Common Peroneal Nerve Paralysis Following Rapid Weight Loss—A Case Report and Literature Review
Abstract
1. Introduction
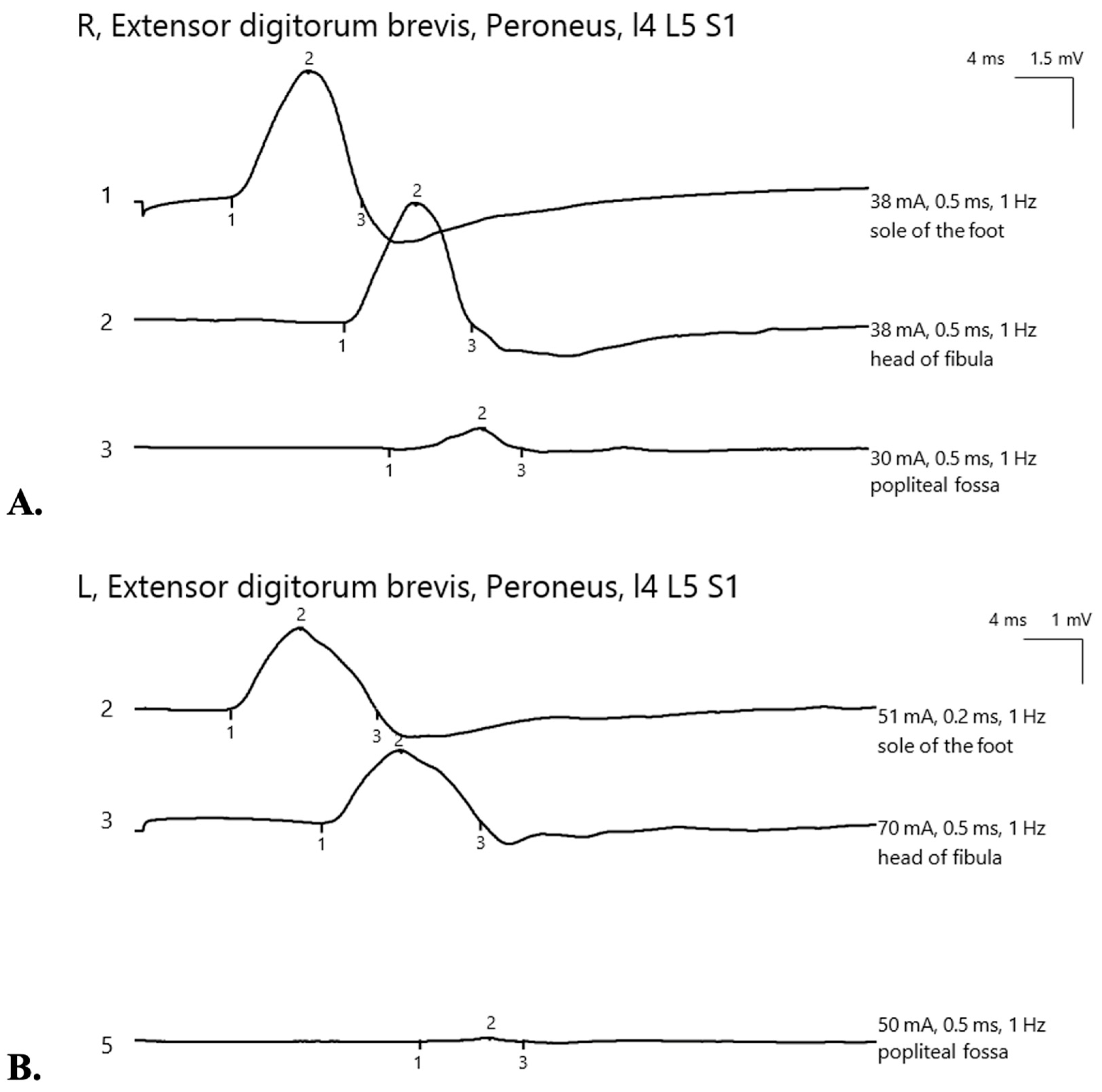
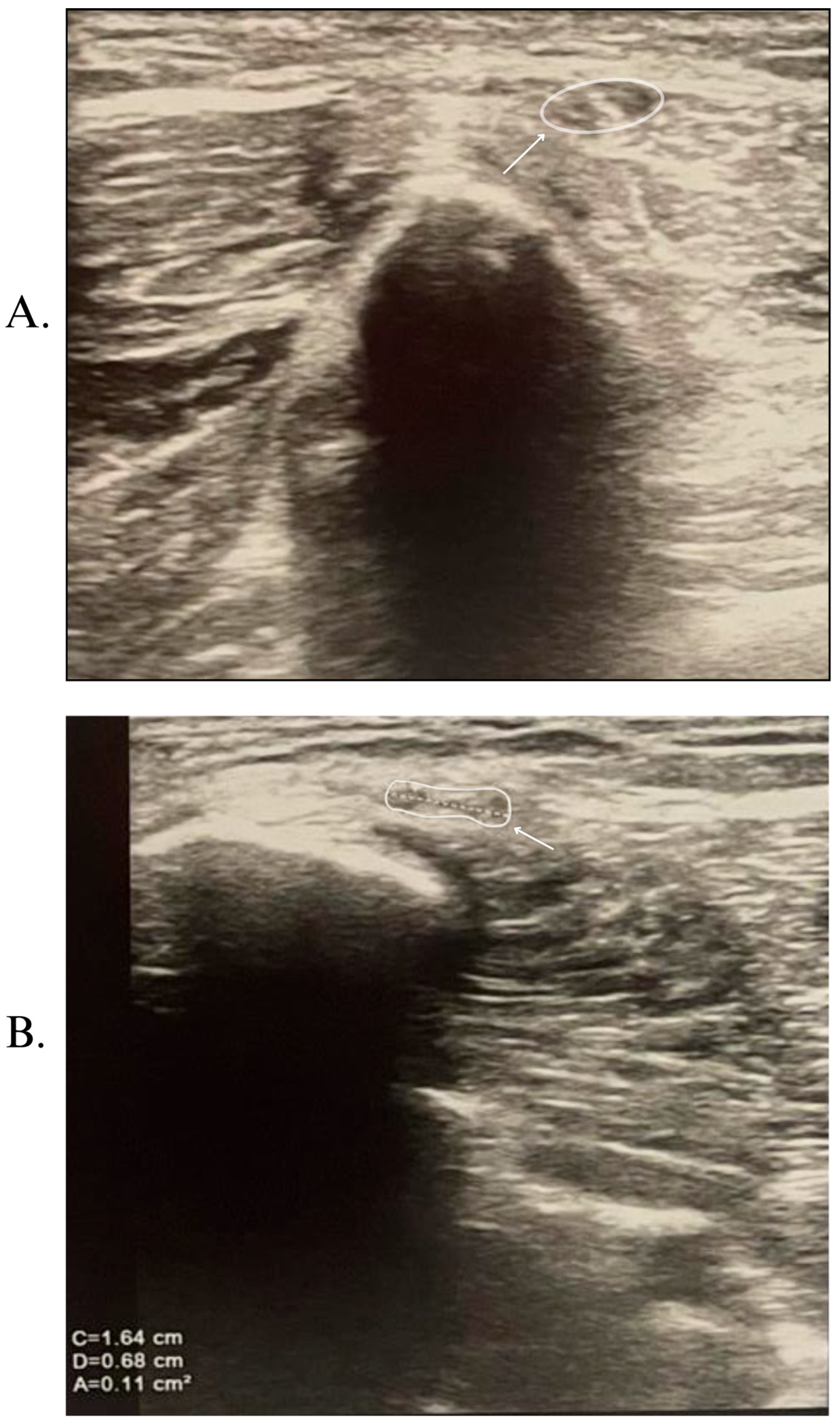
