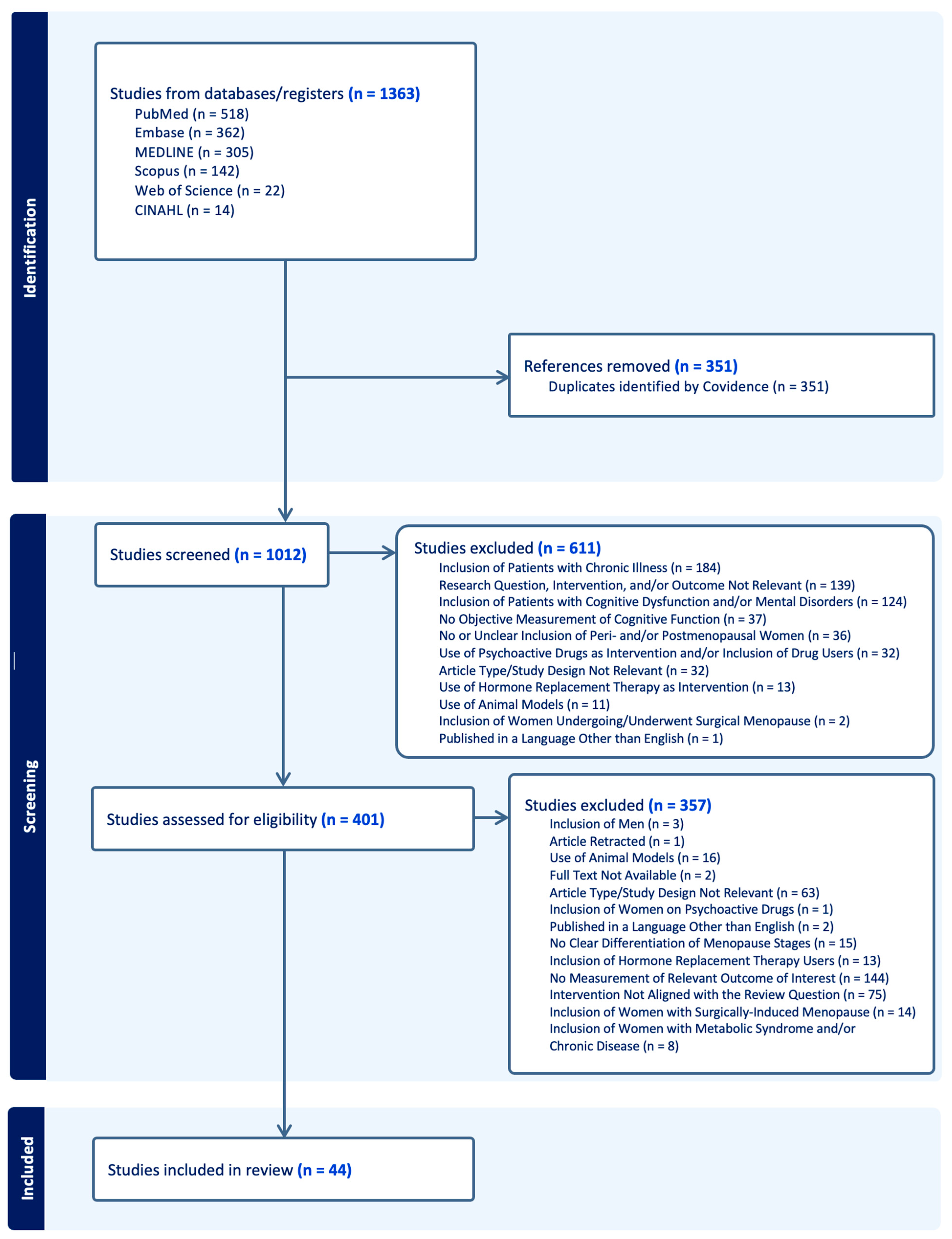
Cognitive Function in Peri- and Postmenopausal Women: Implications for Considering Iron Supplementation
Highlights
- Perimenopausal menorrhagia (PM), or abnormally heavy bleeding that occurs during the menopause transition, affects approximately 25% of women, placing them at risk of iron deficiency.
- Cognitive decline is frequently observed in women during the menopause transition, but the use of hormone replacement therapy is not recommended for the management of cognitive complaints, due to the lack of evidence.
- Treating perimenopausal women experiencing PM with iron supplements has the potential to improve their iron status and ameliorate their cognitive decline.
- Well-designed studies are needed to better understand the role of menopause stage, menopausal signs and symptoms, and the duration of reproductive life in cognitive function in peri- and postmenopausal women, as well as the potential of iron supplementation to improve cognition at this stage of life.
