Using the Rise and Fall of Oxidative Stress and Inflammation Post-Exercise to Evaluate the Effect of Methylsulfonylmethane Supplementation on Immune Response mRNA
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and MSM Supplementation
2.2. Blood Collection, RNA Isolation, and Analysis
2.3. Statistical Methods
3. Results
4. Discussion
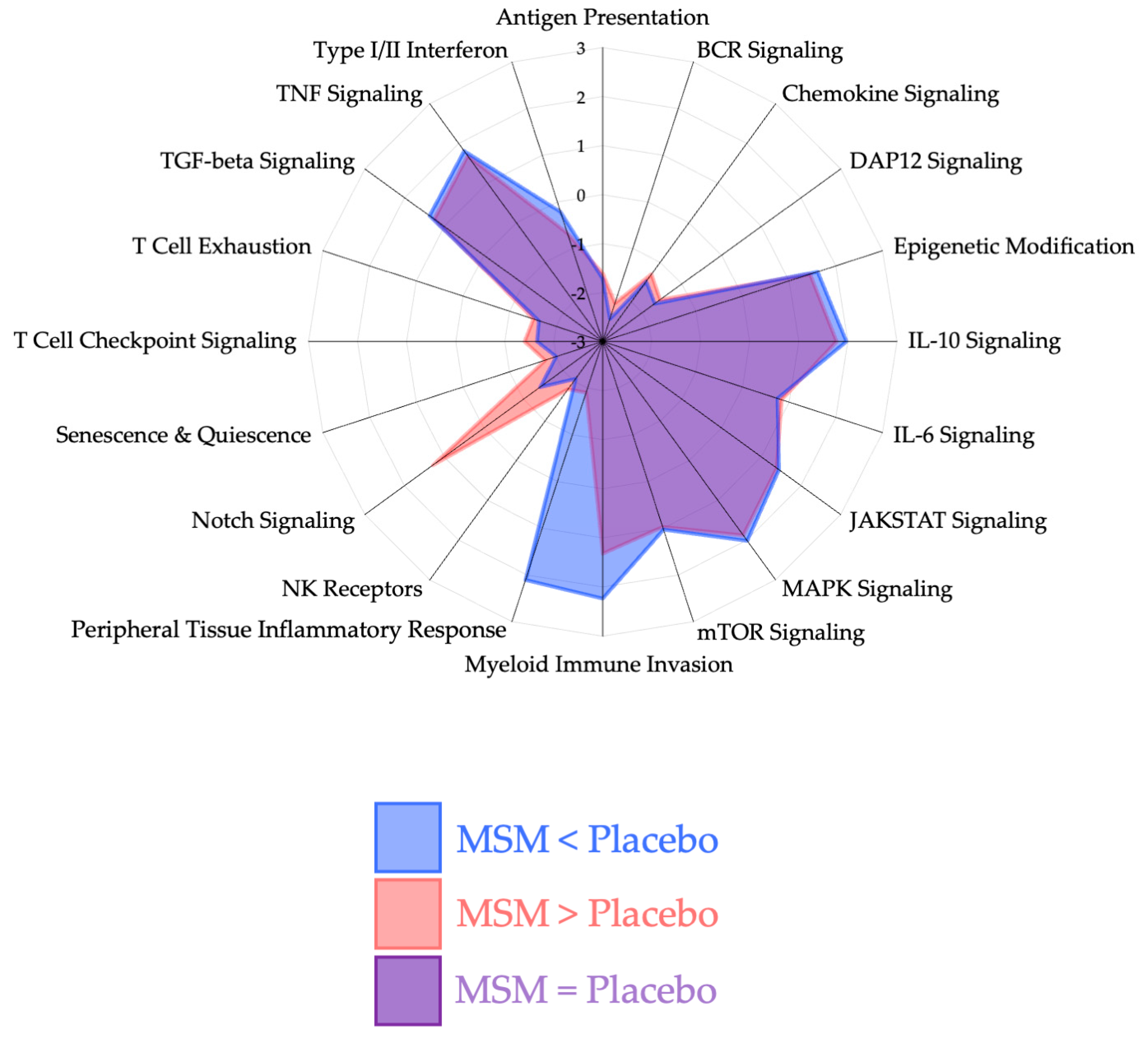
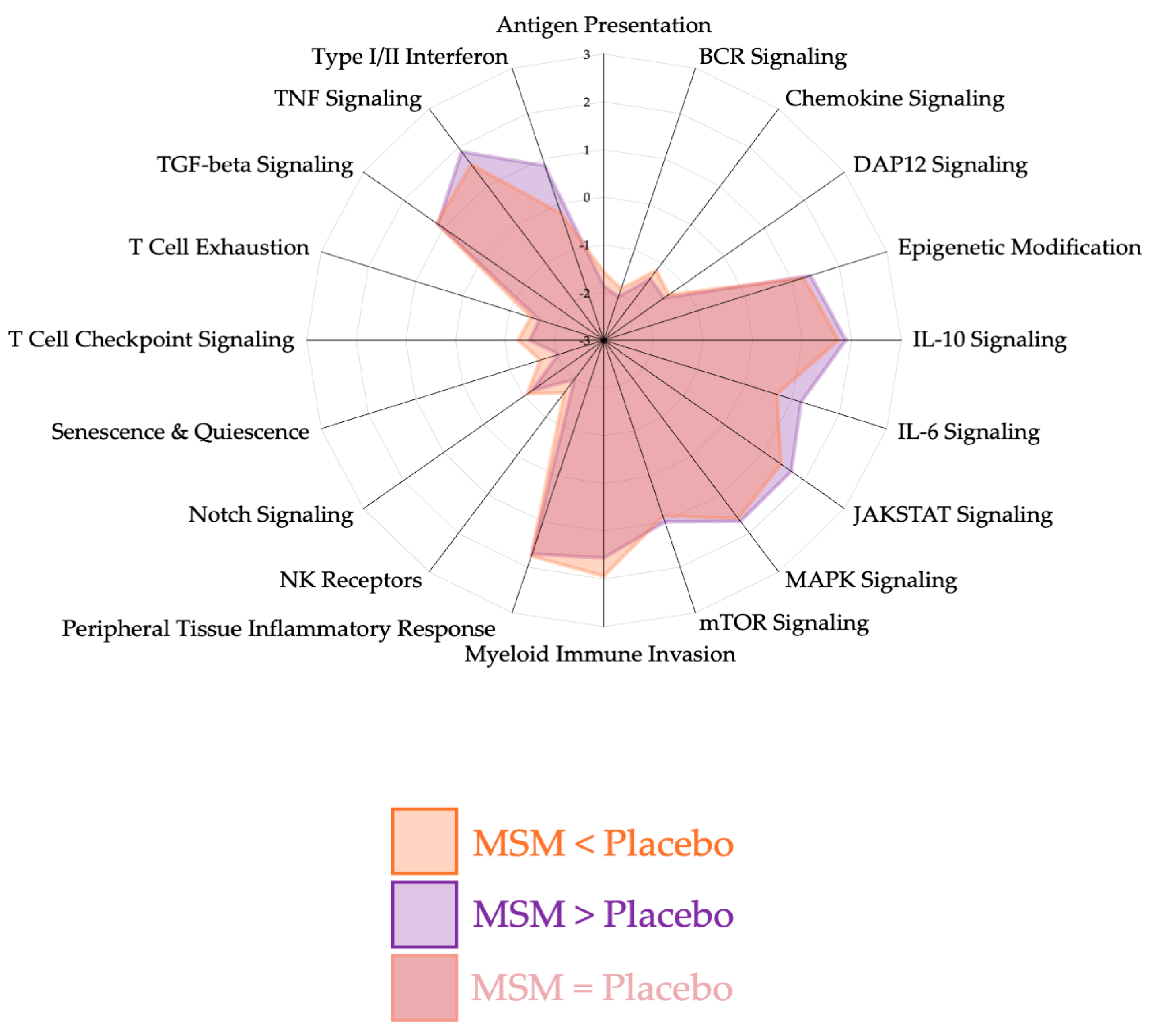
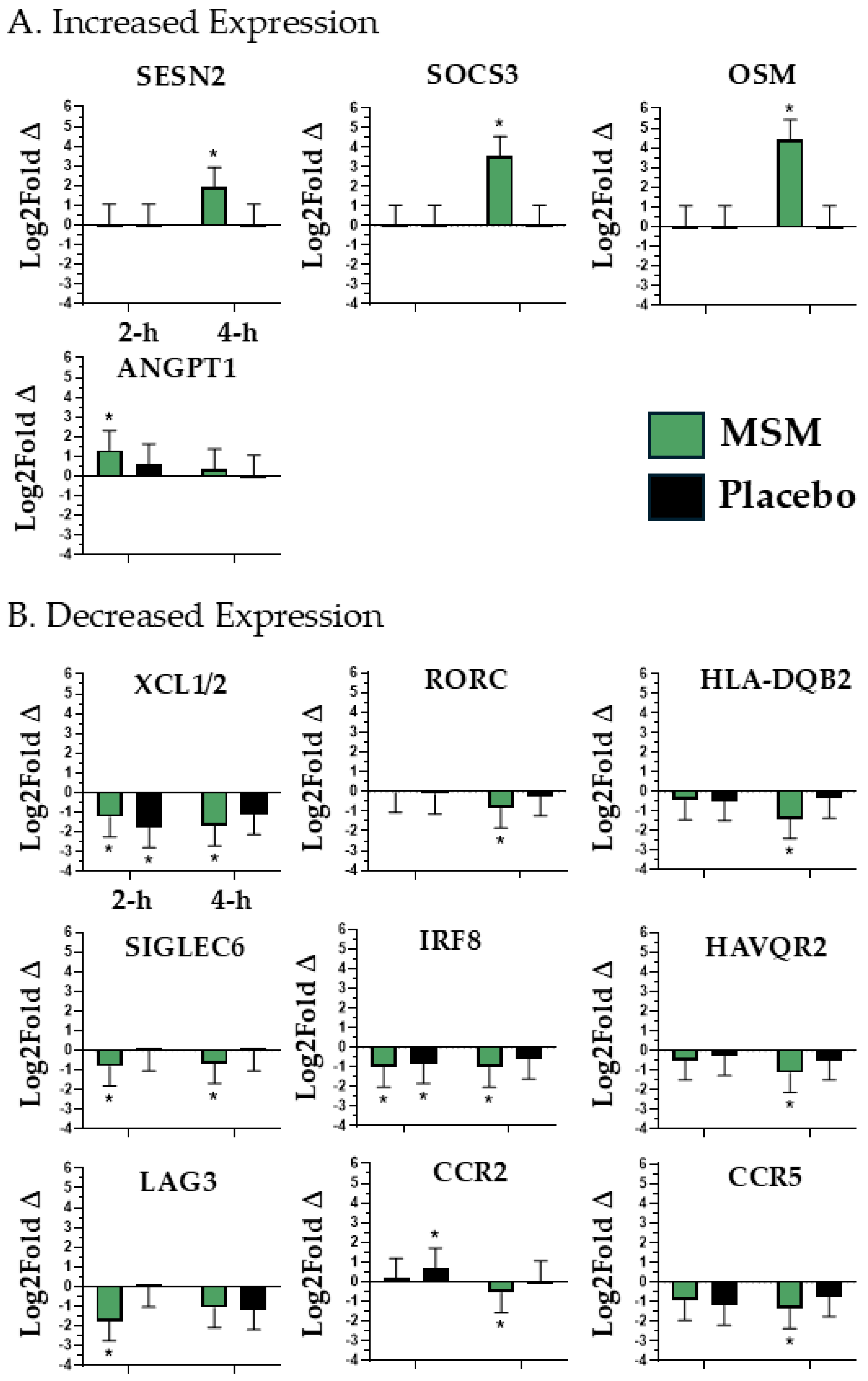
4.1. Peripheral Tissue Inflammatory Response
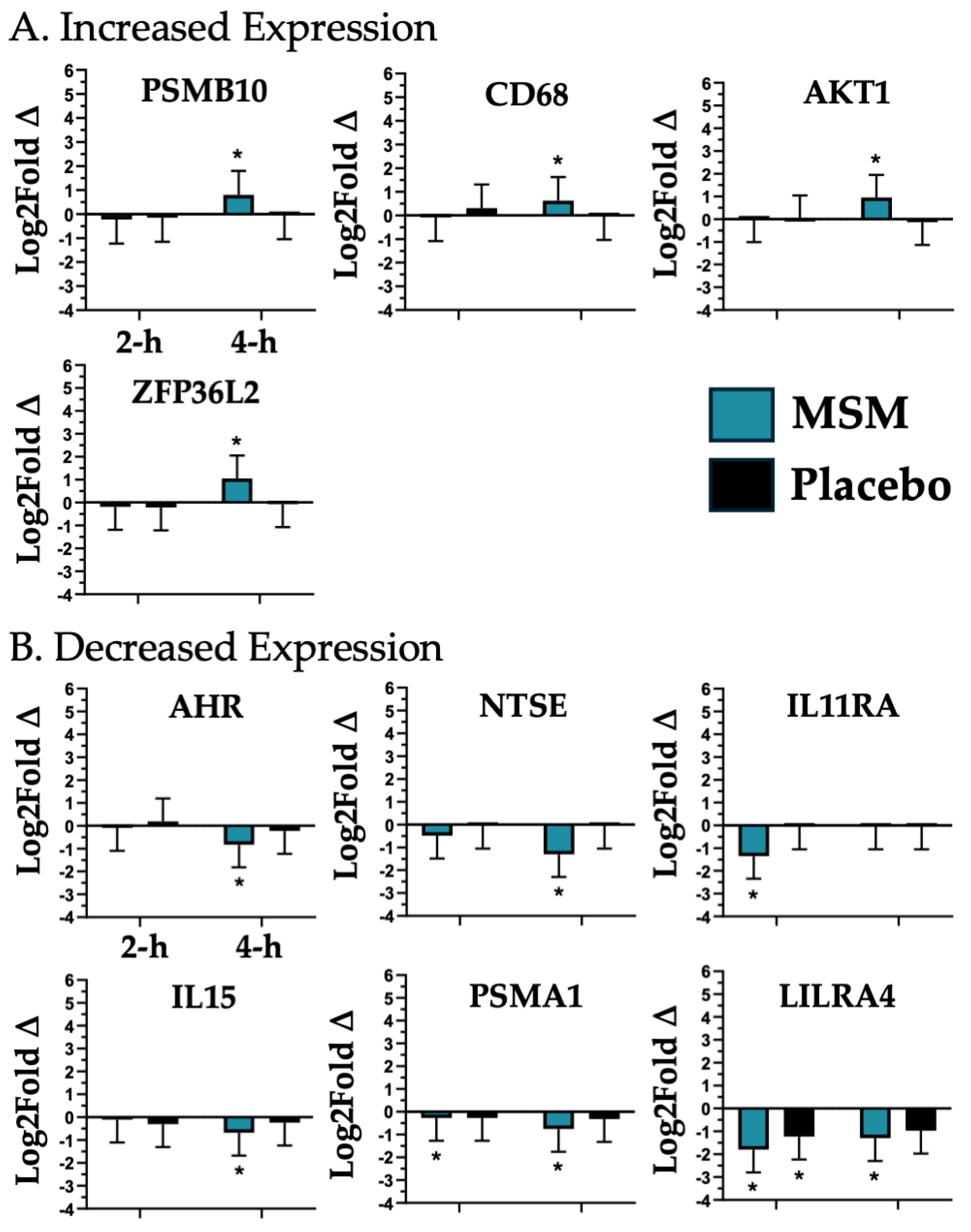
4.2. Myeloid Immune Invasion
4.3. Notch Signaling
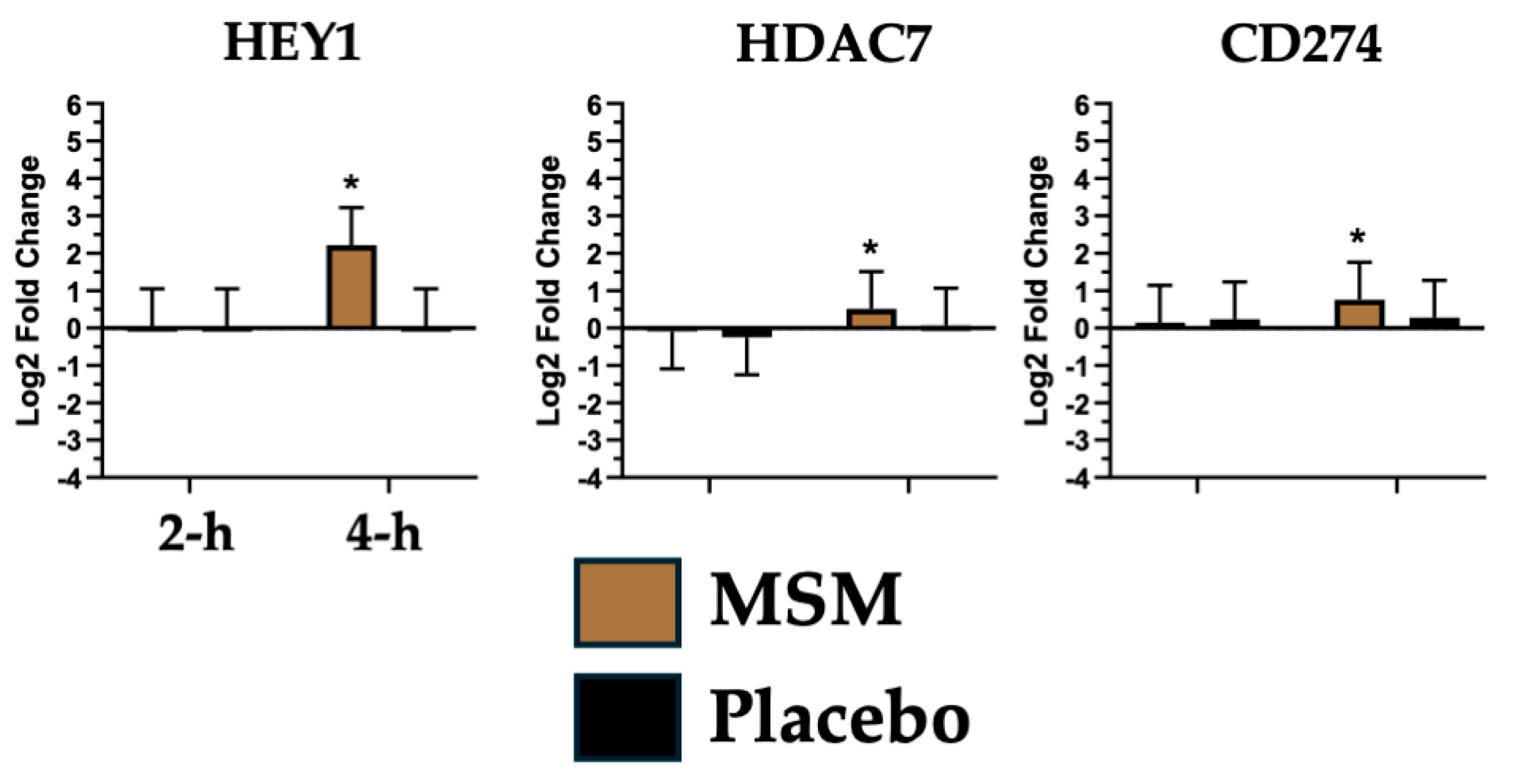
4.4. Natural Killer (NK) Cell Invasion/Activity

5. Study Limitations and Future Opportunities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHR | Aryl hydrocarbon receptor; |
| AKT1 | AKT serine/threonine kinase 1; |
| ANGPT1 | Angiopoietin 1; |
| CCR2 | C-C motif chemokine receptor 2; |
| CCR5 | C-C motif chemokine receptor 5; |
| CD274 | CD274 molecule; |
| CD68 | CD68 molecule; |
| DAMP | Damage-Associated Molecular Pattern; |
| HAVCR2 | Hepatitis A virus cellular receptor 2; |
| HDAC7 | Histone deacetylase 7; |
| HEY1 | Hes-related family bHLH transcription factor with YRPW motif 1; |
| HLA-DQB2 | Major histocompatibility complex, class II, DQ beta 2; |
| IL11RA | Interleukin 11 receptor subunit alpha; |
| IL15 | Interleukin 15; |
| IRB | Institutional Review Board; |
| IRF8 | Interferon regulatory factor 8; |
| KIR2DL3/4 | Killer cell immunoglobulin-like receptor, two Ig domains and long cytoplasmic tail ¾; |
| KLRC1 | Killer cell lectin-like receptor C1; |
| LAG3 | Lymphocyte activation gene-3; |
| LILRA4 | Leukocyte immunoglobulin-like receptor A4; |
| LPS | Lipopolysaccharide; |
| MSM | Methylsulfonylmethane; |
| NCR3 | Natural cytotoxicity triggering receptor 3; |
| NT5E | Interferon regulatory factor 8 5′-nucleotidase ecto; |
| OSM | Oncostatin M; |
| PAMP | Pathogen-Associated Molecular Pattern; |
| PSMA1 | Proteasome 20S subunit alpha 1; |
| PSMB10 | Proteasome 20S subunit beta 10; |
| RORC | RAR-related orphan receptor C; |
| SESN2 | Sestrin 2; |
| SIGLEC6 | Sialic acid-binding Ig-like lectin 6; |
| SOCS3 | Suppressor of cytokine signaling 3; |
| XCL1/2 | Lymphotactin; |
| ZFP36L2 | ZFP36 ring finger protein like 2 |
References