2. Case Report
3. Literature Review
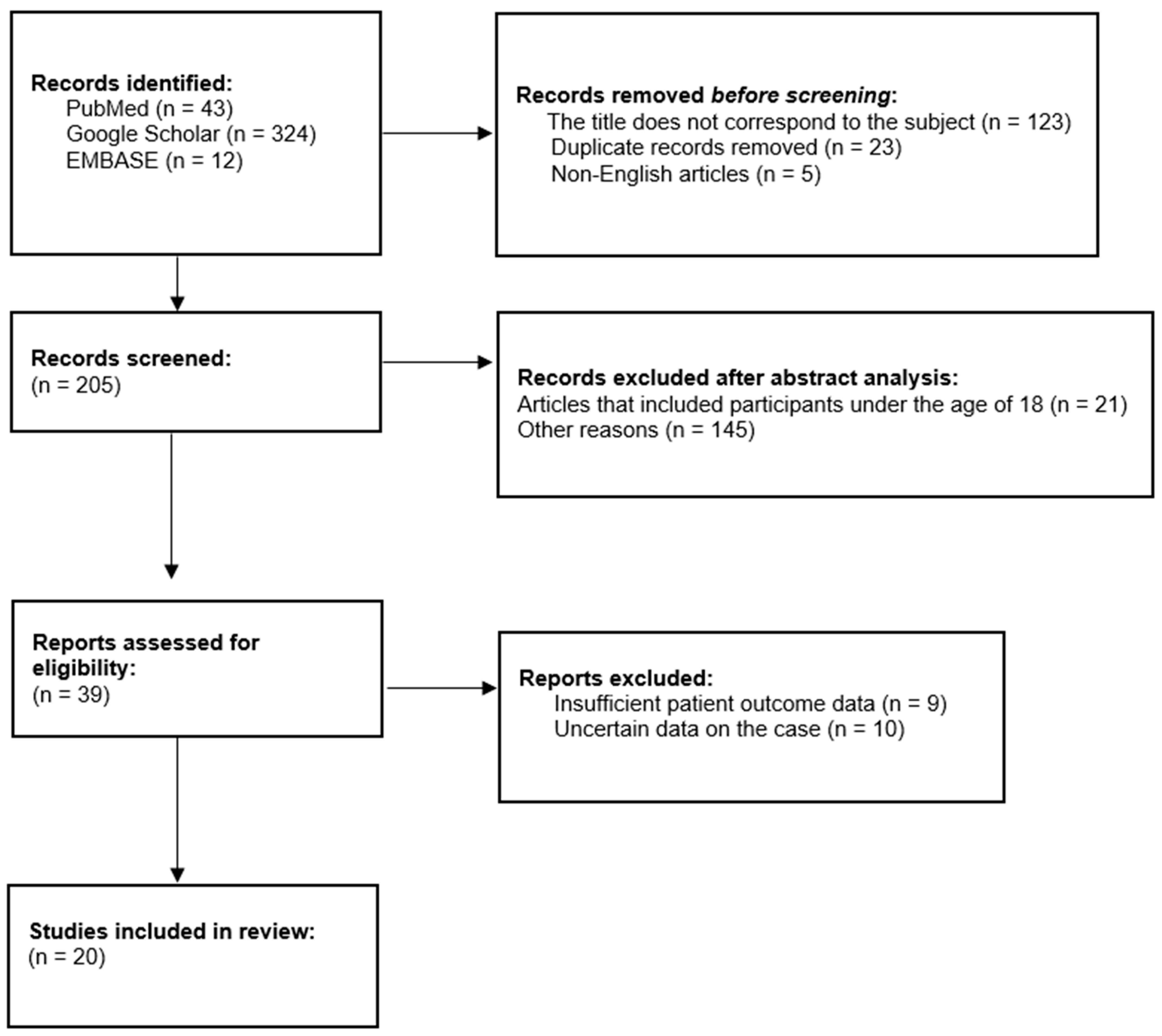
3.1. Materials and Methods
3.2. Results
4. Discussions
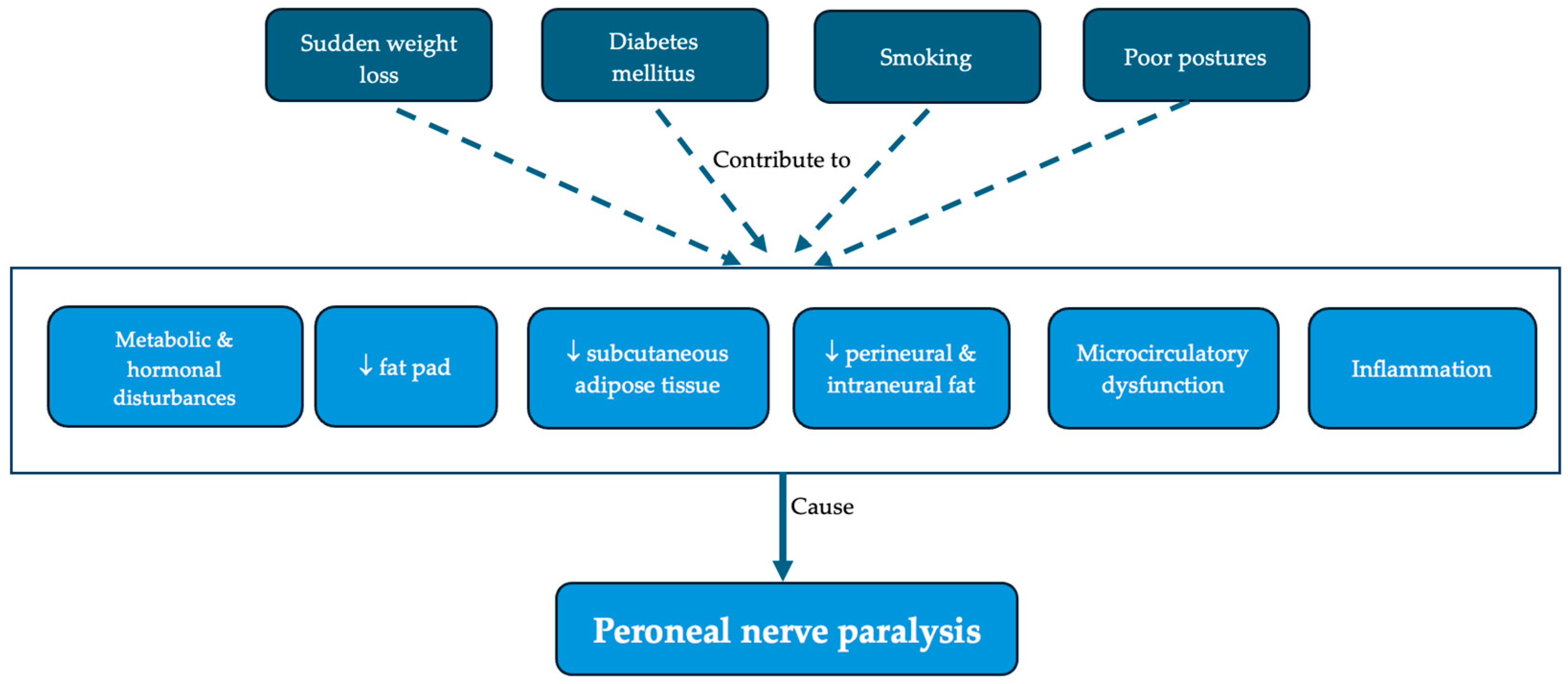
4.1. Risk Factors