Abstract
1. Introduction
2. Literature Search
3. Iron in the Peri- and Postmenopausal Periods
4. Cognitive Function in the Peri- and Postmenopausal Periods
| Author | Study Design | Sample Size | Menopause Stage Investigated | Menopause Stage Categorization Criteria | Cognition-Related Measure(s) | Findings |
|---|---|---|---|---|---|---|
| Chalise et al. (2022) [74] | Cross- Sectional (Nepal) | 180 | Perimenopause | Menstrual bleeding patterns/history |
|
|
| Chen et al. (2007) [70] | Cross- Sectional (China) | 353 | Peri- and postmenopause | Menstrual bleeding patterns/history |
|
|
| Coslov et al. (2021) [75] | Cross- Sectional (US) | 1529 (583 being perimenopausal) | Late pre- and perimenopause | STRAW+10 |
|
|
| Fuh et al. (2006) [69] | Longitudinal (Taiwan) | 495 | Pre- and perimenopause | Menstrual bleeding patterns/history |
|
|
| Greendale et al. (2009) [68] | Longitudinal (US) | 2362 | Pre-, early peri-, late peri-, post-, and postmenopause with current hormone use | SWAN criteria (similar to STRAW) |
|
|
| He et al. (2021) [78] | Cross- Sectional (China) | 57 (25 being perimenopausal) | Pre- and perimenopause | STRAW+10 |
|
|
| Maki et al. (2021) [67] | Longitudinal (US) | 443 | Pre-, early peri-, late peri-, and postmenopause | SWAN criteria (similar to STRAW) |
|
|
| Mathew et al. (2021) [76] | Cross- Sectional (India) | 315 | Peri- and postmenopause | Menstrual bleeding patterns/history |
|
|
| Meyer et al. (2003) [73] | Longitudinal (US) | 868 | Pre-, early peri-, late peri-, and postmenopause | Menstrual bleeding patterns/history |
|
|
| Zhang et al. (2021) [77] | Prospective (China) | 4063 (2107 being perimenopausal) | Peri- and postmenopause | STRAW+10 |
|
|
| Zhang et al. (2021) [80] | Cross- Sectional (China) | 50 (25 being perimenopausal) | Peri- and postmenopause | STRAW+10 |
|
|
| Zhang et al. (2021) [79] | Cross- Sectional (China) | 99 (45 being perimenopausal) | Pre- and perimenopause |
|
|
|
| Author | Study Design | Sample Size | Menopause Stage Investigated | Menopause Stage Categorization Criteria | Hormones Measured | Cognition-Related Measure(s) | Findings |
|---|---|---|---|---|---|---|---|
| Berent- Spillson et al. (2012) [82] | Cross- Sectional (US) | 67 (32 being postmenopausal) | Pre-, peri-, postmenopause |
|
|
|
|
| Epperson et al. (2013) [87] | Longitudinal (US) | 403 | Pre-, late pre-, early peri-, late peri-, early postmenopause |
|
|
|
|
| Herlitz et al. (2007) [86] | Longitudinal (Sweden) | 242 (55 being postmenopausal) | Pre-, peri-, postmenopause | Self-reported stages | Estrogen |
|
|
| Jacobs et al. (2016) [90] | Cross- Sectional (US) | 186 (31 being postmenopausal) | Pre-, peri-, postmenopause | STRAW+10 |
|
|
|
| Jacobs et al. (2016) [89] | Cross- Sectional (US) | 142 (20 being postmenopausal) | Pre-, peri-, postmenopause | STRAW+10 |
| Working Memory N-Back Task completed during fMRI |
|
| Lissaman et al. (2024) [88] | Cross- Sectional (Canada) | 96 (34 being postmenopausal) | Pre- and postmenopause | STRAW+10 |
| Spatial context memory task during the fMRI scanning (Face-Location Memory Paradigm) |
|
| Luetters et al. (2007) [85] | Cross- Sectional (US) | 1657 (342 being postmenopausal) | Pre-, early peri-, late peri-, and postmenopause | SWAN criteria (similar to STRAW) |
|
|
|
| Ryan et al. (2012) [84] | Longitudinal (Australia) | 148 | Postmenopause | Does not specify |
|
|
|
| Seitz et al. (2019) [92] | Cross- Sectional (US) | 94 (32 being postmenopausal) | Pre-, peri-, and postmenopause | STRAW+10 |
|
|
|
| Spets et al. (2024) [93] | Cross- Sectional (US) | 180 (29 being postmenopausal) | Pre-, peri-, and postmenopause | STRAW+10 |
|
|
|
| Weber et al. (2013) [43] | Cross- Sectional (US) | 117 (14 being postmenopausal) | Late pre-, early peri-, late peri-, early postmenopause | STRAW+10 |
|
|
|
| Zhang et al. (2018) [91] | Cross- Sectional (China) | 87 (43 being postmenopausal) | Pre- and postmenopause |
| FSH |
|
|
| Author | Study Design | Sample Size | Menopause Stage Investigated | Menopause Stage Categorization Criteria | Menopausal Signs/Symptoms | Cognition-Related Measure(s) | Findings |
|---|---|---|---|---|---|---|---|
| Bojar et al. (2020) [95] | Cross- Sectional (Poland) | 300 (143 being perimenopausal) | Peri- and postmenopause | STRAW+10 | Insomnia (Athens Insomnia Scale) |
|
|
| Greendale et al. (2010) [72] | Longitudinal (US) | 1903 (59.39% being early perimenopausal) | Pre, early peri-, late peri-, and postmenopause | Menstrual bleeding patterns/history |
|
|
|
| Grummisch et al. (2023) [96] | Cross- Sectional (Canada) | 43 | Perimenopause | STRAW+10 |
|
|
|
| Jaff et al. (2020) [102] | Cross- Sectional (South Africa) | 702 (121 being perimenopausal and 277 being postmenopausal) | Late pre-, early peri-, late peri-, early post-, late postmenopause | STRAW+10 |
| Processing speed and incidental recall (Symbol Digit Modalities Test) |
|
| Kalleinen et al. (2008) [98] | Cross- Sectional (Finland) | 61 (29 being postmenopausal) | Pre- and postmenopause |
|
