The Significance and Process of Inflammation Involving Eicosapentaenoic and Docosahexaenoic Derivatives in Hashimoto’s Disease
Abstract
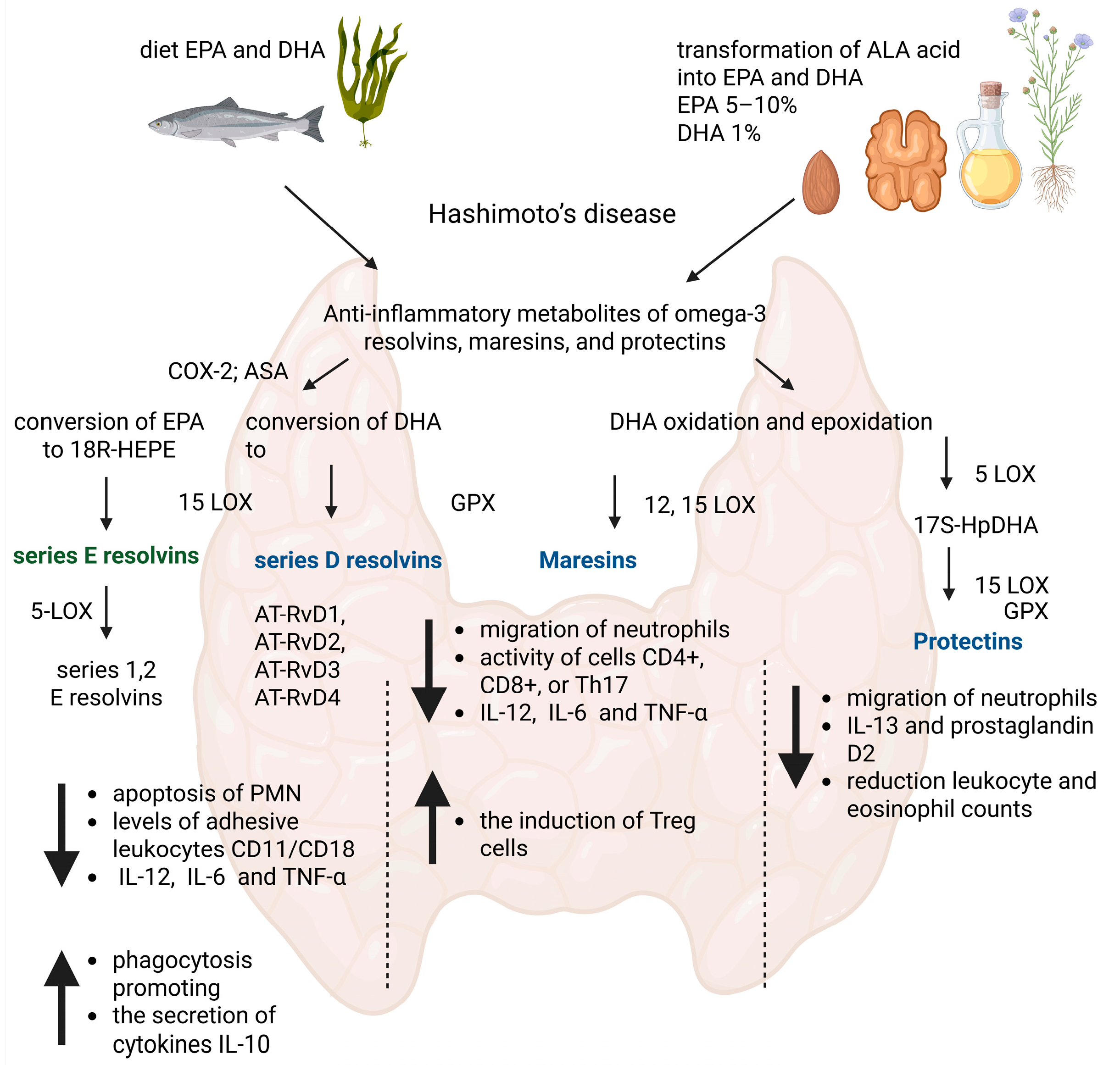
1. Introduction
2. Materials and Methods
2.1. Research Methods and Tools
2.2. Statistical Analysis
3. Results
Study Group
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liontiris, M.I.; Mazokopakis, E.E. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Points that need more investigation. Hell J. Nucl. Med. 2017, 20, 51–56. [Google Scholar] [CrossRef]
- Puszkarz, I.; Guty, E.; Stefaniak, I.; Bonarek, A. Role of Food and Nutrition in Pathogenesis and Prevention of Hashimoto’s Thyroiditis. J. Educ. Health Sport 2018, 8, 394–401. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Famà, F.; Perdichizzi, L.G.; Antonelli, A.; Brenta, G.; Vermiglio, F.; Moleti, M. Fish and the Thyroid: A Janus Bifrons Relationship Caused by Pollutants and the Omega-3 Polyunsaturated Fatty Acids. Front. Endocrinol. 2022, 13, 891233. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2021, 11, 623052. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic Variation, Diet, Inflammation, and the Risk for COVID-19. Life Style Genom. 2021, 14, 37–42. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Bennett, M.; Gilroy, D.W. Lipid Mediators in Inflammation. Microbiol. Spectr. 2016, 4, 2016. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules 2021, 11, 1873. [Google Scholar] [CrossRef]
- Abdolmaleki, F.; Kovanen, P.T.; Mardani, R.; Gheibi-hayat, S.M.; Bo, S.; Sahebkar, A. Resolvins: Emerging Players in Autoimmune and Inflammatory Diseases. Clin. Rev. Allergy Immunol. 2020, 58, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Zhu, M.; Vlasenko, N.A.; Deng, B.; Haeggström, J.Z.; Petasis, N.A.; Serhan, C.N. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013, 27, 2573–2583. [Google Scholar] [CrossRef]
- Deng, B.; Wang, C.W.; Arnardottir, H.H.; Li, Y.; Cheng, C.Y.; Dalli, J.; Serhan, C.N. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS ONE 2014, 9, e102362. [Google Scholar] [CrossRef]
- Saito-Sasaki, N.; Sawada, Y.; Nakamura, M. Maresin-1 and Inflammatory Disease. Int. J. Mol. Sci. 2022, 23, 1367. [Google Scholar] [CrossRef]
- Rubbert-Roth, A.; Enejosa, J.; Pangan, A.L.; Haraoui, B.; Rischmueller, M.; Khan, N.; Zhang, Y.; Martin, N.; Xavier, R.M. Trial of Upadacitinib or Abatacept in Rheumatoid Arthritis. N. Engl. J. Med. 2020, 383, 1511–1521. [Google Scholar] [CrossRef]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef]