4.2. Proposed Pathophysiological Mechanisms
4.3. Clinical Manifestation
4.4. Investigations
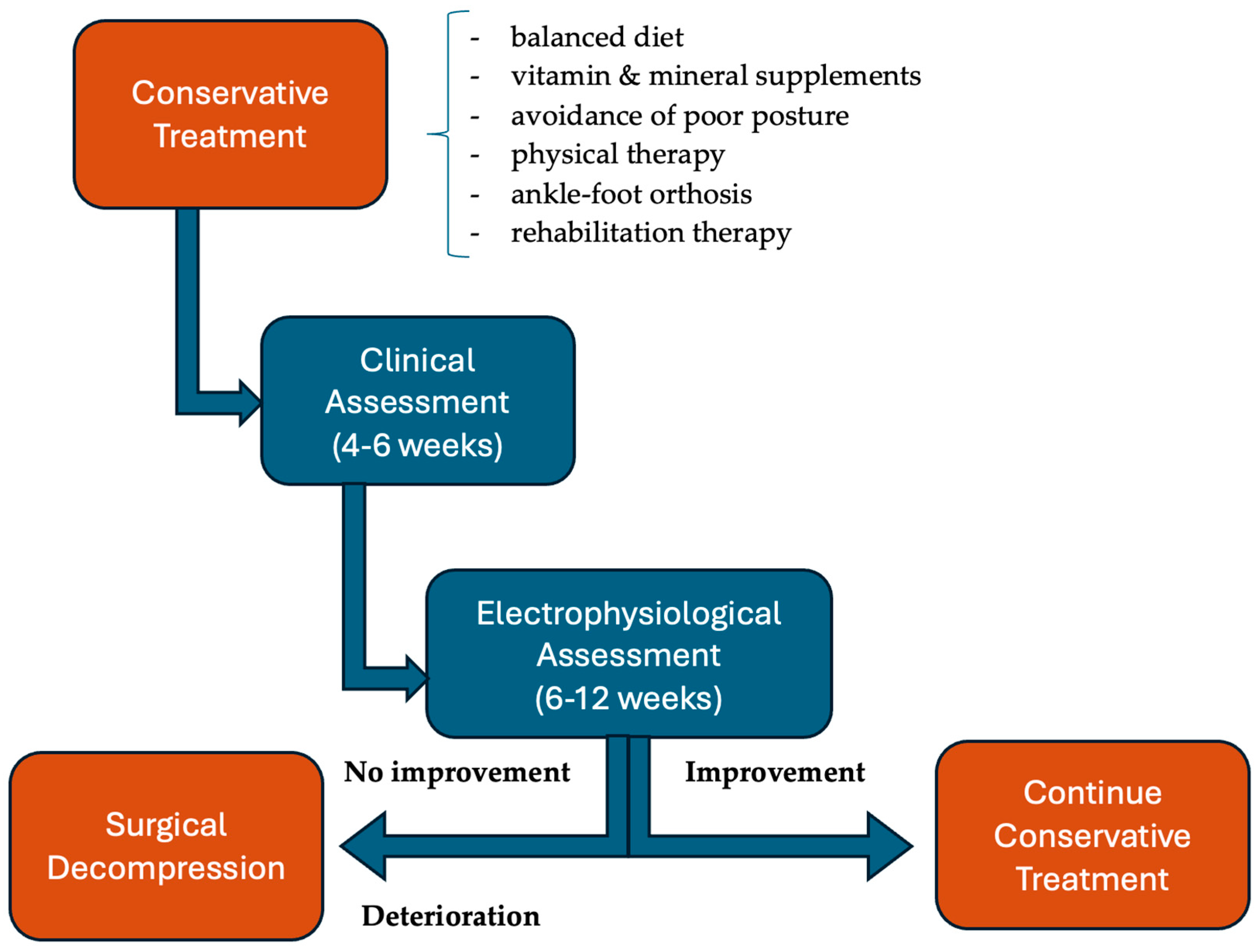
4.5. Treatment
4.6. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lostein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Federation, World Obesity Atlas 2023. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023 (accessed on 23 September 2024).