| Attention/vigilance (CogniSpeed) |
|
| Raczkiewicz et al. (2024) [103] | Cross- Sectional (Poland) | 287 (141 being perimenopausal) | Peri- and postmenopause | STRAW+10 |
|
|
|
| Raczkiewicz et al. (2017) [99] | Cross- Sectional (Poland) | 300 (143 being perimenopausal) | Early peri-, late peri-, and postmenopause |
| Stress (serum cortisol concentration) |
|
|
| Schaafsma et al. (2010) [104] | Cross- Sectional (Australia) | 120 (48 being perimenopausal and 38 being postmenopausal) | Pre-, peri-, and postmenopause | STRAW |
|
|
|
| Triantafyllou et al. (2016) [100] | Cross- Sectional (Greece) | 39 | Postmenopause |
|
|
|
|
| Unkenstein et al. (2016) [106] | Cross- Sectional (Australia) | 130 (54 being perimenopausal and 40 being postmenopausal) | Pre-, peri-, and postmenopause | STRAW |
|
|
|
| Weber et al. (2021) [94] | Longitudinal (US) | 85 | Early peri-, late peri-, and early postmenopause |
|
|
|
|
| Weber et al. (2012) [105] | Cross- Sectional (US) | 75 | Perimenopause | Menstrual bleeding patterns/history |
|
|
|
| Weber et al. (2009) [101] | Cross- Sectional (US) | 24 | Perimenopause | STRAW |
|
|
|
| Yu et al. (2024) [97] | Cross- Sectional (China) | 76 | Perimenopause | STRAW+10 | Insomnia (International Classification of Sleep Disorders) | Sensory processing and attention (Event-Related Potentials) |
|
| Author | Study Design | Sample Size | Menopause Stage Investigated | Menopause Stage Categorization Criteria | Reproductive History-Related Measures | Cognition-Related Measure(s) | Findings |
|---|---|---|---|---|---|---|---|
| Gholizadeh et al. (2018) [110] | Cross-Sectional (Iran) | 209 | Postmenopause |
|
|
|
|
| Karim et al. (2016) [107] | Cross-Sectional (US) | 830 | Postmenopause |
|
|
|
|
| Kuh et al. (2018) [108] | Population-based (UK) | 1315 | Postmenopause | Menstrual bleeding history |
|
|
|
| Tierney et al. (2013) [109] | Cross-Sectional (Canada) | 126 | Postmenopause | Does not specify |
|
|
|
5. Discussion and Implications for Future Studies
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CNS | Computerized Neurocognitive Assessment Software |
| DHEA | Dehydroepiandrosterone |
| DLPFC | Dorsolateral Prefrontal Cortex |
| fMRI | Functional Magnetic Resonance Imaging |
| FSH | Follicle-Stimulating Hormone |
| GCS | Greene Climacteric Scale |
| HRT | Hormone Replacement Therapy |
| ID | Iron Deficiency |
| IDA | Iron Deficiency Anemia |
| IQ | Intelligence Quotient |
| LH | Luteinizing Hormone |
| MCI | Mild Cognitive Impairment |
| MMSE | Mini-Mental State Examination |
| MRI | Magnetic Resonance Imaging |
| MRS | Menopause Rating Scale |
| PM | Perimenopausal Menorrhagia |
| QOL | Quality of Life |
| rsDMN | Resting-State Default Mode Network |
| SHBG | Sex Hormone-Binding Globulin |
| STRAW | Stages of Reproductive Aging Workshop |
| SWAN | Study of Women’s Health Across the Nation |
| RCT | Randomized Controlled Trial |
| ReHo | Regional Homogeneity |
| rs-fMRI | Resting State-Functional Magnetic Resonance Imaging |
| UK | United Kingdom |
| US | United States |
| WHO | World Health Organization |
| WMS-III | Wechsler Memory Scales-III |
References
- The Global Strategy and Action Plan on Ageing and Health 2016–2020: Towards a World in Which Everyone Can Live a Long and Healthy Life. 2016. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA73/A73_INF2-en.pdf (accessed on 16 June 2024).
- Beard, J.R.; Araujo De Carvalho, I.; Sumi, Y.; Officer, A.; Thiyagarajan, J.A. Healthy Ageing: Moving Forward. Bull. World Health Organ. 2017, 95, 730. [Google Scholar] [CrossRef]
- Gilmer, G.; Hettinger, Z.R.; Tuakli-Wosornu, Y.; Skidmore, E.; Silver, J.K.; Thurston, R.C.; Lowe, D.A.; Ambrosio, F. Female Aging: When Translational Models Don’t Translate. Nat. Aging 2023, 3, 1500–1508. [Google Scholar] [CrossRef]
- Hall, J.E. Endocrinology of the Menopause. Endocrinol. Metab. Clin. N. Am. 2015, 44, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.J.; Mehta, J.M.; Manson, J.E. Management of Menopausal Symptoms: A Review. JAMA 2023, 329, 405. [Google Scholar] [CrossRef]
- Peacock, K.; Carlson, K.; Ketvertis, K.M. Menopause. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hill, K. The Demography of Menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Caughey, A.B.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; et al. Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Persons: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 328, 1740. [Google Scholar] [CrossRef]
- Faubion, S.S.; Enders, F.; Hedges, M.S.; Chaudhry, R.; Kling, J.M.; Shufelt, C.L.; Saadedine, M.; Mara, K.; Griffin, J.M.; Kapoor, E. Impact of Menopause Symptoms on Women in the Workplace. Mayo Clin. Proc. 2023, 98, 833–845. [Google Scholar] [CrossRef]
- Delamater, L.; Santoro, N. Management of the Perimenopause. Clin. Obs. Gynecol. 2018, 61, 419–432. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J. Executive Summary of the Stages of Reproductive Aging Workshop+10: Addressing the Unfinished Agenda of Staging Reproductive Aging. Menopause 2012, 19, 387–395. [Google Scholar] [CrossRef]
