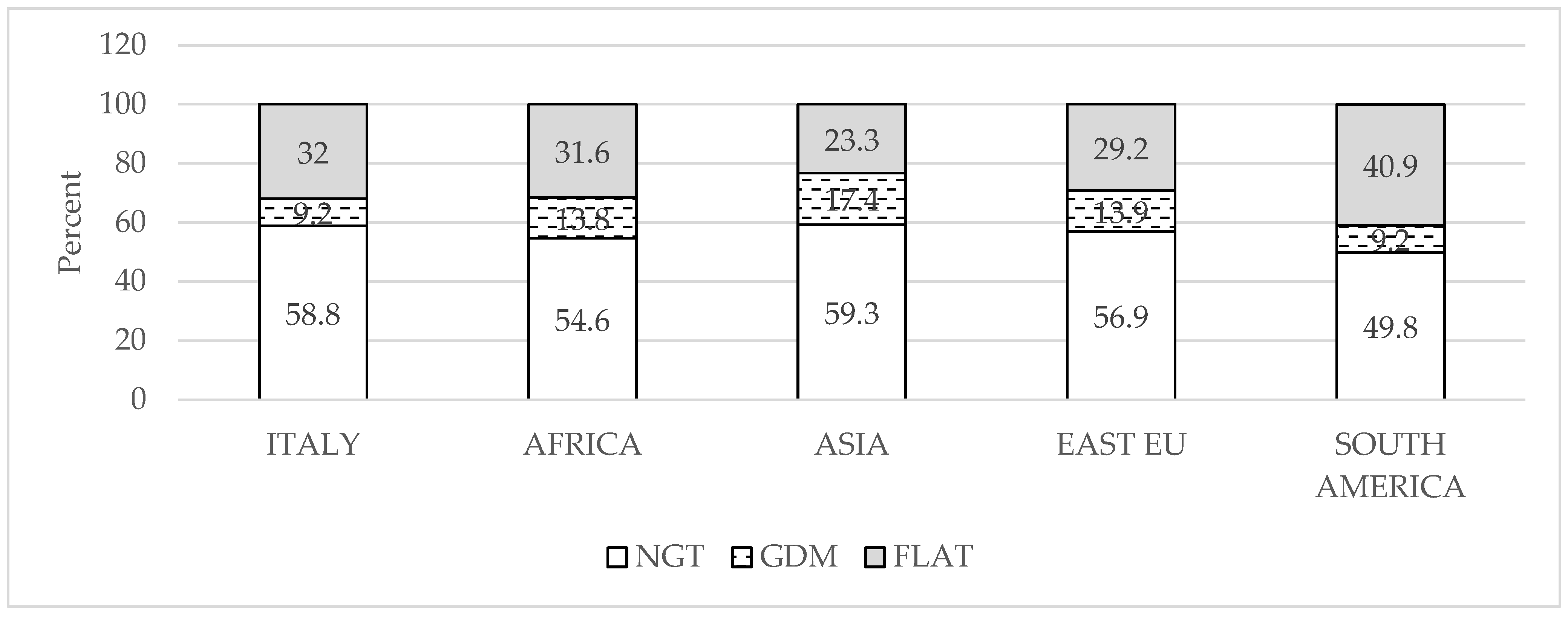
Participant Characteristics
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Assisted Reproduction Technique |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| GA | Gestational Age |
| GDM | Gestational Diabetes Mellitus |
| GWG | Gestational Weight Gain |
| HEI | Healthy Eating Index |
| NGT | Normal Glucose Tolerance |
| OGTT | Oral Glucose Tolerance Test |
| SGA | Small for Gestational Age |
| VD | Vaginal Delivery |
| WHO | World Health Organization |
References
- ACOG. Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef] [PubMed]
- NICE. Guideline Diabetes in Pregnancy: Management from Preconception to the Postnatal Period; National Institute for Health and Care Excellence (NICE): London, UK, 2020. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. 1), S20–S42. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, Switzerland, 2013; pp. 1–63. Available online: http://apps.who.int/iris/bitstream/10665/85975/1/WHO_NMH_MND_13.2_eng.pdf (accessed on 21 May 2025).
