Impact of Mediterranean Diet Adherence During Pregnancy on Preeclampsia, Gestational Diabetes Mellitus, and Excessive Gestational Weight Gain: A Systematic Review of Observational Studies and Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria, Information Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Definitions of Outcomes
2.5. Assessment of Risk of Bias
3. Results
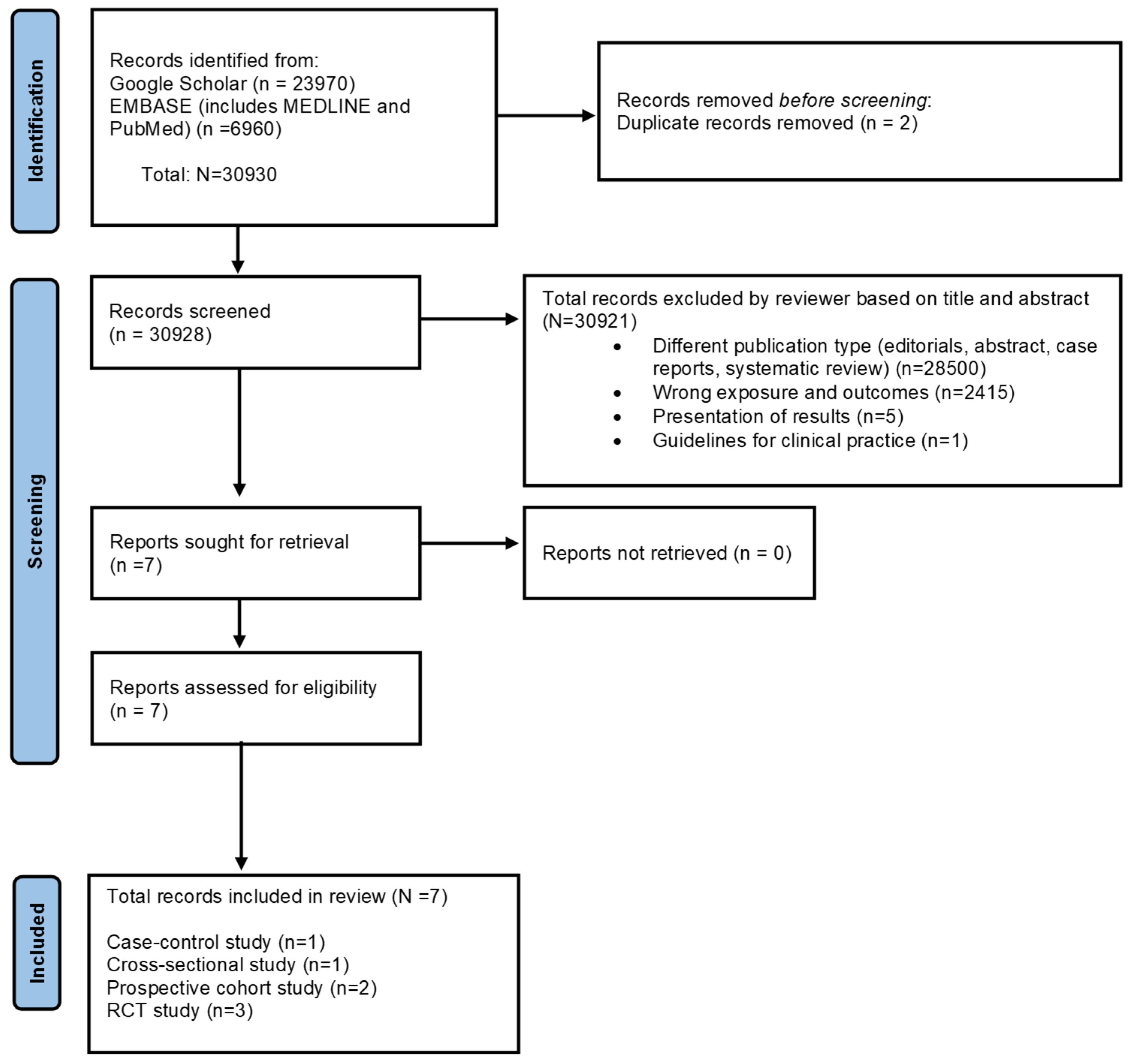
3.1. Study Selection Findings
3.2. Characteristics of the Included Studies
3.3. Results of the Included Studies
3.4. Risk of Bias in Included Studies
4. Discussion
4.1. Comparison with the Existing Literature
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MD | Mediterranean diet |
| BMI | Body mass index |
| eGWG | Excessive gestational weight gain |
| GDM | Gestational diabetes mellitus |
| OR | Odds ratio |
References
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89. [Google Scholar] [CrossRef]
- Mockridge, A.; Maclennan, K. Physiology of pregnancy. Anaesth. Intensive Care Med. 2022, 23, 347–351. [Google Scholar] [CrossRef]
- Abdollahi, S.; Soltani, S.; de Souza, R.J.; Forbes, S.C.; Toupchian, O.; Salehi-Abargouei, A. Associations between Maternal Dietary Patterns and Perinatal Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. Adv. Nutr. Int. Rev. J. 2021, 12, 1332–1352. [Google Scholar] [CrossRef]
- Chia, A.-R.; Chen, L.-W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F.-F. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2019, 10, 685–695. [Google Scholar] [CrossRef]
- Paknahad, Z.; Fallah, A.; Moravejolahkami, A.R. Maternal Dietary Patterns and Their Association with Pregnancy Outcomes. Clin. Nutr. Res. 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.; McAuliffe, F.M. Impact of maternal nutrition on pregnancy outcome–does it matter what pregnant women eat? Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Rottenstreich, A.; Elazary, R.; Goldenshluger, A.; Pikarsky, A.J.; Elchalal, U.; Ben-Porat, T. Maternal nutritional status and related pregnancy outcomes following bariatric surgery: A systematic review. Surg. Obes. Relat. Dis. 2019, 15, 324–332. [Google Scholar] [CrossRef]
- Ahmed, F.; Tseng, M. Diet and nutritional status during pregnancy. Public Health Nutr. 2013, 16, 1337. [Google Scholar] [CrossRef]
- Ciulei, M.A.; Smith, E.R.; Perumal, N.; Jakazi, C.S.; Sudfeld, C.R.; Gernand, A.D. Nutritious Supplemental Foods for Pregnant Women from Food Insecure Settings: Types, Nutritional Composition, and Relationships to Health Outcomes. Curr. Dev. Nutr. 2023, 7, 100094. [Google Scholar] [CrossRef]
- Brough, L.; Rees, G.A.; Crawford, M.A.; Morton, R.H.; Dorman, E.K. Effect of multiple-micronutrient supplementation on maternal nutrient status, infant birth weight and gestational age at birth in a low-income, multi-ethnic population. Br. J. Nutr. 2010, 104, 437–445. [Google Scholar] [CrossRef]
- Oh, C.; Keats, E.C.; Bhutta, Z.A. Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 491. [Google Scholar] [CrossRef]
- de Seymour, J.V.; Beck, K.L.; Conlon, C.A.; Jones, M.B.; Colombo, J.; Xia, Y.Y.; Baker, P.N. An Investigation of the Relationship Between Dietary Patterns in Early Pregnancy and Maternal/Infant Health Outcomes in a Chinese Cohort. Front. Nutr. 2022, 9, 775557. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Bhutta, Z.A. Effect of balanced protein energy supplementation during pregnancy on birth outcomes. BMC Public Health 2011, 11, S17. [Google Scholar] [CrossRef] [PubMed]