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Evans, R.M. Exercise Mimetics: Impact on Health and Performance. Cell Metab. 2017, 25, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.A.; Gary, M.A.; Michalik, S.; Davis, A.A.; McFarlin, B.K. Optimized Curcumin, Pomegranate Extract, and Methylsulfonylmethane Reduce Acute, Systemic Inflammatory Response to a Half-Marathon Race. Altern. Ther. Health Med. 2022, 28, 72–81. [Google Scholar] [PubMed]
- McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Sampson, J.N.B.; Pennel, K.; Vingren, J.L.; Hill, D.W. Reduced Inflammatory and Muscle Damage Biomarkers Following Oral Supplementation with Bioavailable Curcumin. BBA Clin. 2016, 5, 72–78. [Google Scholar] [CrossRef]
- Akpinar, K.; Simsek, E.K.; Ozen, O.I.; Haberal, B. The Effect of Msm in the Treatment of Ankle Arthrosis: Is Msm as Effective as Methylprednisolone or Hyaluronic Acid? J. Orthop. Res. 2024, 42, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Beder, M.; Yemenoglu, H.; Bostan, S.A.; Kose, O.; Karakas, S.M.; Mercantepe, T.; Yılmaz, A.; Tumkaya, L. Investigation of the Preventive Effect of Methylsulfonylmethane on Alveolar Bone Loss and Oxidative Stress in a Rat Model of Periodontitis. BMC Oral Health 2025, 25, 78. [Google Scholar] [CrossRef]
- Toguchi, A.; Noguchi, N.; Kanno, T.; Yamada, A. Methylsulfonylmethane Improves Knee Quality of Life in Participants with Mild Knee Pain: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 2995. [Google Scholar] [CrossRef]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary Supplements for Treating Osteoarthritis: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef]
- Miller, L.; Thompson, K.; Pavlenco, C.; Mettu, V.S.; Haverkamp, H.; Skaufel, S.; Basit, A.; Prasad, B.; Larsen, J. The Effect of Daily Methylsulfonylmethane (Msm) Consumption on High-Density Lipoprotein Cholesterol in Healthy Overweight and Obese Adults: A Randomized Controlled Trial. Nutrients 2021, 13, 3620. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Kim, H.D.; Rugamba, A.; Jo, E.S.; Park, J.C.; Bae, S.W.; Lee, J.M.; Jang, K.J. Natural Sulfurs Inhibit Lps-Induced Inflammatory Responses through Nf-Kappab Signaling in Ccd-986sk Skin Fibroblasts. Life 2021, 11, 427. [Google Scholar] [CrossRef]
- Jo, E.S.; Sp, N.; Kang, D.Y.; Rugamba, A.; Kim, I.H.; Bae, S.W.; Liu, Q.; Jang, K.J.; Yang, Y.M. Sulfur Compounds Inhibit High Glucose-Induced Inflammation by Regulating Nf-Kappab Signaling in Human Monocytes. Molecules 2020, 25, 2342. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E. Methylsulfonylmethane Decreases Inflammatory Response to Tumor Necrosis Factor-Alpha in Cardiac Cells. Am. J. Cardiovasc. Dis. 2018, 8, 31–38. [Google Scholar] [PubMed]