- Das Gupta, D.S.; Whitehouse, F.W. Significance of the flat oral glucose tolerance test. Postgrad. Med. 1971, 49, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lepore, M.J. The clinical significance of the low or “flat” oral glucose tolerance curve. Ann. Intern. Med. 1941, 14, 2008–2013. [Google Scholar] [CrossRef]
- Naeh, A.; Wilkof-Segev, R.; Jaffe, A.; Maor-Sagie, E.; Hallak, M.; Gabbay-Benziv, R. Flat Oral Glucose Tolerance Test During Pregnancy: Maternal Characteristics and Risk for Adverse Outcomes. Clin. Diabetes 2021, 39, 313–319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Navon, I.; Romano, A.; Pardo, A.; Matot, R.; Toledano, Y.; Barbash Hazan, S.; Hadar, E. Flat maternal glucose response curve and adverse pregnancy outcome. J. Perinatol. 2023, 43, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.; Stephan, T.; Khurana, K.C.; Morgan, C.R.; Danowski, T.S. Low profile (flat) glucose tolerances. Am. J. Med. Sci. 1972, 264, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Pakhetra, R.; Garg, M.K.; Saini, J.S. Is beta cell dysfunction responsible for flat glucose tolerance curve in primary hypothyroidism? (a hypothesis). Med. J. Armed Forces India 2001, 57, 120–125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szoke, D.; Robbiano, C.; Dolcini, R.; Montefusco, L.; Aiello, G.B.; Caruso, S.; Ottolenghi, A.; Birindelli, S.; Panteghini, M. Incidence and status of insulin secretion in pregnant women with flat plasma glucose profiles during oral glucose tolerance test. Clin. Biochem. 2022, 109–110, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Thaysen, T.H.E.; Norgaard, A. The regulation of blood sugar in idiopathic steatorrhea (sprue and Gee-Herter’s disease): I. The low blood sugar curve. Arch. Intern. Med. 1929, 44, 17–28. [Google Scholar] [CrossRef]
- Brody, H.A.; Prendergast, J.J.; Silverman, S., Jr. The relationship between oral symptoms, insulin release, and glucose intolerance. Oral Surg. Oral Med. Oral Pathol. 1971, 31, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Thaysen, T.H.E. Ten cases of idiopathic steatorrhoea. Q. J. Med. 1935, 4, 359–396. [Google Scholar]
- Langer, O.; Damus, K.; Maiman, M.; Divon, M.; Levy, J.; Bauman, W. A link between relative hypoglycemia-hypoinsulinemia during oral glucose tolerance tests and intrauterine growth retardation. Am. J. Obstet. Gynecol. 1986, 155, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Valensise, H.; Romanini, C. Second-trimester uterine artery flow velocity waveform and oral glucose tolerance test as a means of predicting intrauterine growth retardation. Ultrasound Obstet. Gynecol. 1993, 3, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Abell, D.A.; Beischer, N.A. Evaluation of the three-hour oral glucose tolerance test in detection of significant hyperglycemia and hypoglycemia in pregnancy. Diabetes 1975, 24, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Baht, R.G.; Bhagya, K.V.; Kumar, P. Association of Low Maternal Plasma Glucose after Oral Glucose Challenge Test with Small for Gestational Age Neonate. Int. J. Infertil. Fetal Med. 2012, 3, 22–25. [Google Scholar]