- Liberato, S.C.; Singh, G.; Mulholland, K. Effects of protein energy supplementation during pregnancy on fetal growth: A review of the literature focusing on contextual factors. Food Nutr. Res. 2013, 57, 20499. [Google Scholar] [CrossRef]
- de Kok, B.; Toe, L.C.; Hanley-Cook, G.; Argaw, A.; Ouédraogo, M.; Compaoré, A.; Lachat, C. Prenatal fortified balanced energy-protein supplementation and birth outcomes in rural Burkina Faso: A randomized controlled efficacy trial. PLoS Med. 2022, 19, e1004002. [Google Scholar] [CrossRef]
- Stevens, B.; Buettner, P.; Watt, K.; Clough, A.; Brimblecombe, J.; Judd, J. The effect of balanced protein energy supplementation in undernourished pregnant women and child physical growth in low- and middle-income countries: A systematic review and meta-analysis. Matern. Child. Nutr. 2015, 11, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Paula, W.O.; Patriota, E.S.O.; Gonçalves, V.S.S.; Pizato, N. Maternal Consumption of Ultra-Processed Foods-Rich Diet and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3242. [Google Scholar] [CrossRef]
- Costanza, J.; Camanni, M.; Ferrari, M.M.; De Cosmi, V.; Tabano, S.; Fontana, L.; Radaelli, T.; Privitera, G.; Alberico, D.; Colapietro, P.; et al. Assessment of pregnancy dietary intake and association with maternal and neonatal outcomes. Pediatr. Res. 2021, 91, 1890–1896. [Google Scholar] [CrossRef]
- Chen, X.; Maguire, B.; Brodaty, H.; O’leary, F. Dietary Patterns and Cognitive Health in Older Adults: A Systematic Review. J. Alzheimer’s Dis. 2019, 67, 583–619. [Google Scholar] [CrossRef]
- Papandreou, P.; Amerikanou, C.; Vezou, C.; Gioxari, A.; Kaliora, A.C.; Skouroliakou, M. Improving Adherence to the Mediterranean Diet in Early Pregnancy Using a Clinical Decision Support System; A Randomised Controlled Clinical Trial. Nutrients 2023, 15, 432. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, D.; Mao, X.; Xia, Y.; Baker, P.N.; Zhang, H. Maternal Dietary Patterns and Pregnancy Outcome. Nutrients 2016, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Schulze, M.B.; Solomon, C.G.; Hu, F.B. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006, 49, 2604–2613. [Google Scholar] [CrossRef]
- Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: Results from an Australian population-based prospective cohort study. Diabetologia 2015, 58, 2726–2735. [Google Scholar] [CrossRef] [PubMed]
- Brantsæter, A.L.; Haugen, M.; Samuelsen, S.O.; Torjusen, H.; Trogstad, L.; Alexander, J.; Magnus, P.; Meltzer, H.M. A Dietary Pattern Characterized by High Intake of Vegetables, Fruits, and Vegetable Oils Is Associated with Reduced Risk of Preeclampsia in Nulliparous Pregnant Norwegian Women. J. Nutr. 2009, 139, 1162–1168. [Google Scholar] [CrossRef]
- Rasmussen, M.A.; Maslova, E.; Halldorsson, T.I.; Olsen, S.F. Characterization of Dietary Patterns in the Danish National Birth Cohort in Relation to Preterm Birth. PLoS ONE 2014, 9, e93644. [Google Scholar] [CrossRef] [PubMed]
- Englund-Ögge, L.; Brantsaeter, A.L.; Sengpiel, V.; Haugen, M.; Birgisdottir, B.E.; Myhre, R.; Meltzer, H.M.; Jacobsson, B.; Brantsæter, A.L. Maternal dietary patterns and preterm delivery: Results from large prospective cohort study. BMJ 2014, 348, g1446. [Google Scholar] [CrossRef]
- Timmermans, S.; Steegers-Theunissen, R.P.; Vujkovic, M.; Bakker, R.; Breeijen, H.D.; Raat, H.; Russcher, H.; Lindemans, J.; Hofman, A.; Jaddoe, V.W.; et al. Major dietary patterns and blood pressure patterns during pregnancy: The Generation R Study. Am. J. Obstet. Gynecol. 2011, 205, 337.e1–337.e12. [Google Scholar] [CrossRef]