- Santoro, N. Perimenopause: From Research to Practice. J. Women’s Health 2016, 25, 332–339. [Google Scholar] [CrossRef]
- Dhakal, A.; Bobrin, B.D. Cognitive Deficits. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Murman, D. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, N.; Shelton, S.; Halbreich, U. Perimenopausal Mental Disorders: Epidemiology and Phenomenology. CNS Spectr. 2005, 10, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Tepper, P.G.; Randolph, J.F.; McConnell, D.S.; Crawford, S.L.; El Khoudary, S.R.; Joffe, H.; Gold, E.B.; Zheng, H.; Bromberger, J.T.; Sutton-Tyrrell, K. Trajectory Clustering of Estradiol and Follicle-Stimulating Hormone during the Menopausal Transition among Women in the Study of Women’s Health across the Nation (SWAN). J. Clin. Endocrinol. Metab. 2012, 97, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The Menopause Transition: Signs, Symptoms, and Management Options. J. Clin. Endocrinol. Metab. 2021, 106, 1–15. [Google Scholar] [CrossRef]
- Metcalf, C.A.; Duffy, K.A.; Page, C.E.; Novick, A.M. Cognitive Problems in Perimenopause: A Review of Recent Evidence. Curr. Psychiatry Rep. 2023, 25, 501–511. [Google Scholar] [CrossRef]
- Brinton, R.D.; Yao, J.; Yin, F.; Mack, W.J.; Cadenas, E. Perimenopause as a Neurological Transition State. Nat. Rev. Endocrinol. 2015, 11, 393–405. [Google Scholar] [CrossRef]
- Wilson, R.S.; Leurgans, S.E.; Boyle, P.A.; Bennett, D.A. Cognitive Decline in Prodromal Alzheimer Disease and Mild Cognitive Impairment. Arch. Neurol. 2011, 68, 351–356. [Google Scholar] [CrossRef]
- Camaschella, C. Iron Deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef]
- Gaskell, H.; Derry, S.; Andrew Moore, R.; McQuay, H.J. Prevalence of Anaemia in Older Persons: Systematic Review. BMC Geriatr. 2008, 8, 1. [Google Scholar] [CrossRef]
- Wenger, M.J.; DellaValle, D.M.; Murray-Kolb, L.E.; Haas, J.D. Effect of Iron Deficiency on Simultaneous Measures of Behavior, Brain Activity, and Energy Expenditure in the Performance of a Cognitive Task. Nutr. Neurosci. 2019, 22, 196–206. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The Role of Iron in Brain Ageing and Neurodegenerative Disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L.; Connor, J.R. Iron Status and Neural Functioning. Annu. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Jimenez, E.; Wolf, A.W. Long-Term Developmental Outcome of Infants with Iron Deficiency. N. Engl. J. Med. 1991, 325, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin Receptor on Endothelium of Brain Capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef]
- Farrall, A.J.; Wardlaw, J.M. Blood–Brain Barrier: Ageing and Microvascular Disease—Systematic Review and Meta-Analysis. Neurobiol. Aging 2009, 30, 337–352. [Google Scholar] [CrossRef]
- Conde, J.R.; Streit, W.J. Microglia in the Aging Brain. J. Neuropathol. Exp. Neurol. 2006, 65, 199–203. [Google Scholar] [CrossRef]
- McClung, J.P.; Murray-Kolb, L.E. Iron Nutrition and Premenopausal Women: Effects of Poor Iron Status on Physical and Neuropsychological Performance. Annu. Rev. Nutr. 2013, 33, 271–288. [Google Scholar] [CrossRef]
- Murray-Kolb, L.E. Iron Status and Neuropsychological Consequences in Women of Reproductive Age: What Do We Know and Where Are We Headed? J. Nutr. 2011, 141, 747S–755S. [Google Scholar] [CrossRef]
- Scott, S.P.; Murray-Kolb, L.E. Iron Status Is Associated with Performance on Executive Functioning Tasks in Nonanemic Young Women. J. Nutr. 2016, 146, 30–37. [Google Scholar] [CrossRef]
- Jáuregui-Lobera, I. Iron Deficiency and Cognitive Functions. Neuropsychiatr. Dis. Treat. 2014, 10, 2087–2095. [Google Scholar] [CrossRef]
- Murray-Kolb, L.E.; Beard, J.L. Iron Deficiency and Child and Maternal Health. Am. J. Clin. Nutr. 2009, 89, 946S–950S. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.B.; Cankurtaran, M.; Haznedaroglu, I.C.; Halil, M.; Ulger, Z.; Altun, B.; Ariogul, S. Iron Deficiency Can Cause Cognitive Impairment in Geriatric Patients. J. Nutr. Health Aging 2012, 16, 220–224. [Google Scholar] [CrossRef]
- Whiteman, M.K.; Kuklina, E.; Jamieson, D.J.; Hillis, S.D.; Marchbanks, P.A. Inpatient Hospitalization for Gynecologic Disorders in the United States. Am. J. Obstet. Gynecol. 2010, 202, e1–e541. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, J.; DiBonaventura, M.d.; Wagner, J.-S.; Alvir, J.; Shah, S. The Impact of Menopausal Symptoms on Quality of Life, Productivity, and Economic Outcomes. J. Women’s Health 2013, 22, 983–990. [Google Scholar] [CrossRef]
- Firquet, A.; Kirschner, W.; Bitzer, J. Forty to Fifty-Five-Year-Old Women and Iron Deficiency: Clinical Considerations and Quality of Life. Gynecol. Endocrinol. 2017, 33, 503–509. [Google Scholar] [CrossRef]