- Bienstock, J.L.; Holcroft, C.J.; Althaus, J. Small fetal abdominal circumference in the second trimester and subsequent low maternal plasma glucose after a glucose challenge test is associated with the delivery of a small-for-gestational age neonate. Ultrasound Obstet. Gynecol. 2008, 31, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Delibas, I.B.; Tanriverdi, S.; Cakmak, B. Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? Ginekol. Pol. 2018, 89, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, J.H.; Magann, E.F.; Morrison, J.C.; Holman, J.R.; Polizzotto, M.J. Does maternal hypoglycemia during screening glucose assessment identify a pregnancy at-risk for adverse perinatal outcome? J. Perinatol. 2005, 25, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Khouzami, V.A.; Ginsburg, D.S.; Daikoku, N.H.; Johnson, J.W. The glucose tolerance test as a means of identifying intrauterine growth retardation. Am. J. Obstet. Gynecol. 1981, 139, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Hay, J.; Liu, G.; Zhang, J.; Wang, J.; Liu, H.; Yang, X.; Liu, J. Small-for-gestational age and its association with maternal blood glucose, body mass index and stature: A perinatal cohort study among Chinese women. BMJ Open 2016, 6, e010984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melamed, N.; Hiersch, L.; Peled, Y.; Hod, M.; Wiznitzer, A.; Yogev, Y. The association between low 50 g glucose challenge test result and fetal growth restriction. J. Matern. Fetal Neonatal Med. 2013, 26, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.U.; Vijay, A.M.A.; Indusekhar, R.; Kalidindi, S.; Katreddy, V.M.; Varadhan, L. Association of hypoglycaemia in screening oral glucose tolerance test in pregnancy with low birth weight fetus. World J. Diabetes 2019, 10, 304–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pugh, S.K.; Doherty, D.A.; Magann, E.F.; Chauhan, S.P.; Hill, J.B.; Morrison, J.C. Does hypoglycemia following a glucose challenge test identify a high risk pregnancy? Reprod. Health 2009, 6, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shinohara, S.; Uchida, Y.; Hirai, M.; Hirata, S.; Suzuki, K. Relationship between maternal hypoglycaemia and small-for-gestational-age infants according to maternal weight status: A retrospective cohort study in two hospitals. BMJ Open 2016, 6, e013749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weissman, A.; Solt, I.; Zloczower, M.; Jakobi, P. Hypoglycemia during the 100-g oral glucose tolerance test: Incidence and perinatal significance. Obstet. Gynecol. 2005, 105, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Yuen, L.; Bontempo, S.; Wong, V.W.; Russell, H. Hypoglycaemia on an oral glucose tolerance test in pregnancy—Is it clinically significant? Diabetes Res. Clin. Pract. 2019, 147, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef] [PubMed]