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement. Nutrients 2017, 9, 290. [Google Scholar] [CrossRef]
- Withee, E.D.; Tippens, K.M.; Dehen, R.; Tibbitts, D.; Hanes, D.; Zwickey, H. Effects of Methylsulfonylmethane (Msm) on Exercise-Induced Oxidative Stress, Muscle Damage, and Pain Following a Half-Marathon: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 2017, 14, 24. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Hill, D.W.; Vingren, J.L.; Curtis, J.H.; Tanner, E.A. Dietary Polyphenol and Methylsulfonylmethane Supplementation Improves Immune, Damp Signaling, and Inflammatory Responses During Recovery from All-out Running Efforts. Front. Physiol. 2021, 12, 712731. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Vingren, J.L.; Hill, D.W.; Bridgeman, E.A. Msm Supplementation Is Associated with Reduced Inflammation and Improved Innate Immune Response Following in Vitro Lps-Stimulation in Humans after a Bout of Downhill Running. Muscles 2023, 2, 204–217. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Curtis, J.H.; Vingren, J.L.; Hill, D.W.; Bridgeman, E.A. Discovery of Innate Immune Response Mrnas That Are Impacted by Structure-Specific Oral Baker’’s Yeast Beta Glucan Consumption. BioTech 2025, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, B.K.; Sass, T.N.; Bridgeman, E.A. Optimization of Rna Extraction from Dry Blood Spots for Nanostring Analysis. Curr. Protoc. 2023, 3, e708. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Bridgeman, E.A.; Sass, T.N. Magnetic Isolation, Rna Extraction, and Nanostring Analysis of Human Peripheral Blood Leukocytes Subsets. Curr. Protoc. 2023, 3, e703. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Bridgeman, E.A.; Curtis, J.H.; Vingren, J.L.; Hill, D.W. Baker’s Yeast Beta Glucan Supplementation Was Associated with an Improved Innate Immune Mrna Expression Response after Exercise. Methods 2024, 230, 68–79. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Deemer, S.E.; Bridgeman, E.A. Oral Spore-Based Probiotic Supplementation Alters Post-Prandial Expression of Mrna Associated with Gastrointestinal Health. Biomedicines 2024, 12, 2386. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Bridgeman, E.A.; Vingren, J.L.; Hill, D.W. Dry Blood Spot Samples to Monitor Immune-Associated Mrna Expression in Intervention Studies: Impact of Baker’s Yeast Beta Glucan. Methods 2023, 219, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.A.; Gary, M.A.; Davis, A.A.; Michalik, S.; McFarlin, B.K. Alterations in Systemic Inflammatory Response Following a Half-Marathon Race with a Combined Curcumin and Pomegranate Supplement: A Feasibility Study. J. Diet. Suppl. 2021, 18, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Seok, S.J.; Lee, E.S.; Kim, G.T.; Hyun, M.; Lee, J.H.; Chen, S.; Choi, R.; Kim, H.M.; Lee, E.Y.; Chung, C.H. Blockade of Ccl2/Ccr2 Signalling Ameliorates Diabetic Nephropathy in Db/Db Mice. Nephrol. Dial. Transplant. 2013, 28, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.-D.; Yang, X.-P.; Liu, Y.-H.; Shesely, E.G.; Cavasin, M.A.; Kuziel, W.A.; Pagano, P.J.; Carretero, O.A. Role of Inflammation in the Development of Renal Damage and Dysfunction in Angiotensin Ii-Induced Hypertension. Hypertension 2008, 52, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Barlic, J.; Murphy, P.M. Chemokine Regulation of Atherosclerosis. J. Leukoc. Biol. 2007, 82, 226–236. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, D.H.; Park, M.S.; Jung, Y.S.; Hong, J.T. C-C Chemokine Receptor Type 5 Deficiency Exacerbates Alcoholic Fatty Liver Disease through Pro-Inflammatory Cytokines and Chemokines-Induced Hepatic Inflammation. J. Gastroenterol. Hepatol. 2017, 32, 1258–1264. [Google Scholar] [CrossRef]
- Costa, R.M.; Cerqueira, D.M.; Bruder-Nascimento, A.; Alves, J.V.; Awata, W.M.; Singh, S.; Kufner, A.; Prado, D.S.; Johny, E.; Cifuentes-Pagano, E.; et al. Role of the Ccl5 and Its Receptor, Ccr5, in the Genesis of Aldosterone-Induced Hypertension, Vascular Dysfunction, and End-Organ Damage. Hypertension 2024, 81, 776–786. [Google Scholar] [CrossRef]
- O’sullivan, J.A.; Youngblood, B.A.; Schleimer, R.P.; Bochner, B.S. Siglecs as Potential Targets of Therapy in Human Mast Cell- and/or Eosinophil-Associated Diseases. Semin. Immunol. 2023, 69, 101799. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, K.M.; Kim, M.G.; Seo, K.H.; Han, J.Y.; Ka, S.O.; Park, B.H.; Shin, S.M.; Ku, S.K.; Cho, I.J.; et al. Role of Sestrin2 in the Regulation of Proinflammatory Signaling in Macrophages. Free Radic. Biol. Med. 2015, 78, 156–167. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, K.Y.; Kim, S.R.; Park, H.S.; Park, S.J.; Min, K.H.; Cho, C.H.; Koh, G.Y.; Park, H.S.; Lee, Y.C. Blockade of Airway Inflammation and Hyper-Responsiveness by an Angiopoietin-1 Variant, Comp-Ang1. Exp. Mol. Med. 2007, 39, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.; Salem, D.; Gros, P. Role of Irf8 in Immune Cells Functions, Protection against Infections, and Susceptibility to Inflammatory Diseases. Hum. Genet. 2020, 139, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Morikawa, Y. Essential Roles of the Cytokine Oncostatin M in Crosstalk between Muscle Fibers and Immune Cells in Skeletal Muscle after Aerobic Exercise. J. Biol. Chem. 2022, 298, 102686. [Google Scholar] [CrossRef]
- He, W.; Wang, H.; Yang, G.; Zhu, L.; Liu, X. The Role of Chemokines in Obesity and Exercise-Induced Weight Loss. Biomolecules 2024, 14, 1121. [Google Scholar] [CrossRef] [PubMed]
- Leiter, O.; Bernas, S.N.; Seidemann, S.; Overall, R.W.; Horenburg, C.; Kowal, S.; Kempermann, G.; Walker, T.L. The Systemic Exercise-Released Chemokine Lymphotactin/Xcl1 Modulates in Vitro Adult Hippocampal Precursor Cell Proliferation and Neuronal Differentiation. Sci. Rep. 2019, 9, 11831. [Google Scholar] [CrossRef]
- Wang, F.; Mao, Z.; Liu, D.; Yu, J.; Wang, Y.; Ye, W.; Lin, D.; Zhou, N.; Xie, Y. Overexpression of Tim-3 Reduces Helicobacter Pylori-Associated Inflammation through Tlr4/Nfkappab Signaling in Vitro. Mol. Med. Rep. 2017, 15, 3252–3258. [Google Scholar] [CrossRef]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting Edge: Molecular Analysis of the Negative Regulatory Function of Lymphocyte Activation Gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef]
- Babon, J.J.; Varghese, L.N.; Nicola, N.A. Inhibition of Il-6 Family Cytokines by Socs3. Semin. Immunol. 2014, 26, 13–19. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Chothani, S.; Viswanathan, S.; Goh, J.W.T.; Lim, W.-W.; Cook, S.A. Il11 Stimulates Il33 Expression and Proinflammatory Fibroblast Activation across Tissues. Int. J. Mol. Sci. 2022, 23, 8900. [Google Scholar] [CrossRef]
- Nishina, T.; Komazawa-Sakon, S.; Yanaka, S.; Piao, X.; Zheng, D.-M.; Piao, J.-H.; Kojima, Y.; Yamashina, S.; Sano, E.; Putoczki, T.; et al. Interleukin-11 Links Oxidative Stress and Compensatory Proliferation. Sci. Signal. 2012, 5, ra5. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L. Elevated Il-23r Expression and Foxp3+Rorgt+ Cells in Intestinal Mucosa During Acute and Chronic Colitis. Med. Sci. Monit. 2016, 22, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Lenormand, C.; Bausinger, H.; Gross, F.; Signorino-Gelo, F.; Koch, S.; Peressin, M.; Fricker, D.; Cazenave, J.