- Mosconi, L.; Berti, V.; Dyke, J.; Schelbaum, E.; Jett, S.; Loughlin, L.; Jang, G.; Rahman, A.; Hristov, H.; Pahlajani, S.; et al. Menopause Impacts Human Brain Structure, Connectivity, Energy Metabolism, and Amyloid-Beta Deposition. Sci. Rep. 2021, 11, 10867. [Google Scholar] [CrossRef]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of Menopause—Global Prevalence, Physiology and Implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Sullivan Mitchell, E.; Fugate Woods, N. Midlife Women’s Attributions about Perceived Memory Changes: Observations from the Seattle Midlife Women’s Health Study. J. Women’s Health Gend. Based Med. 2001, 10, 351–362. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Greendale, G.; Crawford, S.L.; Avis, N.E.; Brooks, M.M.; Thurston, R.C.; Karvonen-Gutierrez, C.; Waetjen, L.E.; Matthews, K. The Menopause Transition and Women’s Health at Midlife: A Progress Report from the Study of Women’s Health Across the Nation (SWAN). Menopause 2019, 26, 1213–1227. [Google Scholar] [CrossRef]
- Weber, M.T.; Rubin, L.H.; Maki, P.M. Cognition in Perimenopause: The Effect of Transition Stage. Menopause 2013, 20, 511–517. [Google Scholar] [CrossRef]
- Maki, P.M.; Dennerstein, L.; Clark, M.; Guthrie, J.; LaMontagne, P.; Fornelli, D.; Little, D.; Henderson, V.W.; Resnick, S.M. Perimenopausal Use of Hormone Therapy Is Associated with Enhanced Memory and Hippocampal Function Later in Life. Brain Res. 2011, 1379, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Crandall, C.J.; Davis, L.; El Khoudary, S.R.; Hodis, H.N.; Lobo, R.A.; Maki, P.M.; Manson, J.E.; Pinkerton, J.V.; Santoro, N.F.; et al. The 2022 Hormone Therapy Position Statement of The North American Menopause Society. Menopause 2022, 29, 767–794. [Google Scholar] [CrossRef]
- Alsugeir, D.; Wei, L.; Adesuyan, M.; Cook, S.; Panay, N.; Brauer, R. Hormone Replacement Therapy Prescribing in Menopausal Women in the UK: A Descriptive Study. BJGP Open 2022, 6, BJGPO.2022.0126. [Google Scholar] [CrossRef]
- Hickey, M.; Elliott, J.; Davison, S.L. Hormone Replacement Therapy. BMJ 2012, 344, e763. [Google Scholar] [CrossRef]
- Nguyen, M.; Tadi, P. Iron Supplementation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Pilar, G.; Josiane, A.; Chantal, J.; Serge, H.; Emmanuelle, K.G. Midlife Iron Status Is Inversely Associated with Subsequent Cognitive Performance, Particularly in Perimenopausal Women. J. Nutr. 2013, 143, 1974–1981. [Google Scholar] [CrossRef]
- Barnett, A.L.; Wenger, M.J.; Miles, P.; Wu, D.; Isingizwe, Z.R.; Benbrook, D.M.; Yuan, H. Cognitive Performance in Relation to Systemic and Brain Iron at Perimenopause. Nutrients 2025, 17, 745. [Google Scholar] [CrossRef]
- Koothirezhi, R.; Ranganathan, S. Postmenopausal Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Von Holle, A.; O’Brien, K.M.; Sandler, D.P.; Janicek, R.; Karagas, M.R.; White, A.J.; Niehoff, N.M.; Levine, K.E.; Jackson, B.P.; Weinberg, C.R. Toenail and Serum Levels as Biomarkers of Iron Status in Pre- and Postmenopausal Women: Correlations and Stability over Eight-Year Follow-Up. Sci. Rep. 2024, 14, 1682. [Google Scholar] [CrossRef]
- Jian, J.; Pelle, E.; Huang, X. Iron and Menopause: Does Increased Iron Affect the Health of Postmenopausal Women? Antioxid. Redox Signal. 2009, 11, 2939–2943. [Google Scholar] [CrossRef] [PubMed]
- Zacharski, L.R.; Ornstein, D.L.; Woloshin, S.; Schwartz, L.M. Association of Age, Sex, and Race with Body Iron Stores in Adults: Analysis of NHANES III Data. Am. Heart J. 2000, 140, 98–104. [Google Scholar] [CrossRef]
- Ahanchi, N.S.; Khatami, F.; Llanaj, E.; Quezada-Pinedo, H.G.; Dizdari, H.; Bano, A.; Glisic, M.; Eisenga, M.F.; Vidal, P.-M.; Muka, T. The Complementary Roles of Iron and Estrogen in Menopausal Differences in Cardiometabolic Outcomes. Clin. Nutr. 2024, 43, 1136–1150. [Google Scholar] [CrossRef]
- Fleming, D.J.; Jacques, P.F.; Tucker, K.L.; Massaro, J.M.; D’Agostino, R.B.; Wilson, P.W.; Wood, R.J. Iron Status of the Free-Living, Elderly Framingham Heart Study Cohort: An Iron-Replete Population with a High Prevalence of Elevated Iron Stores. Am. J. Clin. Nutr. 2001, 73, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Carlson, K.; Abramovitz, A. Postmenopausal Bleeding. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Saboor, M.; Qamar, K.; Qudsia, F.; Khosa, S.M.; Moinuddin, M.; Usman, M. Malabsorption of Iron as a Cause of Iron Deficiency Anemia in Postmenopausal Women. Pak. J. Med. Sci. 2015, 31, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Duman, T.T.; Aktas, G.; Meryem Atak, B.; Kocak, M.Z.; Kurtkulagi, O.; Bilgin, S. General Characteristics of Anemia in Postmenopausal Women and Elderly Men. Aging Male 2020, 23, 780–784. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Alves, S.E.; Bulloch, K.; Weiland, N.G. Ovarian Steroids and the Brain: Implications for Cognition and Aging. Neurology 1997, 48, 8S–15S. [Google Scholar] [CrossRef]
- Comasco, E.; Frokjaer, V.G.; Sundström-Poromaa, I. Functional and Molecular Neuroimaging of Menopause and Hormone Replacement Therapy. Front. Neurosci. 2014, 8, 388. [Google Scholar] [CrossRef]
- Rice, M.M.; Graves, A.B.; McCurry, S.M.; Larson, E.B. Estrogen Replacement Therapy and Cognitive Function in Postmenopausal Women Without Dementia. Am. J. Med. 1997, 103, 26S–35S. [Google Scholar] [CrossRef]