- The International Fetal and Newborn Growth Consortium for the 21st Century. INTERGROWTH-21st 2009. Available online: https://intergrowth21.tghn.org/ (accessed on 21 May 2025).
- Tarashandegan, D.; Naeh, A.; Hallak, M.; Toledano, Y.; Gabbay-Benziv, R.; Maor-Sagie, E. Flat Oral Glucose Tolerance Test during Pregnancy and Risk for Type 2 Diabetes: A 5-Year Cohort Study. Am. J. Perinatol. [CrossRef] [PubMed]
- Cryer, P.E. Glucose counterregulation: Prevention and correction of hypoglycemia in humans. Am. J. Physiol. 1993, 264 Pt 1, E149–E155. [Google Scholar] [CrossRef] [PubMed]
- Lopian, M.; Segal, E.; Neiger, R.; Many, A.; Kashani Ligumsky, L. The Implications of a “Flat” Oral Glucose Tolerance Test Curve in Pregnancy. Am. J. Perinatol. 2025, 42, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Phelps, R.L.; Metzger, B.E.; Freinkel, N. Carbohydrate metabolism in pregnancy. XVII. Diurnal profiles of plasma glucose, insulin, free fatty acids, triglycerides, cholesterol, and individual amino acids in late normal pregnancy. Am. J. Obstet. Gynecol. 1981, 140, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Roman-Drago, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy. Am. J. Obstet. Gynecol. 1998, 179, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, F.C.; Meschia, G. An Introduction to Fetal Physiology; Academic Press INC: Cambridge, MA, USA, 1986; 257p. [Google Scholar]
| NGT N = 5066 | GDM N = 953 | FLAT N = 2791 | NGT vs. GDM | NGT vs. FLAT | FLAT vs. GDM | |
|---|---|---|---|---|---|---|
| G T0, mg/dL | 77.8 ± 6.6 | 88.4 ± 10.4 | 77.5 ± 6.1 | 0.001 | 0.04 | 0.001 |
| G T60, mg/dL | 132.7 ± 18.3 | 166.0 ± 32.7 | 92.0 ± 13.4 | 0.001 | 0.001 | 0.001 |
| G T120, mg/dL | 107.4 ± 19.6 | 143.7 ± 40.7 | 90.4 ± 17.0 | 0.001 | 0.001 | 0.001 |
| G T60–T0, mg/dL | 55.0 ± 17.1 | 77.5 ± 35.8 | 14.5 ± 11.9 | 0.001 | 0.001 | 0.001 |
| G AUC mg/dL | 317.9 ± 35.1 | 398.1 ± 56.6 | 259.8 ± 27.9 | 0.001 | 0.001 | 0.001 |
| Age, years | 32.1 ± 5.5 | 33.0 ± 5.6 | 30.9 ± 5.8 | 0.001 | 0.001 | 0.001 |
| Age ≥ 35 years (%) | 1807 (35.7) | 397 (41.7) | 795 (28.5) | 0.001 | 0.001 | 0.001 |
| Height, cm | 163.1 ± 6.6 | 162.3 ± 6.5 | 163.7 ± 6.5 | 0.002 | 0.001 | 0.001 |
| Weight, kg | 62.8 ± 12.6 | 65.6 ± 13.3 | 61.5 ± 12.1 | 0.001 | 0.001 | 0.001 |
| GWG, kg | 12.4 ± 4.6 | 10.3 ± 5.1 | 12.7 ± 4.5 | 0.001 | 0.02 | 0.001 |
| BMI, kg/m2 | 23.6 ± 4.5 | 24.9 ± 5.0 | 23.0 ± 4.3 | 0.001 | 0.001 | 0.001 |
| Underweight, (%) | 363 (7.4) | 51 (5.6) | 255 (9.5) | 0.05 | 0.002 | 0.001 |
| Obese (%) | 460 (9.3) | 140 (15.3) | 202 (7.5) | 0.001 | 0.01 | 0.001 |
| Gravida 1, (%) | 1818 (35.9) | 308 (32.3) | 980 (35.1) | 0.04 | 0.5 | 0.1 |
| Immigrants (%) non Italian | 1725 (34.1) | 430 (45.1) | 966 (34.6) | 0.001 | 0.6 | 0.001 |
| Tobacco use (%) | 393 (11.2) | 65 (10.8) | 216 (10.9) | 0.8 | 0.8 | 1.0 |
| ART (%) | 220 (4.3) | 46 (4.8) | 77 (2.8) | 0.5 | 0.001 | 0.003 |
| NGT N = 5066 | GDM N = 953 | FLAT N = 2791 | NGT vs. GDM | NGT vs. FLAT | FLAT vs. GDM | |
|---|---|---|---|---|---|---|