- Hashimoto, A.; Murakami, Y.; Kitasato, H.; Hayashi, I.; Endo, H. Glucocorticoids co-interact with lipoxin A4 via lipoxin A4 receptor (ALX) up-regulation. Biomed. Pharmacother. 2007, 61, 81–85. [Google Scholar] [CrossRef]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Tylutka, A.; Gołąb-Janowska, M.; Drozd, A. Lipoxins, RevD1 and 9, 13 HODE as the most important derivatives after an early incident of ischemic stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef]
- Purdel, C.; Ungurianu, A.; Margina, D. Metabolic and Metabolomic Insights Regarding the Omega-3 PUFAs Intake in Type 1 Diabetes Mellitus. Front. Mol. Biosci. 2021, 8, 783065. [Google Scholar] [CrossRef] [PubMed]
- Barbalace, M.C.; Talotta, R.; Rapisarda, F.; D’Amico, V.; Laganà, M.; Malaguti, M.; Campennì, A.; Cannavò, S.; Hrelia, S.; Ruggeri, R.M. Unlocking the Power of the Mediterranean Diet: Two in One-Dual Benefits for Rheumatic and Thyroid Autoimmune Diseases. Nutrients 2025, 17, 1383. [Google Scholar] [CrossRef] [PubMed]
- Koller-Smith, L.; Mehdi, A.M.; March, L.; Tooth, L.; Mishra, G.D.; Thomas, R. Rheumatoid arthritis is a preventable disease: 11 ways to reduce your patients’ risk. Intern. Med. J. 2022, 52, 711–716. [Google Scholar] [CrossRef]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef] [PubMed]
- Gkiouras, K.; Grammatikopoulou, M.G.; Myrogiannis, I.; Papamitsou, T.; Rigopoulou, E.I.; Sakkas, L.I.; Bogdanos, D.P. Efficacy of n-3 fatty acid supplementation on rheumatoid arthritis’ disease activity indicators: A systematic review and meta-analysis of randomized placebo-controlled trials. Crit. Rev. Food Sci. Nutr. 2024, 64, 16–30. [Google Scholar] [CrossRef]
- Sciascia, S.; Ferrara, G.; Roccatello, L.; Rubini, E.; Foddai, S.G.; Radin, M.; Cecchi, I.; Rossi, D.; Barinotti, A.; Ricceri, F.; et al. The Interconnection Between Systemic Lupus Erythematosus and Diet: Unmet Needs, Available Evidence, and Guidance-A Patient-Driven, Multistep-Approach Study. Nutrients 2024, 16, 4132. [Google Scholar] [CrossRef]
- Song, J.; Sun, R.; Zhang, Y.; Ke, J.; Zhao, D. Serum resolvin E1 levels and its relationship with thyroid autoimmunity in Hashimoto’s thyroiditis: A preliminary study. BMC Endocr. Disord. 2021, 21, 66. [Google Scholar] [CrossRef]
- Song, J.; Sun, R.; Zhang, Y.; Fu, Y.; Zhao, D. Role of the Specialized Pro-resolving Mediator Resolvin D1 in Hashimoto’s Thyroiditis. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2021, 129, 791–797. [Google Scholar] [CrossRef]
- Benvenga, S.; Vigo, M.T.; Metro, D.; Granese, R.; Vita, R.; Le Donne, M. Type of fish consumed and thyroid autoimmunity in pregnancy and postpartum. Endocrine 2016, 52, 120–129. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, X.; Jia, M. Efficacy of omega-3 polyunsaturated fatty acids on hormones, oxidative stress, and inflammatory parameters among polycystic ovary syndrome: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 8. [Google Scholar] [CrossRef]