- Shaywitz, S.E. Effect of Estrogen on Brain Activation Patterns in Postmenopausal Women During Working Memory Tasks. JAMA 1999, 281, 1197. [Google Scholar] [CrossRef]
- Yao, J.; Hamilton, R.T.; Cadenas, E.; Brinton, R.D. Decline in Mitochondrial Bioenergetics and Shift to Ketogenic Profile in Brain during Reproductive Senescence. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2010, 1800, 1121–1126. [Google Scholar] [CrossRef]
- Brinton, R.D. Estrogen-Induced Plasticity from Cells to Circuits: Predictions for Cognitive Function. Trends Pharmacol. Sci. 2009, 30, 212–222. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Comparative Distribution of Estrogen Receptor-α And -β mRNA in the Rat Central Nervous System. J. Comp. Neurol. 1997, 388, 507–525. [Google Scholar] [CrossRef]
- Maki, P.M.; Springer, G.; Anastos, K.; Gustafson, D.R.; Weber, K.; Vance, D.; Dykxhoorn, D.; Milam, J.; Adimora, A.A.; Kassaye, S.G.; et al. Cognitive Changes during the Menopausal Transition: A Longitudinal Study in Women with and without HIV. Menopause 2021, 28, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Huang, M.-H.; Wight, R.G.; Seeman, T.; Luetters, C.; Avis, N.E.; Johnston, J.; Karlamangla, A.S. Effects of the Menopause Transition and Hormone Use on Cognitive Performance in Midlife Women. Neurology 2009, 72, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Fuh, J.-L.; Wang, S.-J.; Lee, S.-J.; Lu, S.-R.; Juang, K.-D. A Longitudinal Study of Cognition Change during Early Menopausal Transition in a Rural Community. Maturitas 2006, 53, 447–453. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, S.-Q.; Wei, Y.; Gao, H.-L.; Wu, Z.-L. Menopause-Specific Quality of Life Satisfaction in Community-Dwelling Menopausal Women in China. Gynecol. Endocrinol. 2007, 23, 166–172. [Google Scholar] [CrossRef]
- Conde, D.M.; Verdade, R.C.; Valadares, A.L.R.; Mella, L.F.B.; Pedro, A.O.; Costa-Paiva, L. Menopause and Cognitive Impairment: A Narrative Review of Current Knowledge. World J. Psychiatry 2021, 11, 412–428. [Google Scholar] [CrossRef]
- Greendale, G.A.; Wight, R.G.; Huang, M.H.; Avis, N.; Gold, E.B.; Joffe, H.; Seeman, T.; Vuge, M.; Karlamangla, A.S. Menopause-Associated Symptoms and Cognitive Performance: Results From the Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2010, 171, 1214–1224. [Google Scholar] [CrossRef]
- Meyer, P.M.; Powell, L.H.; Wilson, R.S.; Everson-Rose, S.A.; Kravitz, H.M.; Luborsky, J.L.; Madden, T.; Pandey, D.; Evans, D.A. A Population-Based Longitudinal Study of Cognitive Functioning in the Menopausal Transition. Neurology 2003, 61, 801–806. [Google Scholar] [CrossRef]
- Chalise, G.D.; Shrestha, S.; Thapa, S.; Bharati, M.; Pradhan, S.; Adhikari, B. Health Problems Experienced by Peri-Menopausal Women and Their Perception towards Menopause. J. Nepal. Health Res. Counc. 2022, 20, 102–107. [Google Scholar] [CrossRef]
- Coslov, N.; Richardson, M.K.; Woods, N.F. Symptom Experience during the Late Reproductive Stage and the Menopausal Transition: Observations from the Women Living Better Survey. Menopause 2021, 28, 1012–1025. [Google Scholar] [CrossRef]
- Mathew, D.J.; Kumar, S.; Jain, P.K.; Srivastava, D.K.; Singh, V.; Krishnappa, K. Morbidity Patterns among Menopausal Women in Rural Uttar Pradesh, India: A Cross-Sectional Study. J. Menopausal Med. 2021, 27, 24. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, X.; Cui, Y.; Gu, M.; Mueck, A.O. Menopausal Symptoms among Chinese Peri- and Postmenopausal Women: A Large Prospective Single-Center Cohort Study. Gynecol. Endocrinol. 2021, 37, 185–189. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Guo, W.; Qiu, J.; An, X.; Lu, W. Altered Spontaneous Brain Activity in Women During Menopause Transition and Its Association With Cognitive Function and Serum Estradiol Level. Front. Endocrinol. 2021, 12, 652512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, W.; Hu, H.; Wen, L.; Gong, M.; Liu, B.; Hu, J.; Li, G.; Zhang, D. Subcortical Volume Changes in Early Menopausal Women and Correlation With Neuropsychological Tests. Front. Aging Neurosci. 2021, 13, 738679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, W.Q.; Liu, N.N.; Liu, H.J. Alterations of Regional Homogeneity in Perimenopause: A Resting-State Functional MRI Study. Climacteric 2022, 25, 460–466. [Google Scholar] [CrossRef]
- Xu, Z.; Lai, J.; Zhang, H.; Ng, C.H.; Zhang, P.; Xu, D.; Hu, S. Regional Homogeneity and Functional Connectivity Analysis of Resting-State Magnetic Resonance in Patients with Bipolar II Disorder. Medicine 2019, 98, e17962. [Google Scholar] [CrossRef]
- Berent-Spillson, A.; Persad, C.C.; Love, T.; Sowers, M.; Randolph, J.F.; Zubieta, J.-K.; Smith, Y.R. Hormonal Environment Affects Cognition Independent of Age during the Menopause Transition. J. Clin. Endocrinol. Metab. 2012, 97, E1686–E1694. [Google Scholar] [CrossRef]