- Blüher, M.; Aras, M.; Aronne, L.J.; Batterham, R.L.; Giorgino, F.; Ji, L.; Pietiläinen, K.H.; Schnell, O.; Tonchevska, E.; Wilding, J.P.H. New insights into the treatment of obesity. Diabetes Obes. Metab. 2023, 25, 2058–2072. [Google Scholar] [CrossRef] [PubMed]
- Craig, H.C.; Alsaeed, D.; Norris, S.; Holian, J.; Kennedy, C.; Feldman, A.; Le Roux, C. Patient perspectives about treatment preferences for obesity with complications. Obes. Sci. Pract. 2023, 29, e720. [Google Scholar] [CrossRef]
- Denny-Brown, D. Neurological conditions resulting from prolonged and severe dietary restriction. Medicine 1947, 26, 41–113. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Al-Mulhim, A.S. Laparoscopic sleeve gastrectomy and nutrient deficiencies: A prospective study. Surg. Laparosc. Endosc. Percutan Tech. 2016, 26, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Kochkodan, J.; Telem, D.A.; Ghaferi, A.A. Physiologic and psychological gender diferences in bariatric surgery. Surg. Endosc. 2018, 32, 1382–1388. [Google Scholar] [CrossRef]
- Anderson, C.; Peterson, C.B.; Fletcher, L.; Mitchell, J.E.; Thuras, P.; Crow, S.J. Weight loss and gender: An examination of physician attitudes. Obes. Res. 2001, 9, 257–263. [Google Scholar] [CrossRef]
- Woltman, H.W. Crossing the legs as a factor in the production of peroneal palsy. JAMA 1929, 93, 670–672. [Google Scholar] [CrossRef]
- Kaminsky, F. Peroneal palsy by crossing the legs. JAMA 1947, 134, 206. [Google Scholar] [CrossRef]
- Shields, L.B.E.; Iyer, V.G.; Shields, C.B.; Zhang, Y.P.; Rao, A.J. Varied Presentation and Importance of MR Neurography of the Common Fibular Nerve in Slimmer’s Paralysis. Case Rep. Neurol. 2021, 13, 555–564. [Google Scholar] [CrossRef]
- Baima, J.; Krivickas, L. Evaluation and treatment of peroneal neuropathy. Curr. Rev. Musculoskelet. Med. 2008, 1, 147–153. [Google Scholar] [CrossRef]
- Fortier, L.M.; Markel, M.; Thomas, B.G.; Sherman, W.F.; Thomas, B.H.; Kaye, A.D. An Update on Peroneal Nerve Entrapment and Neuropathy. Orthop. Rev. 2021, 13, 24937. [Google Scholar] [CrossRef]
- Dwivedi, N.; Paulson, A.E.; Johnson, J.E.; Dy, C.J. Surgical Treatment of Foot Drop: Patient Evaluation and Peripheral Nerve Treatment Options. Orthop. Clin. North Am. 2022, 53, 223–234. [Google Scholar] [CrossRef]
- Medical Research Council. Aids to the Examination of the Peripheral Nervous System 1975; Pengragon House: London, UK, 1975. [Google Scholar]
- Hallett, M. NINDS myotatic reflex scale. Neurology 1993, 43, 2723. [Google Scholar] [CrossRef]
- Elias, W.J.; Pouratian, N.; Oskouian, R.J.; Schirmer, B.; Burns, T. Peroneal neuropathy following successful bariatric surgery. Case report and review of the literature. J. Neurosurg. 2006, 105, 631–635. [Google Scholar] [CrossRef]
- Şen, O.; Karaca, F.C.; Türkçapar, A. Neurological Complication After Laparoscopic Sleeve Gastrectomy: Foot Drop. Obes. Surg. 2020, 30, 957–960. [Google Scholar] [CrossRef]
- Pennington, N. Slimmer’s Paralysis: A Case Study of Peroneal Neuropathy Following Rapid Weight Loss. J. Nurse Pract. 2024, 20, 105117. [Google Scholar] [CrossRef]
- Tucker, J.M.; Ritchie, J. The Tirzepatide Drop: Beware of Slimmer’s Paralysis. J. Investig. Med. High Impact Case Rep. 2024, 12, 23247096241264635. [Google Scholar] [CrossRef]
- Baharin, J.; Yusof Khan, A.H.K.; Abdul Rashid, A.M.; Loh, W.C.; Ibrahim, A.; Inche Mat, L.N.; Wan Sulaiman, W.A.; Hoo, F.K.; Basri, H. Slimmer’s palsy following an intermittent fasting diet. Egypt. J. Neurol. Psychiat. Neurosurg. 2022, 58, 157. [Google Scholar] [CrossRef]