| GA at delivery, weeks | 39.5 ± 1.1 | 39.0 ± 0.9 | 39.6 ± 1.1 | 0.001 | 0.04 | 0.001 |
| Hypothyroidism (%) | 510 (10.1) | 111 (11.6) | 254 (9.1) | 0.3 | 0.2 | 0.03 |
| Hypertension (%) | 239 (4.7) | 57 (6.0) | 101 (3.6) | 0.1 | 0.02 | 0.003 |
| Induction of labor (%) | 1401 (27.7) | 402 (42.2) | 751 (26.9) | 0.001 | 0.5 | 0.001 |
| Vaginal delivery (%) | 3939 (77.8) | 696 (73) | 2244 (80.4) | 0.002 | 0.01 | 0.001 |
| Primary cesarean (%) | 654 (14.8) | 146 (18.2) | 290 (11.9) | 0.01 | 0.001 | 0.001 |
| Blood loss at VD, mL | 373 ± 378 | 351 ± 291 | 354 ± 322 | 0.1 | 0.02 | 0.9 |
| >500 mL (%) | 19.4 | 16.3 | 17.1 | 0.06 | 0.03 | 0.6 |
| Newborn weight, g | 3327 ± 419 | 3269 ± 411 | 3298 ± 410 | 0.001 | 0.003 | 0.06 |
| >90° centile (%) | 506 (10) | 96 (10.1) | 224 (8.0) | 0.1 | 0.01 | 0.06 |
| <10° centile (%) | 312 (6.2) | 59 (6.2) | 193 (6.9) | 0.9 | 0.2 | 0.5 |
| Newborn length, cm | 50.3 ± 1.9 | 50.0 ± 1.8 | 50.2 ± 1.9 | 0.001 | 0.09 | 0.005 |
| Newborn Ponderal Index, g/l3 | 2.6 ± 0.3 | 2.6 ± 0.2 | 2.6 ± 0.3 | 0.1 | 0.08 | 0.7 |
| Apgar at 5′ | 9.9 ± 0.4 | 9.9 ± 0.4 | 9.9 ± 0.4 | 0.9 | 0.2 | 0.5 |
| Umb artery pH | 7.264 ± 0.09 | 7.267 ± 0.09 | 7.266 ± 0.09 | 0.4 | 0.4 | 0.8 |
| Umb artery BD, mM | −4.9 ± 3.5 | −4.6 ± 3.4 | −4.8 ± 3.4 | 0.03 | 0.4 | 0.1 |
| Variable | OR [95% CI] | p |
|---|---|---|
| Predictors of vaginal delivery | ||
| Flat curve | 1.13 [1.01–1.26] | 0.034 |
| Maternal age, years | 0.97 [0.96–0.98] | 0.001 |
| Maternal body mass index, kg/m2 | 0.94 [0.93–0.95] | 0.001 |
| Birthweight, kg | 1.0005 [1.0003–1.0006] | 0.001 |
| Predictors of primary cesarean | ||
| Flat OGTT | 0.83 [0.72–0.96] | 0.016 |
| Maternal age, years | 1.04 [1.03–1.05] | 0.001 |
| Maternal body mass index, kg/m2 | 1.02 [1.01–1.03] | 0.001 |
| Birthweight, kg | 0.9995 [0.9994–0.9997] | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marconi, A.M.; Alfieri, N.; Garzia, E.; Terzoni, S.; Manodoro, S.; Catalano, P.M. The Lack of a Glucose Peak During the Oral Glucose Tolerance Test in Pregnancy: What Does It Portend for Perinatal Outcomes? Nutrients 2025, 17, 1785. https://doi.org/10.3390/nu17111785
Marconi AM, Alfieri N, Garzia E, Terzoni S, Manodoro S, Catalano PM. The Lack of a Glucose Peak During the Oral Glucose Tolerance Test in Pregnancy: What Does It Portend for Perinatal Outcomes? Nutrients. 2025; 17(11):1785. https://doi.org/10.3390/nu17111785
Chicago/Turabian StyleMarconi, Anna Maria, Nikita Alfieri, Emanuele Garzia, Stefano Terzoni, Stefano Manodoro, and Patrick M. Catalano. 2025. "The Lack of a Glucose Peak During the Oral Glucose Tolerance Test in Pregnancy: What Does It Portend for Perinatal Outcomes?" Nutrients 17, no. 11: 1785. https://doi.org/10.3390/nu17111785
APA StyleMarconi, A. M., Alfieri, N., Garzia, E., Terzoni, S., Manodoro, S., & Catalano, P. M. (2025). The Lack of a Glucose Peak During the Oral Glucose Tolerance Test in Pregnancy: What Does It Portend for Perinatal Outcomes? Nutrients, 17(11), 1785. https://doi.org/10.3390/nu17111785