- Brennan Laing, B.; Cavadino, A.; Ellett, S.; Ferguson, L.R. Effects of an Omega-3 and Vitamin D Supplement on Fatty Acids and Vitamin D Serum Levels in Double-Blinded, Randomized, Controlled Trials in Healthy and Crohn’s Disease Populations. Nutrients 2020, 12, 1139. [Google Scholar] [CrossRef]
- Setty, B.N.Y.; Betal, S.G.; E Miller, R.; Brown, D.S.; Meier, M.; Cahill, M.; Lerner, N.B.; Apollonsky, N.; Stuart, M.J. Relationship of Omega-3 fatty acids DHA and EPA with the inflammatory biomarker hs-CRP in children with sickle cell anemia. Prostaglandins Leukot. Essent. Fat. Acids 2019, 146, 11–18. [Google Scholar] [CrossRef]
- Benvenga, S.; Vita, R.; Di Bari, F.; Granese, R.; Metro, D.; Le Donne, M. Stable consumption of swordfish favors, whereas stable consumption of oily fish protects from, development of postpartum thyroiditis. Endocrine 2019, 65, 94–101. [Google Scholar] [CrossRef]
- Benvenga, S.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Patrizio, A.; Paparo, S.R.; Camastra, S.; Bonofiglio, D.; Antonelli, A.; Fallahi, P. Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies. Nutrients 2020, 12, 1337. [Google Scholar] [CrossRef]
- Szczuko, M.; Szwec-Nadworna, N.; Palma, J.; Tomasik, M.; Ziętek, M. Increased Demand of Obese Women for Protectins, Maresin, and Resolvin D1 in the Last Trimester of Pregnancy. Nutrients 2023, 15, 4340. [Google Scholar] [CrossRef]
- Siddiquee, A.; Patel, M.; Rajalingam, S.; Narke, D.; Kurade, M.; Ponnoth, D.S. Effect of omega-3 fatty acid supplementation on resolvin (RvE1)-mediated suppression of inflammation in a mouse model of asthma. Immunopharmacol. Immunotoxicol. 2019, 41, 250–257. [Google Scholar] [CrossRef]
- Grazda, R.; Seyfried, A.N.; Maddipatti, K.R.; Fredman, G.; MacNamara, K.C. Impaired inflammation resolution in murine bone marrow failure is rescued by Resolvin E1 treatment. BioRxiv Prepr. Serv. Biol. 2023, 2023, 528688. [Google Scholar] [CrossRef]
- Ridker, P.M. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: From concept to clinical practice to clinical benefit. Am. Heart J. 2004, 148, S19–S26. [Google Scholar] [CrossRef]
- Clària, J.; López-Vicario, C.; Rius, B.; Titos, E. Pro-resolving actions of SPM in adipose tissue biology. Mol. Aspects Med. 2017, 58, 83–92. [Google Scholar] [CrossRef]
- Gemperle, C.; Tran, S.; Schmid, M.; Rimann, N.; Marti-Jaun, J.; Hartling, I.; Wawrzyniak, P.; Hersberger, M. Resolvin D1 reduces inflammation in co-cultures of primary human macrophages and adipocytes by triggering macrophages. Rostaglandins Leukot. Essent. Fat. Acids 2021, 174, 102363. [Google Scholar] [CrossRef]