- Özişler, Z.; Akyüz, M.; Yalçın, E. Bilateral peroneal neuropathy after bariatric surgery: A case report. Turk. J. Phys. Med. Rehabil. 2017, 63, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.P. Developed foot drop as a complication of bariatric surgery: Case reports. Einstein 2010, 8, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.W.; Gu, M.S.; Kong, H.H. Bilateral common peroneal neuropathy due to rapid and marked weight loss after biliary surgery: A case report. World J. Clin. Cases 2021, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martinez, A.; Arpa, J.; Palau, F. Peroneal neuropathy after weight loss. J. Peripher. Nerv. Syst. 2000, 5, 101–105. [Google Scholar] [CrossRef]
- Lale, A.; Kirkil, C.; Ozturk, S.; Yur, M.; Can, O.F.; Artaş, G.; Aygen, E. The results of surgical decompression in the treatment of foot drop due to peroneal nerve entrapment after bariatric surgery. Surg. Obes. Relat. Dis. 2020, 16, 1684–1691. [Google Scholar] [CrossRef]
- Meylaerts, L.; Cardinaels, E.; Vandevenne, J.; Velghe, B.; Gelin, G.; Vanormelingen, L.; Weyns, F. Peroneal neuropathy after weight loss: A high-resolution ultrasonographic characterization of the common peroneal nerve. Skelet. Radiol. 2011, 40, 1557–1562. [Google Scholar] [CrossRef]
- Tabbara, M.; Carandina, S.; Bossi, M.; Polliand, C.; Genser, L.; Barrat, C. Rare Neurological Complications After Sleeve Gastrectomy. Obes. Surg. 2016, 26, 2843–2848. [Google Scholar] [CrossRef]
- Aguirre, M.S.; Gibson, J.; Lozowska, D. Bilateral foot drop linked to rapid intentional weight loss and long distance walking. Marshall J. Med. 2019, 5, 27. [Google Scholar] [CrossRef]
- Broekx, S.; Weyns, F. External neurolysis as a treatment for foot drop secondary to weight loss: A retrospective analysis of 200 cases. Acta Neurochir. 2018, 160, 1847–1856. [Google Scholar] [CrossRef]
- Papagianni, A.; Oulis, P.; Zambelis, T.; Kokotis, P.; Koulouris, G.C.; Karandreas, N. Clinical and neurophysiological study of peroneal nerve mononeuropathy after substantial weight loss in patients suffering from major depressive and schizophrenic disorder: Suggestions on patients’ management. J. Brachial Plex. Peripher. Nerve Inj. 2008, 3, e69–e74. [Google Scholar] [CrossRef][Green Version]
- Margulis, M.; Ben Zvi, L.; Bernfeld, B. Bilateral Common Peroneal Nerve Entrapment After Excessive Weight Loss: Case Report and Review of the Literature. J. Foot Ankle Surg. 2018, 57, 632–634. [Google Scholar] [CrossRef]
- Ramos-Leví, A.M.; Matías-Guiu, J.A.; Guerrero, A.; Sánchez-Pernaute, A.; Rubio, M.A. Peroneal palsy after bariatric surgery; is nerve decompression always necessary? Nutr. Hosp. 2013, 28, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Sarıtosun, D.; Çiftci İnceoğlu, S.; Kuran, B. Foot Drop Following Bariatric Surgery: A Case Report. J. Phys. Med. Rehabil. Sci. 2025, 29, 191–194. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kathiravel, Y.; Khullar, S. Unilateral peroneal neuropathy in a patient following laparoscopic sleeve gastrectomy. Postgrad. Med. 2024, 136, 782–787. [Google Scholar] [CrossRef]
- Weyns, F.J.; Beckers, F.; Vanormelingen, L.; Vandersteen, M.; Niville, E. Foot drop as a complication of weight loss after bariatric surgery: Is. it preventable? Obes. Surg. 2007, 17, 1209–1212. [Google Scholar] [CrossRef]
- Algahtani, H.A.; Khan, A.S.; Khan, M.A.; Aldarmahi, A.A.; Lodhi, Y. Neurological complications of bariatric surgery. Neurosciences 2016, 21, 241–245. [Google Scholar] [CrossRef]
- Thaisetthawatkul, P.; Collazo-Clavell, M.L.; Sarr, M.G.; Norell, J.E.; Dyck, P.J. A controlled study of peripheral neuropathy after bariatric surgery. Neurology 2004, 63, 1462–1470. [Google Scholar] [CrossRef]