-P.; Bieber, T.; Hanau, D.; et al. Hla-Dqa2 and Hla-Dqb2 Genes Are Specifically Expressed in Human Langerhans Cells and Encode a New Hla Class Ii Molecule. J. Immunol. 2012, 188, 3903–3911. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V. Cytokines Pre-Determined by Genetic Factors Are Involved in Pathogenesis of Rheumatoid Arthritis. Cytokine 2015, 75, 216–221. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Fernández-Hernando, C.; Cirino, G.; Sessa, W.C. Akt1 Is Critical for Acute Inflammation and Histamine-Mediated Vascular Leakage. Proc. Natl. Acad. Sci. USA 2009, 106, 14552–14557. [Google Scholar] [CrossRef]
- Zhu, K.; Meng, Q.; Zhang, Z.; Yi, T.; He, Y.; Zheng, J.; Lei, W. Aryl Hydrocarbon Receptor Pathway: Role, Regulation and Intervention in Atherosclerosis Therapy (Review). Mol. Med. Rep. 2019, 20, 4763–4773. [Google Scholar] [CrossRef]
- Guarnieri, T.; Abruzzo, P.M.; Bolotta, A. More Than a Cell Biosensor: Aryl Hydrocarbon Receptor at the Intersection of Physiology and Inflammation. Am. J. Physiol. Cell Physiol. 2020, 318, C1078–C1082. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Antioxidative Phytochemicals Accelerate Epidermal Terminal Differentiation Via the Ahr-Ovol1 Pathway: Implications for Atopic Dermatitis. Acta Derm. Venereol. 2018, 98, 918–923. [Google Scholar] [CrossRef] [PubMed]
- King, R.J.; Shukla, S.K.; He, C.; Vernucci, E.; Thakur, R.; Attri, K.S.; Dasgupta, A.; Chaika, N.V.; Mulder, S.E.; Abrego, J.; et al. Cd73 Induces Gm-Csf/Mdsc-Mediated Suppression of T Cells to Accelerate Pancreatic Cancer Pathogenesis. Oncogene 2022, 41, 971–982. [Google Scholar] [CrossRef]
- Fraternale, A.; Green, K.A.; Schiavano, G.F.; Bruschi, M.; Retini, M.; Magnani, M.; Green, W.R. Inhibition of Myeloid-Derived Suppressor Cell (Mdsc) Activity by Redox-Modulating Agents Restores T and B Cell Proliferative Responses in Murine Aids. Int. Immunopharmacol. 2023, 124, 110882. [Google Scholar] [CrossRef]
- Sumida, K.; Ohno, Y.; Ohtake, J.; Kaneumi, S.; Kishikawa, T.; Takahashi, N.; Taketomi, A.; Kitamura, H. Il-11 Induces Differentiation of Myeloid-Derived Suppressor Cells through Activation of Stat3 Signalling Pathway. Sci. Rep. 2015, 5, 13650. [Google Scholar] [CrossRef]