| Parameter | Avg ± SD |
|---|---|
| Age [year] | 37.58 ± 8.41 |
| Height [cm] | 167.32 ± 5.17 |
| Body weight [kg] | 70.59 ± 12.90 |
| BMI [kg/m2] | 25.16 ± 4.13 |
| Fat tissue mass [kg] | 25.181 ± 8.97 |
| % body fat content | 34.89 ± 6.96 |
| ATPO [IU/mL] | 197.72 ± 141.45 |
| ATG [IU/mL] | 326.2 ± 581.1 |
| TSH [µLU/mL] | 3.27 ± 2.91 |
| fT3 [pg/mL] | 2.90 ± 0.47 |
| fT4 [ng/dL] | 1.26 ± 0.21 |
| CRP [mg/L] | 2.24 ± 1.46 |
| Blood platelets (PLT) [tys/mm3] | 245.5 ± 50.79 |
| Leukocytes (WBC) [tys/mm3] | 5.8 ± 1.51 |
| Neutrophils [tys/µL] | 3.04 ± 1.18 |
| Lymphocytes [tys/µL] | 2.01 ± 0.41 |
| Monocytes [tys/µL] | 0.53 ± 0.14 |
| Eosinophils [tys/µL] | 0.17 ± 0.11 |
| Basophils [tys/µL] | 0.03 ± 0.01 |
| Fatty Acids and Their Derivatives | Avg | SD |
|---|---|---|
| C20:5n3 EPA | 4.390 | 5.179 |
| C22:6n3 DHA | 6.751 | 4.105 |
| Resolvin E1 | 0.736 | 0.539 |
| Resolvina D1 | 0.154 | 0.077 |
| Maresina 1 | 1.258 | 2.838 |
| 10S17R DiHDHA | 0.247 | 0.114 |
| 18RS HEPE | 0.376 | 0.281 |
| 17RS HDHA | 3.386 | 1.949 |
| EPA | R | p |
| Age at study entry | 0.24 | 0.248 |
| Growth | 0.085 | 0.6304 |
| Body weight | 0.043 | 0.8091 |
| BMI | 0.03 | 0.168 |
| Fat tissue mass | 0.032 | 0.8552 |
| % body fat content | 0.038 | 0.8301 |
| DHA | R | p |
| Age at study entry | 0.115 | 0.5154 |
| Growth | −0.294 | 0.0259 |
| Body weight | −0.113 | 0.5237 |
| BMI | −0.02 | 0.9093 |
| Fat tissue mass | −0.6 | 0.7336 |
| % body fat content | −0.055 | 0.7536 |
| Resolvin E1 | R | p |
| Age at study entry | 0.26 | 0.882 |
| Growth | 0.024 | 0.891 |
| Body weight | 0.293 | 0.098 |
| BMI | 0.286 | 0.1061 |
| Fat tissue mass | 0.29 | 0.1004 |
| % body fat content | 0.349 | 0.0486 |
| Resolvin D1 | R | p |
| Age at study entry | −0.55 | 0.754 |
| Growth | 0.211 | 0.2334 |
| Body weight | 0.365 | 0.0388 |
| BMI | 0.332 | 0.0601 |
| Fat tissue mass | 0.36 | 0.0418 |
| % body fat content | 0.363 | 0.0402 |
| 10S17R DiHDHA | R | p |
| Age at study entry | 0.042 | 0.8124 |
| Growth | 0.17 | 0.3354 |
| Body weight | 0.201 | 0.2548 |
| BMI | 0.16 | 0.3649 |
| Fat tissue mass | 0.007 | 0.9683 |
| % body fat content | 0.012 | 0.9442 |
| Maresin 1 | R | p |
| Age at study entry | 0.165 | 0.3503 |
| Growth | 0.06 | 0.7329 |
| Body weight | 0.146 | 0.4089 |
| BMI | 0.133 | 0.4532 |
| Fat tissue mass | 0.324 | 0.669 |
| % body fat content | 0.32 | 0.0704 |
| 18RS HEPE | R | p |
| Age at study entry | −0.085 | 0.6317 |
| Growth | 0.135 | 0.4458 |
| Body weight | 0.103 | 0.5619 |
| BMI | 0.04 | 0.8194 |
| Fat tissue mass | 0.14 | 0.4277 |
| % body fat content | 0.192 | 9.2766 |
| 17RS HDHA | R | p |
| Age at study entry | 0.086 | 0.627 |