- Tryggvadottir, E.A.; Medek, H.; Birgisdottir, B.E.; Geirsson, R.T.; Gunnarsdottir, I. Association between healthy maternal dietary pattern and risk for gestational diabetes mellitus. Eur. J. Clin. Nutr. 2016, 70, 237–242. [Google Scholar] [CrossRef]
- De Lorgeril, M. Mediterranean diet and cardiovascular disease: Historical perspective and latest evidence. Curr. Atheroscler. Rep. 2013, 15, 370. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Eng. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Morales-Suárez-Varela, M.; Peraita-Costa, I.; Perales-Marín, A.; Puig, B.M.; Llopis-Morales, J.; Picó, Y. Effect of Adherence to the Mediterranean Diet on Maternal Iron Related Biochemical Parameters during Pregnancy and Gestational Weight Gain. Life 2023, 13, 1138. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive Oil and Red Wine Antioxidant Polyphenols Inhibit Endothelial Activation. Arter. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef]
- Augimeri, G.; Galluccio, A.; Caparello, G.; Avolio, E.; La Russa, D.; De Rose, D.; Morelli, C.; Barone, I.; Catalano, S.; Andò, S.; et al. Potential Antioxidant and Anti-Inflammatory Properties of Serum from Healthy Adolescents with Optimal Mediterranean Diet Adherence: Findings from DIMENU Cross-Sectional Study. Antioxidants 2021, 10, 1172. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Bian, J.; Xu, M.; Jiang, N.; Luo, W.; Zu, P.; Yin, W.; Zhu, P. Association between the Maternal Mediterranean Diet and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2024, 15, 100159. [Google Scholar] [CrossRef]
- Zaragoza-Martí, A.; Ruiz-Ródenas, N.; Herranz-Chofre, I.; Sánchez-SanSegundo, M.; Delgado, V.d.l.C.S.; Hurtado-Sánchez, J.A. Adherence to the Mediterranean Diet in Pregnancy and Its Benefits on Maternal-Fetal Health: A Systematic Review of the Literature. Front. Nutr. 2022, 9, 813942. [Google Scholar] [CrossRef]
- Miller, C.B.; Benny, P.; Riel, J.; Boushey, C.; Perez, R.; Khadka, V.; Qin, Y.; Maunakea, A.K.; Lee, M.-J. Adherence to Mediterranean diet impacts gastrointestinal microbial diversity throughout pregnancy. BMC Pregnancy Childbirth 2021, 21, 558. [Google Scholar] [CrossRef]
- Di Renzo, L.; Marchetti, M.; Rizzo, G.; Gualtieri, P.; Monsignore, D.; Dominici, F.; Mappa, I.; Cavicchioni, O.; Aguzzoli, L.; De Lorenzo, A.; et al. Adherence to Mediterranean Diet and Its Association with Maternal and Newborn Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 8497. [Google Scholar] [CrossRef]
- Khan, B.; Yar, R.A.; Khakwani, A.K.; Karim, S.; Ali, H.A. Preeclampsia Incidence and Its Maternal and Neonatal Outcomes With Associated Risk Factors. Cureus 2022, 14, e31143. [Google Scholar] [CrossRef]
- Brown, J.; Kapurubandara, S.; McGee, T.M. Confounding effect of ethnic diversity on booking-in body mass index and prevalence of gestational diabetes and hypertensive disorders in pregnant women in western Sydney 1997–2016. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 369–375. [Google Scholar] [CrossRef]
- Goławski, K.; Giermaziak, W.; Ciebiera, M.; Wojtyła, C. Excessive Gestational Weight Gain and Pregnancy Outcomes. J. Clin. Med. 2023, 12, 3211. [Google Scholar] [CrossRef]
- National Institute of Child Health and Human Development. NICHD—Eunice Kennedy Shriver National Institute of Child Health and Human Development. What Are Some Common Complications of Pregnancy? 2021. Available online: https://www.nichd.nih.gov/health/topics/pregnancy/conditioninfo/complications (accessed on 1 March 2024).
- Pregnancy Complications|Maternal and Infant Health|CDC. 2023. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications.html (accessed on 1 February 2024).