- Skariah, N.; James, O.J.; Swamy, M. Signalling Mechanisms Driving Homeostatic and Inflammatory Effects of Interleukin-15 on Tissue Lymphocytes. Discov. Immunol. 2024, 3, kyae002. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. Damp-Sensing Receptors in Sterile Inflammation and Inflammatory Diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Bai, J.; Yang, X.-L.; Zhang, Y.-L.; Che, Y.-L.; Li, H.-H.; Yang, Y.-Z. Novel Role for the Immunoproteasome Subunit Psmb10 in Angiotensin Ii-Induced Atrial Fibrillation in Mice. Hypertension 2018, 71, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Kuramochi, M.; Golbar, H.M.; Izawa, T.; Kuwamura, M.; Yamate, J. Acetaminophen-Induced Rat Hepatotoxicity Based on M1/M2-Macrophage Polarization, in Possible Relation to Damage-Associated Molecular Patterns and Autophagy. Int. J. Mol. Sci. 2020, 21, 8998. [Google Scholar] [CrossRef]
- Makita, S.; Takatori, H.; Nakajima, H. Post-Transcriptional Regulation of Immune Responses and Inflammatory Diseases by Rna-Binding Zfp36 Family Proteins. Front. Immunol. 2021, 12, 711633. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y.; Li, P. Novel Insights into Notch Signaling in Tumor Immunity: Potential Targets for Cancer Immunotherapy. Front. Immunol. 2024, 15, 1352484. [Google Scholar] [CrossRef]
- Bubak, M.P.; Stout, K.; Tomtschik, J.; Peterson, E.; Cardozo, C.P.; Graham, Z.A.; Gallagher, P. Notch, Numb and Numb-Like Responses to Exercise-Induced Muscle Damage in Human Skeletal Muscle. Exp. Physiol. 2022, 107, 800–806. [Google Scholar] [CrossRef]
- Pinto, A.P.; Sarni, A.A.J.; Tavares, M.E.A.; da Rocha, A.L.; Carolino, R.O.G.; Neto, I.V.d.S.; Ferreira, D.C.D.S.; Munoz, V.R.; Teixeira, G.R.; Simabuco, F.M.; et al. Combined Exercise-Induced Modulation of Notch Pathway and Muscle Quality in Senescence-Accelerated Mice. Pflugers Arch. 2025, 477, 393–405. [Google Scholar] [CrossRef]
- Chen, D.; Guo, Y.; Zhang, M.; Liu, X.; Zhang, B.; Kou, X. Exercise Alleviates Cognitive Decline of Natural Aging Rats by Upregulating Notch-Mediated Autophagy Signaling. Brain Res. 2025, 1850, 149398. [Google Scholar] [CrossRef]
- Das Gupta, K.; Ramnath, D.; von Pein, J.B.; Curson, J.E.B.; Wang, Y.; Abrol, R.; Kakkanat, A.; Moradi, S.V.; Gunther, K.S.; Murthy, A.M.V.; et al. Hdac7 Is an Immunometabolic Switch Triaging Danger Signals for Engagement of Antimicrobial Versus Inflammatory Responses in Macrophages. Proc. Natl. Acad. Sci. USA 2023, 120, e2212813120. [Google Scholar] [CrossRef]