- Becker, D.A.; Balcer, L.J.; Galetta, S.L. The Neurological Complications of Nutritional Deficiency following Bariatric Surgery. J. Obes. 2012, 2012, 608534. [Google Scholar] [CrossRef]
- Koffman, B.M.; Greenfield, L.J.; Ali, I.I.; Pirzada, N.A. Neurologic complications after surgery for obesity. Muscle Nerve 2006, 33, 166–176. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Alves-Leon, S.V.; Anacleto, A.C.; Brooks, J.B.; Gama, P.D.; Gomes, S.; Gonçalves, M.V.; Lin, K.; Lopes, J.; Kaimen-Maciel, D.R.; et al. Neurological complications following bariatric surgery. Arq. Neuropsiquiatr. 2012, 70, 700–703. [Google Scholar] [CrossRef]
- Singh, S.; Nautiyal, A.; Presutti, R.J. Neurologic Complications of Bariatric Surgery/In reply. Mayo Clin. Proc. 2005, 80, 136–137. [Google Scholar] [CrossRef]
- van der Velde, J.H.P.M.; Koster, A.; Strotmeyer, E.S.; Mess, W.H.; Hilkman, D.; Reulen, J.P.H.; Stehouwer, C.D.A.; Henry, R.M.A.; Schram, M.T.; van der Kallen, C.J.H.; et al. Cardiometabolic risk factors as determinants of peripheral nerve function: The Maastricht Study. Diabetologia 2020, 63, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Poage, C.; Roth, C.; Scott, B. Peroneal Nerve Palsy: Evaluation and Management. J. Am. Acad. Orthop. Surg. 2016, 24, 1–10. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: Cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Endocr. Pract. 2013, 19, 337–372. [Google Scholar] [CrossRef]
- Moulla, Y.; Lyros, O.; Mehdorn, M.; Lange, U.; Hamade, H.; Thieme, R.; Hoffmeister, A.; Feisthammel, J.; Blüher, M.; Jansen-Winkeln, B.; et al. Preoperative Upper-GI Endoscopy Prior to Bariatric Surgery: Essential or Optional? Obes. Surg. 2020, 30, 2076–2084. [Google Scholar] [CrossRef]
- Wolter, S.; Duprée, A.; Miro, J.; Schroeder, C.; Jansen, M.I.; Schulze-Zur-Wiesch, C.; Groth, S.; Izbicki, J.; Mann, O.; Busch, P. Upper Gastrointestinal Endoscopy prior to Bariatric Surgery-Mandatory or Expendable? An Analysis of 801 Cases. Obes. Surg. 2017, 27, 1938–1943. [Google Scholar] [CrossRef]
- Davrieux, C.F.; Palermo, M.; Nedelcu, M.; Nocca, D. Reflux After Sleeve Gastrectomy: An Update. J. Laparoendosc. Adv. Surg. Tech. 2021, 31, 978–982. [Google Scholar] [CrossRef]
- Handelsman, Y.; Bloomgarden, Z.T.; Grunberger, G.; Umpierrez, G.; Zimmerman, R.S.; Bailey, T.S.; Blonde, L.; Bray, G.A.; Cohen, A.J.; Dagogo-Jack, S.; et al. American association of clinical endocrinologists and american college of endocrinology–clinical practice guidelines for developing a diabetes mellitus comprehensive care plan—2015. Endocr. Pract. 2015, 21 (Suppl. S1), 1–87. [Google Scholar] [CrossRef]
- Fabricatore, A.N.; Crerand, C.E.; Wadden, T.A.; Sarwer, D.B.; Krasucki, J.L. How do mental health professionals evaluate candidates for bariatric surgery? Survey results. Obes. Surg. 2006, 16, 567–573. [Google Scholar] [CrossRef]
- Schlottmann, F.; Nayyar, A.; Herbella, F.A.M.; Patti, M.G. Preoperative Evaluation in Bariatric Surgery. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 925–929. [Google Scholar] [CrossRef]
- Gunnarson, G.L.; Frøyen, J.K.; Sandbu, R.; Thomsen, J.B.; Hjelmesæth, J. Plastic surgery after bariatric surgery. Tidsskr. Den Nor. Legeforening 2015, 135, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- de Moura-Grec, P.G.; Yamashita, J.M.; Marsicano, J.A.; Ceneviva, R.; de Souza Leite, C.V.; de Brito, G.B.; Brienze, S.L.; de Carvalho Sales-Peres, S.H. Impact of bariatric surgery on oral health conditions: 6-months cohort study. Int. Dent. J. 2014, 64, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, A.E.; Veyrune, J.L.; Nicolas, E.; Ciangura, C.A.; Chaussain, C.C.; Czernichow, S.; Basdevant, A.; Hennequin, M. Effect of dental status on changes in mastication in patients with obesity following bariatric surgery. PLoS ONE 2011, 6, e22324. [Google Scholar] [CrossRef]