- Simko, M.; Totka, A.; Vondrova, D.; Samohyl, M.; Jurkovicova, J.; Trnka, M.; Cibulkova, A.; Stofko, J.; Argalasova, L. Maternal Body Mass Index and Gestational Weight Gain and Their Association with Pregnancy Complications and Perinatal Conditions. Int. J. Environ. Res. Public Health 2019, 16, 1751. [Google Scholar] [CrossRef]
- Mazumder, T.; Akter, E.; Rahman, S.M.; Islam, T.; Talukder, M.R. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Bangladesh: Findings from Demographic Health Survey 2017–2018. Int. J. Environ. Res. Public Health 2022, 19, 2583. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, N. Gestational Diabetes Mellitus and Preeclampsia: Correlation and Influencing Factors. Front. Cardiovasc. Med. 2022, 9, 831297. [Google Scholar] [CrossRef]
- Lee, S.I.; Hope, H.; O’reilly, D.; Kent, L.; Santorelli, G.; Subramanian, A.; Moss, N.; Azcoaga-Lorenzo, A.; Fagbamigbe, A.F.; Nelson-Piercy, C.; et al. Maternal and child outcomes for pregnant women with pre-existing multiple long-term conditions: Protocol for an observational study in the UK. BMJ Open 2023, 13, e068718. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Ganeshkumar, P. Systematic Reviews and Meta-analysis: Understanding the Best Evidence in Primary Healthcare. J. Fam. Med. Prim. Care 2013, 2, 9. [Google Scholar]
- Vitolins, M.Z.; Case, T.L. What Makes Nutrition Research So Difficult to Conduct and Interpret? Diabetes Spectr. 2020, 33, 113. [Google Scholar] [CrossRef]
- Inskip, H.M.; Crozier, S.R.; Godfrey, K.M.; Borland, S.E.; Cooper, C.; Robinson, S.M. Women’s compliance with nutrition and lifestyle recommendations before pregnancy: General population cohort study. BMJ 2009, 338, 586–589. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Antasouras, G.; Papadopoulou, S.K.; Alexatou, O.; Papandreou, D.; Mentzelou, M.; Migdanis, A.; Giaginis, C. Adherence to the Mediterranean Diet during Pregnancy: Associations with Sociodemographic and Anthropometric Parameters, Perinatal Outcomes, and Breastfeeding Practices. Medicina 2023, 59, 1547. [Google Scholar] [CrossRef]
- Li, M.; Grewal, J.; Hinkle, S.N.; Yisahak, S.F.; Grobman, W.A.; Newman, R.B.; Skupski, D.W.; Chien, E.K.; Wing, D.A.; Grantz, K.L.; et al. Healthy dietary patterns and common pregnancy complications: A prospective and longitudinal study. Am. J. Clin. Nutr. 2021, 114, 1229–1237. [Google Scholar] [CrossRef]
- Minhas, A.S.; Hong, X.; Wang, G.; Rhee, D.K.; Liu, T.; Zhang, M.; Michos, E.D.; Wang, X.; Mueller, N.T. Mediterranean-Style Diet and Risk of Preeclampsia by Race in the Boston Birth Cohort. J. Am. Heart Assoc. 2022, 11, e022589. [Google Scholar] [CrossRef]
- Izadi, V.; Tehrani, H.; Haghighatdoost, F.; Dehghan, A.; Surkan, P.J.; Azadbakht, L. Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition 2016, 32, 1092–1096. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; Garcia De La Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- García de la Torre, N.; Assaf-Balut, C.; Jiménez Varas, I.; Del Valle, L.; Durán, A.; Fuentes, M.; Calle-Pascual, A.L. Effectiveness of Following Mediterranean Diet Recommendations in the Real World in the Incidence of Gestational Diabetes Mellitus (GDM) and Adverse Maternal-Foetal Outcomes: A Prospective, Universal, Interventional Study with a Single Group. The St Carlos Study. Nutrients 2019, 11, 1210. [Google Scholar] [CrossRef]
- Melero, V.; de la Torre, N.G.; Assaf-Balut, C.; Jiménez, I.; del Valle, L.; Durán, A.; Bordiú, E.; Valerio, J.J.; Herraiz, M.A.; Izquierdo, N.; et al. Effect of a Mediterranean Diet-Based Nutritional Intervention on the Risk of Developing Gestational Diabetes Mellitus and Other Maternal-Fetal Adverse Events in Hispanic Women Residents in Spain. Nutrients 2020, 12, 3505. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, E237–E260. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2011, 34, S62–S69. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.d.; Hod, M.; et al. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676. [Google Scholar] [CrossRef]
- Liao, L.-Z.; Xu, Y.; Zhuang, X.-D.; Hong, S.-B.; Wang, Z.-L.; Sandra, D.A.; Liu, B. Evaluation of guidelines on the screening and diagnosis of gestational diabetes mellitus: Systematic review. BMJ Open 2019, 9, e023014. [Google Scholar] [CrossRef]
- Rasmussen, K.; Yaktine, A. Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Martínez-Hortelano, J.A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Garrido-Miguel, M.; Soriano-Cano, A.; Martínez-Vizcaíno, V. Monitoring gestational weight gain and prepregnancy BMI using the 2009 IOM guidelines in the global population: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 649. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 April 2023).