- Wu, Y.; Li, K.; Liang, S.; Lou, X.; Li, Y.; Xu, D.; Wu, Y.; Wang, Y.; Cui, W. An Icd-Associated Damp Gene Signature Predicts Survival and Immunotherapy Response of Patients with Lung Adenocarcinoma. Respir. Res. 2023, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ma, C.; Wei, B.; Aziz, N.; Rajalingam, R.; Yusung, S.; Erlich, H.A.; Trachtenberg, E.A.; Targan, S.R.; McGovern, D.P.; et al. Human Nk Cells Licensed by Killer Ig Receptor Genes Have an Altered Cytokine Program That Modifies Cd4+ T Cell Function. J. Immunol. 2014, 193, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Pazina, T.; Shemesh, A.; Brusilovsky, M.; Porgador, A.; Campbell, K.S. Regulation of the Functions of Natural Cytotoxicity Receptors by Interactions with Diverse Ligands and Alterations in Splice Variant Expression. Front. Immunol. 2017, 8, 369. [Google Scholar] [CrossRef] [PubMed]
| mRNA Biomarker | Pathway Response | Possible Health Outcome |
|---|---|---|
| ANGPT1, CCR2, CCR5, IRF8, OSM, SOCS3, LAG3, SIGLEC6, XCL1/2, RORC, HAVCR2, HLA-DQB2, SESN2 | Peripheral Tissue Inflammatory Response |
|
| AKT1, AHR, CD68, NT5E, IL11RA, PSMA1, IL15, ZFP36L2, and PSMB10 | Myeloid Immune Invasion |
|
| KIR2DL3/4, KLRC1 and NCR3 | Natural Killer (NK) Cell Invasion/Activity |
|
| HDAC7, CD274 and HEY1 | Notch Signaling |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McFarlin, B.K.; Curtis, J.H.; du Preez, H.N.; McFarlin, M.A. Using the Rise and Fall of Oxidative Stress and Inflammation Post-Exercise to Evaluate the Effect of Methylsulfonylmethane Supplementation on Immune Response mRNA. Nutrients 2025, 17, 1761. https://doi.org/10.3390/nu17111761
McFarlin BK, Curtis JH, du Preez HN, McFarlin MA. Using the Rise and Fall of Oxidative Stress and Inflammation Post-Exercise to Evaluate the Effect of Methylsulfonylmethane Supplementation on Immune Response mRNA. Nutrients. 2025; 17(11):1761. https://doi.org/10.3390/nu17111761
Chicago/Turabian StyleMcFarlin, Brian K., John H. Curtis, Heidi N. du Preez, and Meredith A. McFarlin. 2025. "Using the Rise and Fall of Oxidative Stress and Inflammation Post-Exercise to Evaluate the Effect of Methylsulfonylmethane Supplementation on Immune Response mRNA" Nutrients 17, no. 11: 1761. https://doi.org/10.3390/nu17111761
APA StyleMcFarlin, B. K., Curtis, J. H., du Preez, H. N., & McFarlin, M. A. (2025). Using the Rise and Fall of Oxidative Stress and Inflammation Post-Exercise to Evaluate the Effect of Methylsulfonylmethane Supplementation on Immune Response mRNA. Nutrients, 17(11), 1761. https://doi.org/10.3390/nu17111761