- El Soury, M.; Fornasari, B.E.; Carta, G.; Zen, F.; Haastert-Talini, K.; Ronchi, G. The Role of Dietary Nutrients in Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2021, 22, 7417. [Google Scholar] [CrossRef]
- Thaisetthawatkul, P.; Collazo-Clavell, M.L.; Sarr, M.G.; Norell, J.E.; Dyck, P.J. Good nutritional control may prevent polyneuropathy after bariatric surgery. Muscle Nerve 2010, 42, 709–714. [Google Scholar] [CrossRef]

| Parameter | Pre-Treatment Value | 4-Month Follow-Up Value | Reference Range |
|---|---|---|---|
| WBC | 6.78 × 103/mm3 | 4.82 × 103/mm3 | 4.00–10.00 × 103/mm3 |
| RBC | 3.67 × 103/mm3 | 3.79 × 103/mm3 | 3.80–5.80 × 103/mm3 |
| Hb | 11.9 g/dL | 12.1 g/dL | 12.0–16.0 g/dL |
| Ht | 37.0% | 37.6% | 36.0–48.0% |
| MCV | 100.8 fL | 99.1 fL | 78–100 fL |
| MCH | 32.5 pg | 32.0 pg | 25.0–32.0 pg |
| MCHC | 32.2 g/dL | 32.2 g/dL | 32.0–35.0 g/dL |
| RDW-CV | 12.2% | 11.8% | 12–18% |
| PLT | 334 × 103/mm3 | 284 × 103/mm3 | 150–400 × 103/mm3 |
| VSH | 11 mm/h | 9 mm/h | 0–20 mm/h |
| CRP | 0.01 mg/dL | 0.28 mg/dL | 0–1 mg/dL |
| Sodium | 139.10 mmol/L | 142.29 mmol/L | 135–145 mmol/L |
| Potassium | 3.83 mmol/L | 4.43 mmol/L | 3.5–5 mmol/L |
| Magnesium | 2.01 mg/dL | 1.91 mg/dL | 1.6–2.6 mg/dL |
| Calcium (Total) | 9.64 mg/dL | 8.76 mg/dL | 8.4–10.2 mg/dL |
| Ionized Calcium | 4.96 mg/dL | 4.01 mg/dL | 3.82–4.82 mg/dL |
| Iron | 111.2 μg/dL | - | 50–170 μg/dL |
| Glucose | 100.8 mg/dL | 76.38 mg/dL | 70–105 mg/dL |
| A1c% | 4.71% | - | 4–6% |
| TSH | 0.537 μIU/mL | - | 0.55–4.78 μIU/mL |
| fT4 | 1.24 ng/dL | - | 0.89–1.76 ng/dL |
| fT3 | 3.03 pg/mL | - | 2.3–4.2 pg/mL |
| Folate | 8.01 ng/dL | - | >5.38 ng/dL |
| Vitamin B12 | 386 pg/mL | - | 211–911 pg/mL |
| 25-OH-Vitamin D | 20.9 ng/dL | - | 20–50 ng/dL |
| Commonly identified risk factors | Diabetes [11,17,20,31,34,36,43] |
| Smoking [11,19,34,35,36,43] | |
| Maintaining poor postures (leg crossing, prolonged sitting, squatting) [11,19,20,21,22,25,29,31,33,34] | |
| Immobility [11,24,30,31] | |
| Controversial risk factors | Level and rapidity of weight reduction [18,19,21,22,27,28,36] |
| Risk factors requiring further research | Alcohol consumption [31] |
| Polyneuropathy [17] | |
| Minor knee trauma [35] | |
| Excessive walking [29] | |
| Effort [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucu, L.-E.; Popescu, G.; Maștaleru, A.; Ignat, E.B.; Grosu, C.; Bîrsanu, L.; Leon, M.M. Common Peroneal Nerve Paralysis Following Rapid Weight Loss—A Case Report and Literature Review. Nutrients 2025, 17, 1782. https://doi.org/10.3390/nu17111782
Cucu L-E, Popescu G, Maștaleru A, Ignat EB, Grosu C, Bîrsanu L, Leon MM. Common Peroneal Nerve Paralysis Following Rapid Weight Loss—A Case Report and Literature Review. Nutrients. 2025; 17(11):1782. https://doi.org/10.3390/nu17111782
Chicago/Turabian StyleCucu, Laura-Elena, Gabriela Popescu, Alexandra Maștaleru, Emilian Bogdan Ignat, Cristina Grosu, Lenuța Bîrsanu, and Maria Magdalena Leon. 2025. "Common Peroneal Nerve Paralysis Following Rapid Weight Loss—A Case Report and Literature Review" Nutrients 17, no. 11: 1782. https://doi.org/10.3390/nu17111782
APA StyleCucu, L.-E., Popescu, G., Maștaleru, A., Ignat, E. B., Grosu, C., Bîrsanu, L., & Leon, M. M. (2025). Common Peroneal Nerve Paralysis Following Rapid Weight Loss—A Case Report and Literature Review. Nutrients, 17(11), 1782. https://doi.org/10.3390/nu17111782