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- Roberts, J.M.; Lain, K.Y. Recent insights into the pathogenesis of pre-eclampsia. Placenta 2002, 23, 359–372. [Google Scholar] [CrossRef]
- Al Wattar, B.H.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Carreras, F.J.G.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef]
- Willett, W. Commentary: Dietary diaries versus food frequency questionnaires—A case of undigestible data. Int. J. Epidemiol. 2001, 30, 317–319. [Google Scholar] [CrossRef]
- Day, N.; McKeown, N.; Wong, M.; Welch, A.; Bingham, S. Epidemiological assessment of diet: A comparison of a 7-day diary with a food frequency questionnaire using urinary markers of nitrogen, potassium and sodium. Leuk. Res. 2001, 30, 309–317. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxid. Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Kunz, L.H.; King, J.C. Impact of maternal nutrition and metabolism on health of the offspring. Semin. Fetal Neonatal Med. 2007, 12, 71–77. [Google Scholar] [CrossRef]
- Ramamoorthy, T.G.; Allen, T.-J.; Davies, A.; Harno, E.; Sefton, C.; Murgatroyd, C.; White, A. Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int. J. Obes. 2018, 42, 1431–1444. [Google Scholar] [CrossRef]
- Crovetto, F.; Nakaki, A.; Arranz, A.; Borras, R.; Vellvé, K.; Paules, C.; Gratacós, E. Effect of a Mediterranean Diet or Mindfulness-Based Stress Reduction During Pregnancy on Child Neurodevelopment: A Prespecified Analysis of the IMPACT BCN Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2330255. [Google Scholar] [CrossRef]
- Rizk, J.; Andreou, E.; Hileti, D.; Ghaddar, A.; Zampelas, A. Assessing Health Care Providers’ Knowledge and Practices of Nutrition during Pregnancy in Lebanon: A Cross-Sectional Study. Medicina 2023, 59, 1471. [Google Scholar] [CrossRef]
- Taylor, R.M.; Wolfson, J.A.; Lavelle, F.; Dean, M.; Frawley, J.; Hutchesson, M.J.; Collins, C.E.; Shrewsbury, V.A. Impact of preconception, pregnancy, and postpartum culinary nutrition education interventions: A systematic review. Nutr. Rev. 2021, 79, 1186–1203. [Google Scholar] [CrossRef]
- van Dijk, M.R.; Koster, M.P.H.; Oostingh, E.C.; Willemsen, S.P.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. A Mobile App Lifestyle Intervention to Improve Healthy Nutrition in Women Before and During Early Pregnancy: Single-Center Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e15773. [Google Scholar] [CrossRef]
- Ainscough, K.M.; O’Brien, E.C.; Lindsay, K.L.; Kennelly, M.A.; O'Sullivan, E.J.; O’Brien, O.A.; McAuliffe, F.M. Nutrition, Behavior Change and Physical Activity Outcomes From the PEARS RCT—An mHealth-Supported, Lifestyle Intervention Among Pregnant Women With Overweight and Obesity. Front. Endocrinol. 2020, 10, 496789. [Google Scholar] [CrossRef]
- Teweldemedhin, L.G.; Amanuel, H.G.; Berhe, S.A.; Gebreyohans, G.; Tsige, Z.; Habte, E. Effect of nutrition education by health professionals on pregnancy-specific nutrition knowledge and healthy dietary practice among pregnant women in Asmara, Eritrea: A quasi-experimental study. BMJ Nutr. Prev. Health 2021, 4, 181–194. [Google Scholar] [CrossRef]
- Mazloomy-Mahmoodabad, S.; Goodarzi-Khoigani, M.; Moghadam, M.H.B.; Nadjarzadeh, A.; Mardanian, F.; Fallahzadeh, H. Impact of nutrition education in improving dietary pattern during pregnancy based on pender’s health promotion model: A randomized clinical trial. Iran. J. Nurs. Midwifery Res. 2018, 23, 18–25. [Google Scholar] [CrossRef]
- Muktabhant, B.; Lawrie, T.A.; Lumbiganon, P.; Laopaiboon, M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst. Rev. 2015, 6, CD007145. [Google Scholar] [CrossRef]
- Parlapani, E.; Agakidis, C.; Karagiozoglou-Lampoudi, T.; Sarafidis, K.; Agakidou, E.; Athanasiadis, A.; Diamanti, E. The Mediterranean diet adherence by pregnant women delivering prematurely: Association with size at birth and complications of prematurity. J. Matern. Neonatal Med. 2017, 32, 1084–1091. [Google Scholar] [CrossRef]
- Amati, F.; Hassounah, S.; Swaka, A. The Impact of Mediterranean Dietary Patterns During Pregnancy on Maternal and Offspring Health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef]
- Muffone, A.R.M.C.; De Oliveira Lübke, P.D.P.; Rabito, E.I. Mediterranean diet and infertility: A systematic review with meta-analysis of cohort studies. Nutr. Rev. 2023, 81, 775–789. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Castellano, S.; Galvano, F.; Caraci, F.; Ferri, R. Association between diet and sleep quality: A systematic review. Sleep. Med. Rev. 2021, 57, 101430. [Google Scholar] [CrossRef]
- Albataineh, S.R.; Badran, E.F.; Tayyem, R.F. Dietary factors and their association with childhood obesity in the Middle East: A systematic review. Nutr. Health 2019, 25, 53–60. [Google Scholar] [CrossRef]
- Sharma, S.; Di Castelnuovo, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M. Diet Quality and Risk of SARS-CoV-2 Infection or COVID-19: A Systematic Review of Observational Studies. Adv. Nutr. Int. Rev. J. 2023, 14, 1596–1616. [Google Scholar] [CrossRef]
- Mamikutty, R.; Aly, A.S.; Marhazlinda, J. Selecting Risk of Bias Tools for Observational Studies for a Systematic Review of Anthropometric Measurements and Dental Caries among Children. Int. J. Environ. Res. Public Health 2021, 18, 8623. [Google Scholar] [CrossRef]