- Weber, M.T.; Maki, P.M.; McDermott, M.P. Cognition and Mood in Perimenopause: A Systematic Review and Meta-Analysis. J. Steroid Biochem. Mol. Biol. 2014, 142, 90–98. [Google Scholar] [CrossRef]
- Ryan, J.; Stanczyk, F.Z.; Dennerstein, L.; Mack, W.J.; Clark, M.S.; Szoeke, C.; Kildea, D.; Henderson, V.W. Hormone Levels and Cognitive Function in Postmenopausal Midlife Women. Neurobiol. Aging 2012, 33, 1138–1147.e2. [Google Scholar] [CrossRef]
- Luetters, C.; Huang, M.-H.; Seeman, T.; Buckwalter, G.; Meyer, P.M.; Avis, N.E.; Sternfeld, B.; Johnston, J.M.; Greendale, G.A. Menopause Transition Stage and Endogenous Estradiol and Follicle-Stimulating Hormone Levels Are Not Related to Cognitive Performance: Cross-Sectional Results from the Study of Women’s Health across the Nation (SWAN). J. Women’s Health 2007, 16, 331–344. [Google Scholar] [CrossRef]
- Herlitz, A.; Thilers, P.; Habib, R. Endogenous Estrogen Is Not Associated with Cognitive Performance before, during, or after Menopause. Menopause 2007, 14, 425–431. [Google Scholar] [CrossRef]
- Epperson, C.N.; Sammel, M.D.; Freeman, E.W. Menopause Effects on Verbal Memory: Findings From a Longitudinal Community Cohort. J. Clin. Endocrinol. Metab. 2013, 98, 3829–3838. [Google Scholar] [CrossRef] [PubMed]
- Lissaman, R.; Rajagopal, S.; Kearley, J.; Pasvanis, S.; Rajah, M.N. Menopause Status- and Sex-Related Differences in Age Associations with Spatial Context Memory and White Matter Microstructure at Midlife. Neurobiol. Aging 2024, 141, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.G.; Weiss, B.; Makris, N.; Whitfield-Gabrieli, S.; Buka, S.L.; Klibanski, A.; Goldstein, J.M. Reorganization of Functional Networks in Verbal Working Memory Circuitry in Early Midlife: The Impact of Sex and Menopausal Status. Cereb. Cortex 2016, 27, 2857–2870. [Google Scholar] [CrossRef]
- Jacobs, E.G.; Weiss, B.K.; Makris, N.; Whitfield-Gabrieli, S.; Buka, S.L.; Klibanski, A.; Goldstein, J.M. Impact of Sex and Menopausal Status on Episodic Memory Circuitry in Early Midlife. J. Neurosci. 2016, 36, 10163–10173. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, J.; Fan, W.; Liu, B.; Wen, L.; Wang, G.; Gong, M.; Yang, C.; Zhang, D. Aberrant Cerebral Activity in Early Postmenopausal Women: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Cell. Neurosci. 2018, 12, 454. [Google Scholar] [CrossRef]
- Seitz, J.; Kubicki, M.; Jacobs, E.G.; Cherkerzian, S.; Weiss, B.K.; Papadimitriou, G.; Mouradian, P.; Buka, S.; Goldstein, J.M.; Makris, N. Impact of Sex and Reproductive Status on Memory Circuitry Structure and Function in Early Midlife Using Structural Covariance Analysis. Hum. Brain Mapp. 2019, 40, 1221–1233. [Google Scholar] [CrossRef]
- Spets, D.S.; Cohen, J.E.; Konishi, K.; Aroner, S.; Misra, M.; Lee, H.; Goldstein, J.M. Impact of Sex and Reproductive Status on the Default Mode Network in Early Midlife: Implications for Aging of Memory Circuitry and Function. Cereb. Cortex 2024, 34, bhae088. [Google Scholar] [CrossRef]
- Weber, M.T.; Rubin, L.H.; Schroeder, R.; Steffenella, T.; Maki, P.M. Cognitive Profiles in Perimenopause: Hormonal and Menopausal Symptom Correlates. Climacteric 2021, 24, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Bojar, I.; Raczkiewicz, D.; Gujski, M.; Humeniuk, E.; Wdowiak, A.; Owoc, A.; Pinkas, J. Oestrogen Receptor α Gene Polymorphisms, Insomnia, and Cognitive Functions in Perimenopausal and Postmenopausal Women in Non-Manual Employment. Arch. Med. Sci. 2020, 18, 1318–1328. [Google Scholar] [CrossRef]
- Grummisch, J.A.; Sykes Tottenham, L.; Gordon, J.L. Within-Person Changes in Reproductive Hormones and Cognition in the Menopause Transition. Maturitas 2023, 177, 107804. [Google Scholar] [CrossRef]
- Yu, L.; Luo, Y.; Lin, W.; Dou, Z.; Hu, D.; Wei, W.; He, Y.; Zhu, K.; Hong, X.; Zhang, Q.; et al. Deciphering the Impairment of Perimenopausal Insomnia on Visual Search from a Neurocognitive Processing Perspective. SLEEP 2024, 47, zsae188. [Google Scholar] [CrossRef]
- Kalleinen, N.; Polo-Kantola, P.; Himanen, S.-L.; Alhola, P.; Joutsen, A.; Urrila, A.S.; Polo, O. Sleep and the Menopause—Do Postmenopausal Women Experience Worse Sleep than Premenopausal Women? Menopause Int. 2008, 14, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Raczkiewicz, D.; Sarecka-Hujar, B.; Owoc, A.; Bojar, I. Cognitive Functions and Serum Cortisol Concentration in Perimenopausal and Postmenopausal Women Working Non-Manually. Neuro Endocrinol. Lett. 2017, 38, 269–274. [Google Scholar]
- Triantafyllou, N.; Armeni, E.; Christidi, F.; Rizos, D.; Kaparos, G.; Palaiologou, A.; Augoulea, A.; Alexandrou, A.; Zalonis, I.; Tzivgoulis, G.; et al. The Intensity of Menopausal Symptoms Is Associated with Episodic Memory in Postmenopausal Women. Climacteric 2016, 19, 393–399. [Google Scholar] [CrossRef]
- Weber, M.; Mapstone, M. Memory Complaints and Memory Performance in the Menopausal Transition. Menopause 2009, 16, 694–700. [Google Scholar] [CrossRef]