| Growth | 0.219 | 0.2159 |
| Body weight | 0.018 | 0.9168 |
| BMI | −0.04 | 0.8231 |
| Fat tissue mass | 0.124 | 0.4813 |
| % body fat content | 0.201 | 0.2554 |
| EPA | R | p |
| ATPO | −0.196 | 0.2685 |
| ATG | −0.081 | 0.6483 |
| TSH | 0.058 | 0.7411 |
| fT3 | −0.117 | 0.5084 |
| fT4 | −0.078 | 0.6592 |
| CRP | 0.242 | 0.1716 |
| DHA | R | p |
| ATPO | −0.031 | 0.8601 |
| ATG | −0.009 | 0.9574 |
| TSH | −0.071 | 0.6875 |
| fT3 | 0.257 | 0.1466 |
| fT4 | 0.162 | 0.3589 |
| CRP | −0.045 | 0.8 |
| RvE1 | R | p |
| ATPO | −0.149 | 0.3999 |
| ATG | −0.141 | 0.4258 |
| TSH | −0.207 | 0.2405 |
| fT3 | 0.151 | 0.3925 |
| fT4 | 0.055 | 0.7554 |
| CRP | 0.395 | 0.0254 |
| RvD1 | R | p |
| ATPO | −0.039 | 0.823 |
| ATG | −0.071 | 0.6875 |
| TSH | 0.042 | 0.8135 |
| fT3 | 0.079 | 0.6561 |
| fT4 | 0.079 | 0.6534 |
| CRP | 0.336 | 0.057 |
| 10S17R DiHDHA | R | p |
| ATPO | −0.067 | 0.7043 |
| ATG | 0.034 | 0.8482 |
| TSH | −0.028 | 0.8575 |
| fT3 | 0.057 | 0.7454 |
| fT4 | −0.085 | 0.6321 |
| CRP | 0.523 | 0.0031 |
| Maresina 1 | R | p |
| ATPO | −0.072 | 0.6854 |
| ATG | −0.017 | 0.9228 |
| TSH | −0.256 | 0.1474 |
| fT3 | 0.068 | 0.7015 |
| fT4 | 0.355 | 0.0447 |
| CRP | 0.023 | 0.8962 |
| 18RS HEPE | R | p |
| ATPO | −0.228 | 0.1981 |
| ATG | −0.205 | 0.2459 |
| TSH | −0.098 | 0.5799 |
| fT3 | 0.004 | 0.9842 |
| fT4 | 0.15 | 0.397 |
| CRP | 0.494 | 0.0052 |
| 17RS HDHA | R | p |
| ATPO | −0.202 | 0.2541 |
| ATG | −0.024 | 0.8913 |
| TSH | −0.347 | 0.0499 |
| fT3 | 0.131 | 0.4572 |
| fT4 | 0.309 | 0.0801 |
| CRP | 0.179 | 0.3113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczuko, M.; Zawadzka, K.; Szczuko, U.; Rudak, L.; Pobłocki, J. The Significance and Process of Inflammation Involving Eicosapentaenoic and Docosahexaenoic Derivatives in Hashimoto’s Disease. Nutrients 2025, 17, 1715. https://doi.org/10.3390/nu17101715
Szczuko M, Zawadzka K, Szczuko U, Rudak L, Pobłocki J. The Significance and Process of Inflammation Involving Eicosapentaenoic and Docosahexaenoic Derivatives in Hashimoto’s Disease. Nutrients. 2025; 17(10):1715. https://doi.org/10.3390/nu17101715
Chicago/Turabian StyleSzczuko, Małgorzata, Klaudia Zawadzka, Urszula Szczuko, Leon Rudak, and Jakub Pobłocki. 2025. "The Significance and Process of Inflammation Involving Eicosapentaenoic and Docosahexaenoic Derivatives in Hashimoto’s Disease" Nutrients 17, no. 10: 1715. https://doi.org/10.3390/nu17101715
APA StyleSzczuko, M., Zawadzka, K., Szczuko, U., Rudak, L., & Pobłocki, J. (2025). The Significance and Process of Inflammation Involving Eicosapentaenoic and Docosahexaenoic Derivatives in Hashimoto’s Disease. Nutrients, 17(10), 1715. https://doi.org/10.3390/nu17101715