- Jiao, H.; Acar, G.; Robinson, G.A.; Ciurtin, C.; Jury, E.C.; Kalea, A.Z. Diet and Systemic Lupus Erythematosus (SLE): From Supplementation to Intervention. Int. J. Environ. Res. Public Health 2022, 19, 11895. [Google Scholar] [CrossRef]
- Cespedes, E.M.; Hu, F.B. Dietary patterns: From nutritional epidemiologic analysis to national guidelines. Am. J. Clin. Nutr. 2015, 101, 899. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. Int. Rev. J. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Subar, A.F.; Kipnis, V.; Troiano, R.P.; Midthune, D.; Schoeller, D.A.; Bingham, S.; Sharbaugh, C.O.; Trabulsi, J.; Runswick, S.; Ballard-Barbash, R.; et al. Using Intake Biomarkers to Evaluate the Extent of Dietary Misreporting in a Large Sample of Adults: The OPEN Study. Am. J. Epidemiol. 2003, 158, 1–13. [Google Scholar] [CrossRef]
- Freedman, L.S.; Midthune, D.; Arab, L.; Prentice, R.L.; Subar, A.F.; Willett, W.; Neuhouser, M.L.; Tinker, L.F.; Kipnis, V. Combining a Food Frequency Questionnaire with 24-Hour Recalls to Increase the Precision of Estimation of Usual Dietary Intakes—Evidence From the Validation Studies Pooling Project. Am. J. Epidemiol. 2018, 187, 2227–2232. [Google Scholar] [CrossRef]
| Parameter | Inclusion Criterion |
|---|---|
| Participants | Pregnant women |
| Intervention or exposure | Consumption of Mediterranean diet |
| Comparison | Any other type of diet |
| Outcome | Excessive gestational weight gain/preeclampsia/gestational diabetes mellitus |
| Study design | Randomized controlled trials, observational studies |
| CROSS-SECTIONAL STUDIES | ||||||||
|---|---|---|---|---|---|---|---|---|
| Serial Number | Author, Year; Country | Period When Study Was Conducted, Recruitment Period of Pregnant Women | Sample Size (N), GDM/Preeclampsia/eGWG Cases (%) | Study Design, Study Population | Dietary Assessment | Exposure: Mediterranean Diet (MD) Score Format Used | Outcome of Interest | Guideline/Method Used to Ascertain eGWG/GDM/ Preeclampsia |
| 1. | Antasouras et al., 2023 [56]; Greece | May 2016 to September 2020, third trimester | N = 5688, Overweight N = 1060 (18.6%), Obese N = 322 (5.7%), GDM N = 372 (6.5%) | Online survey of a general population of pregnant women. | MediDiet questionnaire comprised of 11 food groups. The period when dietary data was recorded is not reported. | MediDiet score ranging 0–55: a posteriori-derived dietary pattern. | Risk of GDM and GWG | GWG: WHO method used for GWG GDM: Participants’ gestational diabetes diagnoses were recovered from their medical records. A standardized oral glucose tolerance test (OGTT) during gestation was performed, specifically, a fasting OGTT with 75 g of glucose with a cut-off plasma glucose level of >140 mg/dL after 2 h for the first trimester and the following trimester at 24–28 weeks of pregnancy. |
| PROSPECTIVE COHORT STUDIES | ||||||||
| 1. | Li et al., 2021 [57]; USA | 2009–2013 | N = 1887 GDM, N= 85 (5% of N = 1718 women assessed), preeclampsia, N= 61 (3.5% of the N = 1752 women assessed). | Prospective cohort study, general population of pregnant women. | 124-item FFQ GDM: Dietary data recorded at 8–13 weeks and 16–22 weeks. Preeclampsia: 8–13 weeks, 16–22 weeks, and 24–29 weeks. Dietary data at 8–13 weeks: Diet History Questionnaire II (modified version) 16–22 weeks, and 24–29 weeks: Automated Self-Administered 24-h (ASA24) Dietary Assessment Tool. | Adherence to Mediterranean Diet by the aMED score ranging from 0 to 9. | Risk and severity of GDM and preeclampsia. | GDM: Gestational diabetes was defined by women’s oral glucose challenge test results using the Carpenter-Coustan criteria (at least 2 values met or exceeded: fasting—95 mg/dL, 1 h—180 mg/dL, 2 h—155 mg/dL, 3 h—140 mg/dL), and/or by receipt of GDM medications. Preeclampsia: The 2002 ACOG criteria defined preeclampsia is a new onset of elevated blood pressure (≥140 mm Hg or a diastolic blood pressure ≥ 90 mm Hg) after 20 weeks with proteinuria (≥0.3 g of protein in a 24 h urine specimen). |
| 2. | Minhas et al., 2022 [58]; USA | 1998–2016 Maternal age: 28 (23–33 y) Mixed race/ethnicity, majority black: (4030/47%), Hispanic: (2423/28%). | N = 8507, Preeclampsia N = 848 (10%) | Prospective cohort study, participants recruited from a medical center. | 16-item FFQ Dietary data was recorded after 24–72 h of delivery and covered the dietary intake during pregnancy. | Mediterranean-style diet score (MSDS) (4–38) | Risk of preeclampsia. | Preeclampsia included in any form, including mild or severe preeclampsia, eclampsia, or HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome. |
| CASE-CONTROL STUDIES | ||||||||
| 1. | Izadi et al., 2016 [59]; Iran | No year indicated, between 5 and 28 weeks of gestation. | N = 463, N of GDM cases = 200 (43%) | Hospital-based case-control study, general population of pregnant women. | Three 24 h dietary recalls. Dietary data recorded between 5 and 28 weeks of pregnancy. | MedDiet score ranging 0–9 by Trichopoulou et al. [31] | Risk of GDM | GDM was ascertained if the pregnant women had abnormal fasting glucose (FG; >95 mg/dL or 1-h postprandial glucose > 140 mg/dL for the first time in pregnancy). |
| RANDOMIZED CONTROLLED TRIALS | ||||||||