- Jaff, N.G.; Rubin, L.H.; Crowther, N.J.; Norris, S.A.; Maki, P.M. Menopausal Symptoms, Menopausal Stage and Cognitive Functioning in Black Urban African Women. Climacteric 2020, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Raczkiewicz, D.; Gujski, M.; Sarecka-Hujar, B.; Suski, K.; Pedrycz-Wieczorska, A.; Wdowiak, A.; Bojar, I. Impact of Serum Vitamin D, B6, and B12 and Cognitive Functions on Quality of Life in Peri- and Postmenopausal Polish Women. Med. Sci. Monit. 2024, 30, e943249. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, M.; Homewood, J.; Taylor, A. Subjective Cognitive Complaints at Menopause Associated with Declines in Performance of Verbal Memory and Attentional Processes. Climacteric 2010, 13, 84–98. [Google Scholar] [CrossRef]
- Weber, M.T.; Mapstone, M.; Staskiewicz, J.; Maki, P.M. Reconciling Subjective Memory Complaints with Objective Memory Performance in the Menopausal Transition. Menopause 2012, 19, 735–741. [Google Scholar] [CrossRef]
- Unkenstein, A.E.; Bryant, C.A.; Judd, F.K.; Ong, B.; Kinsella, G.J. Understanding Women’s Experience of Memory over the Menopausal Transition: Subjective and Objective Memory in Pre-, Peri-, and Postmenopausal Women. Menopause 2016, 23, 1319–1329. [Google Scholar] [CrossRef]
- Karim, R.; Dang, H.; Henderson, V.W.; Hodis, H.N.; St. John, J.; Brinton, R.D.; Mack, W.J. Effect of Reproductive History and Exogenous Hormone Use on Cognitive Function in Mid- and Late Life. J. Am. Geriatr. Soc. 2016, 64, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Kuh, D.; Cooper, R.; Moore, A.; Richards, M.; Hardy, R. Age at Menopause and Lifetime Cognition: Findings from a British Birth Cohort Study. Neurology 2018, 90, e1673–e1681. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.C.; Ryan, J.; Ancelin, M.-L.; Moineddin, R.; Rankin, S.; Yao, C.; MacLusky, N.J. Lifelong Estrogen Exposure and Memory in Older Postmenopausal Women. J. Alzheimer’s Dis. 2013, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, S.; Jahanian Sadatmahalleh, S.; Ziaei, S. The Association between Estradiol Levels and Cognitive Function in Postmenopausal Women. Int. J. Reprod. Biomed. (IJRM) 2018, 16, 455–458. [Google Scholar] [CrossRef]
- Hammerton, G.; Munafò, M.R. Causal Inference with Observational Data: The Need for Triangulation of Evidence. Psychol. Med. 2021, 51, 563–578. [Google Scholar] [CrossRef]
- Burger, H.G. Hormonal Changes in the Menopause Transition. Recent. Prog. Horm. Res. 2002, 57, 257–275. [Google Scholar] [CrossRef]
- Mehta, K.M.; Simonsick, E.M.; Rooks, R.; Newman, A.B.; Pope, S.K.; Rubin, S.M.; Yaffe, K.; for the Health, Aging and Body Composition Study. Black and White Differences in Cognitive Function Test Scores: What Explains the Difference? J. Am. Geriatr. Soc. 2004, 52, 2120–2127. [Google Scholar] [CrossRef]
- Metcalf, C.A.; Johnson, R.L.; Novick, A.M.; Freeman, E.W.; Sammel, M.D.; Anthony, L.G.; Epperson, C.N. Adverse Childhood Experiences Interact with Inflammation and Menopause Transition Stage to Predict Verbal Memory in Women. Brain Behav. Immun. Health 2022, 20, 100411. [Google Scholar] [CrossRef]
- deLeyer-Tiarks, J.M.; Caemmerer, J.M.; Bray, M.A.; Kaufman, A.S. Assessment of Human Intelligence—The State of the Art in the 2020s. J. Intell. 2024, 12, 72. [Google Scholar] [CrossRef]
- Holden, L.R.; Tanenbaum, G.J. Modern Assessments of Intelligence Must Be Fair and Equitable. J. Intell. 2023, 11, 126. [Google Scholar] [CrossRef]
- Lawrence, J.A.; Hsu, Y.-T.; Cory, H.J.; Kawachi, I. Racial Discrimination and Cognitive Function: An Instrumental Variable Analysis. Soc. Sci. Med. 2024, 363, 117447. [Google Scholar] [CrossRef] [PubMed]
| Author | Study Design | Sample Size | Menopause Stage Investigated | Menopause Stage Categorization Criteria | Iron Biomarkers | Cognition-Related Measure(s) | Findings |
|---|---|---|---|---|---|---|---|
| Valentina et al. (2013) [49] | Observational follow-up of double-blind, placebo- controlled RCT (France) | 3932 (1431 being pre- and perimenopausal) | Pre-, peri-, and postmenopause | Does not specify |
|
|
|
| Barnett et al. (2025) [50] | Cross- sectional * (US) | 27 (8 being early perimenopausal) | Perimenopause |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.S.; Seiger, E.R.; Murray-Kolb, L.E. Cognitive Function in Peri- and Postmenopausal Women: Implications for Considering Iron Supplementation. Nutrients 2025, 17, 1762. https://doi.org/10.3390/nu17111762
Choi MS, Seiger ER, Murray-Kolb LE. Cognitive Function in Peri- and Postmenopausal Women: Implications for Considering Iron Supplementation. Nutrients. 2025; 17(11):1762. https://doi.org/10.3390/nu17111762
Chicago/Turabian StyleChoi, Mun Sun, Emily R. Seiger, and Laura E. Murray-Kolb. 2025. "Cognitive Function in Peri- and Postmenopausal Women: Implications for Considering Iron Supplementation" Nutrients 17, no. 11: 1762. https://doi.org/10.3390/nu17111762
APA StyleChoi, M. S., Seiger, E. R., & Murray-Kolb, L. E. (2025). Cognitive Function in Peri- and Postmenopausal Women: Implications for Considering Iron Supplementation. Nutrients, 17(11), 1762. https://doi.org/10.3390/nu17111762