| Serial Number | Author, Year; Country | Period When Study Was Conducted, Recruitment Period of Pregnant Women | Sample Size (N), Control Group (CG) (n)/Intervention Group (IG) (n), GDM/Preeclampsia/eGWG cases (N) (%), (n/CG, n/IG) | Control Group Diet | Dietary Assessment | Exposure: Mediterranean Diet (MD) Score Format Used for Interventional Group | Outcome of Interest | Guideline/Method Used to Ascertain eGWG/GDM/ Preeclampsia |
| 1. | Assaf-Balut et al., 2017 [60]; Spain | January–December 2015, 8–12 weeks of pregnancy. | N = 874, 440/434 GDM N = 177 (20.2%), (103/440, 74/434) GDM was distributed at random between two control and interventional groups. | A standard diet with limited fat intake. | 7-day food diaries Dietary data recorded during 8–12 weeks of pregnancy. | 14-point Mediterranean Diet Adherence Screener (MEDAS). MedDiet supplemented with a recommendation of a daily consumption of at least 40 mL of EVOO and a handful (25–30 g) of pistachios. | Risk of GDM | IADPSG criteria were used to diagnose GDM at 24 ± 28 GW with a single 2 h 75 g oral glucose tolerance test. |
| 2. | de la Torre et al., 2019 [61]; Spain | January–November 2017, 8–12 weeks of pregnancy | N = 932 GDM N = 130 (13.9%), (Non-GDM control group = 802/GDM interventional group = 130) GWG:
NGT group: 9/802, (1.1%) GDM group: 1/130 (0.8%) | No control diet administered. All participants were educated regarding Mediterranean diet guidelines with exclusive consumption of EVOO, and a daily handful of nuts (not provided). | 7-day food diaries Dietary data recorded during 8–12 weeks of pregnancy. | 14-point Mediterranean Diet Adherence Screener (MEDAS). Education on the implementation of Mediterranean diet guidelines supplemented with exclusive consumption of EVOO, and a daily handful of nuts (not provided). | Risk of excessive GWG and preeclampsia. | GDM: IADPSG and WHO 2013 criteria was used to diagnose GDM at 24 ± 28 GW with a single 2-h 75-g oral glucose tolerance test. eGWG: eGWG was defined as a gestational weight gain 3 Kg above the designated target according to pre-gestational BMI. Preeclampsia: >140 mmHg systolic/90 mmHg diastolic with proteinuria > 300 mg in 24 hr after 20 gestational weeks. |
| 3. | Melero et al., 2020 [62]; Spain | 2016–2017, 8–12 weeks of pregnancy | N = 600 CG: 142 (23.6%), IG: 143 (23.8%) Real world group (RWG): 315 (52.5%) GDM N = 91/544 (16.7%) CG N = 34/132 (25.8%), IG N = 19/128 (14.8%), RWG N = 38/284 (13.4%) Preeclampsia N = 15 (2.7%) CG N = 6/132 (4.5%), IG N = 5/128 (3.9%), RWG N = 4/284 (1.4%) GWG outcome was not categorized in the study. | CG was advised to restrict fat intake, with the consumption of extra virgin olive oil (EVOO) limited to a maximum of 40 mL/day, and nuts < 3 days per week as usually recommended. | Two semi-quantitative FFQ Dietary data recorded during 8–12 weeks of pregnancy. | 14-point Mediterranean Diet Adherence Screener (MEDAS). MedDiet supplemented with the recommendation of daily consumption of at least 40 mL of EVOO and a handful (25–30 g) of pistachios at least 3 days a week. | Risk of GDM, excessive GWG, and preeclampsia. | GDM: IADPSG criteria were used to diagnose GDM at 24 ± 28 GW with a single 2-h 75 g oral glucose tolerance test. GWG: No evidence of an association. Preeclampsia: >140 mmHg systolic/90 mmHg diastolic with proteinuria > 300 mg in 24 hr after 20 gestational weeks. |
| CROSS-SECTIONAL STUDIES | ||||||
|---|---|---|---|---|---|---|
| Serial Number | Author, Year | Outcome of Interest | Covariates | Statistical Methods | Results | Conclusion |
| 1. | Antasouras et al., 2023 [56]; Greece | Risk of GDM and eGWG | Maternal age, educational and economic status, nationality, type of residence, smoking habits, parity, pre-pregnancy BMI status, preterm birth, gestational diabetes, gestational hypertension, type of delivery, and exclusive breastfeeding. | A multivariate binary logistic regression analysis was applied to evaluate whether compliance with the MD may exert an independent impact on sociodemographic and anthropometric parameters, perinatal outcomes, and breastfeeding practices. | Decreased adherence to MD was associated with an increased risk of eGWG (OR: 1.78; 95% CI 1.51 to 2.02) and GDM (OR: 2.32; 95% CI 2.13 to 2.57). | The MedDiet score was inversely associated with GDM risk and excessive eGWG. |
| PROSPECTIVE STUDIES | ||||||
| 1. | Li et al., 2021 [57]; USA | Risk of GDM, preeclampsia, and common pregnancy complications | Maternal age, race (non-Hispanic white, non-Hispanic black, Hispanic, Asian), education (<high school, high school, some college, bachelor, graduate), marriage/cohabiting (yes, no), nulliparity (yes, no), pre-pregnancy BMI (kg/m2), family history of diabetes (yes, no), light to vigorous physical activities (hour/week, sleep durations (5–6, 7, 8–9, 10+ h/day), and total energy intake (kcal/day). | Log-binomial regression models to explore associations between aMED adherence scores and risk of GDM. | GDM: High aMED score adherence was not associated with a lower risk of GDM during 8–13 weeks of pregnancy (Q4 vs. Q1: RR 0.61; 95% CI: 0.25 to 1.48) and during 16–22 weeks of pregnancy(Q4 vs. Q1: RR 0.61; 95% CI: 0.33 to 1.15). Preeclampsia: High aMED score adherence was not associated with a lower risk of preeclampsia during 8–13 weeks of pregnancy (Q4 vs. Q1: RR 0.68; 95% CI: 0.25 to 1.85), during 16–22 weeks of pregnancy. (Q4 vs. Q1: RR 0.67; 95% CI: 0.34 to 1.32) and during 24–29 weeks of pregnancy (Q4 vs. Q1: RR 0.47; 95% CI: 0.18 to 1.21). | GDM: High MD adherence was not associated with a lower risk of GDM. Preeclampsia: High MD adherence was not associated with a lower risk of preeclampsia. |
| 2. | Minhas et al., 2022 [58] | Risk of preeclampsia | Age (categorical: <21, 21–30, ≥30 years), race and ethnicity (White, Black, Hispanic, and other), education (categorical: 1 = no, school/ elementary school, 2 = high school, 3 = some college or above), marital status (categorical: 1 = married, 2 = unmarried, 3 = unknown), smoking status (binary: 0 = never smoker during pregnancy, 1 = smoking during pregnancy), parity (binary: 0 = nulliparous, 1 = parous), and pre-pregnancy obesity (binary: 0 = body mass index < 30 kg/m2, 1 = body mass index ≥ 30 kg/m2). | Multivariable adjusted logistic regression models were used to evaluate the association between adherence to the Mediterranean-style diet, and the risk of preeclampsia. | Compared with women who scored in the lowest tertile of the MSDS, women in the middle tertile (OR 0.72; 95% CI: 0.59 to 0.89) and highest tertile had (OR 0.78; 95% CI: 0.64 to 0.96) were inversely associated with lower odds of preeclampsia. | Possible protective effect of the Mediterranean-style diet on risk of preeclampsia. |
| CASE-CONTROL STUDIES | ||||||
| 1. | Izadi et al., 2016 [59] | Risk of GDM | Age, energy, number of children, and socioeconomic status. | Multiple logistic regression models to assess MD adherence and the risk of GDM. | Inverse association between high MedDiet score and the risk of GDM (Tertile 3 vs. tertile 1: OR: 0.20, 95% CI 0.50 to 0.70). | An inverse association between the MedDiet score and the risk of GDM. |
| RANDOMIZED CONTROLLED TRIALS | ||||||
| 1. | Assaf-Balut et al., 2017 [60]; Spain | Risk of GDM | Age, ethnicity, parity, BMI (continuous), gestational, personal and family history, and smoker (categorical: never, current, former smoker). | Logistic regression analyses were used to assess the effect of the intervention on the risk of GDM. | Inverse association between high MEDAS score supplemented with extra virgin olive oil and pistachios, and the risk of GDM (OR: 0.75, 95% CI 0.57 to 0.98) in the intervention group. | Potential protective effect of high Mediterranean diet adherence, extra virgin olive oil, and pistachios on risk of GDM. |
| 2. | de la Torre et al., 2019 [61]; Spain | Risk of eGWG and preeclampsia, and adverse maternal-fetal outcomes. | None. | Logistic binary regression analyses were used to assess the effect of GDM on eGWG and preeclampsia. | Amongst GDM women, a high adherence to Mediterranean diet score was associated with a lesser risk of eGWG, (RR: 0.91, 95% CI 0.86 to 0.96). However, it was not associated with a lesser risk of preeclampsia. | Potential protective effect of high Mediterranean diet adherence amongst GDM women on eGWG, but not preeclampsia. |
| 3. | Melero et al., 2020 [62]; Spain | Risk of GDM, GWG and preeclampsia, and other adverse maternal-fetal events. | Age, parity, and BMI. | Logistic regression was used to assess the effect of the MD nutritional therapy for the GDM, GWG, and preeclampsia. | Participants with higher adherence to MD plus EVOO and pistachios intervention were associated with lower risk of GDM (RR: 0.72, 95% CI 0.50 to 0.97) in the IG and (RR: 0.77, 95% CI 0.61 to 0.97) in the RWG, respectively. However, it is not associated with preeclampsia. | High adherence to the Mediterranean diet was associated with a lower risk of GDM but not preeclampsia and GWG. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Esposito, S.; Di Castelnuovo, A.; Gialluisi, A.; De Domenico, P.; de Gaetano, G.; Bonaccio, M.; Iacoviello, L. Impact of Mediterranean Diet Adherence During Pregnancy on Preeclampsia, Gestational Diabetes Mellitus, and Excessive Gestational Weight Gain: A Systematic Review of Observational Studies and Randomized Controlled Trials. Nutrients 2025, 17, 1723. https://doi.org/10.3390/nu17101723
Sharma S, Esposito S, Di Castelnuovo A, Gialluisi A, De Domenico P, de Gaetano G, Bonaccio M, Iacoviello L. Impact of Mediterranean Diet Adherence During Pregnancy on Preeclampsia, Gestational Diabetes Mellitus, and Excessive Gestational Weight Gain: A Systematic Review of Observational Studies and Randomized Controlled Trials. Nutrients. 2025; 17(10):1723. https://doi.org/10.3390/nu17101723
Chicago/Turabian StyleSharma, Sukshma, Simona Esposito, Augusto Di Castelnuovo, Alessandro Gialluisi, Paola De Domenico, Giovanni de Gaetano, Marialaura Bonaccio, and Licia Iacoviello. 2025. "Impact of Mediterranean Diet Adherence During Pregnancy on Preeclampsia, Gestational Diabetes Mellitus, and Excessive Gestational Weight Gain: A Systematic Review of Observational Studies and Randomized Controlled Trials" Nutrients 17, no. 10: 1723. https://doi.org/10.3390/nu17101723
APA StyleSharma, S., Esposito, S., Di Castelnuovo, A., Gialluisi, A., De Domenico, P., de Gaetano, G., Bonaccio, M., & Iacoviello, L. (2025). Impact of Mediterranean Diet Adherence During Pregnancy on Preeclampsia, Gestational Diabetes Mellitus, and Excessive Gestational Weight Gain: A Systematic Review of Observational Studies and Randomized Controlled Trials. Nutrients, 17(10), 1723. https://doi.org/10.3390/nu17101723






