A Novel Camel Milk-Derived Peptide LLPK Improves Glucose-Lipid Metabolism in db/db Mice via PPAR Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Experimental Design
2.4. Biochemical Assays
2.5. Liver Histological Analysis
2.6. Liver Protein Preparation and Digestion
2.7. Nano-UPLC MS/MS Analysis
2.8. Data and Statistical Analysis
3. Results
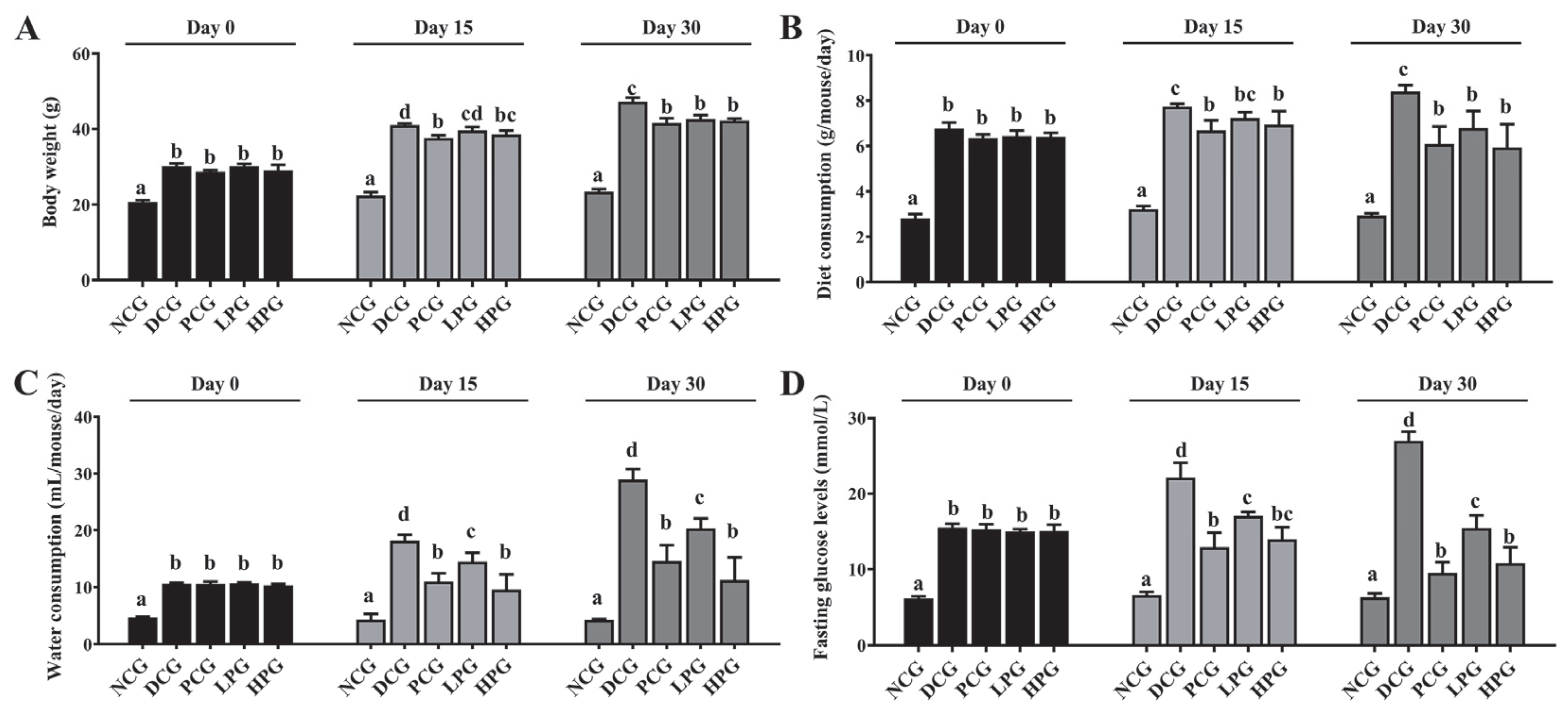
3.1. The Camel Milk-Derived Peptide LLPK Effectively Ameliorated Diabetic Symptoms in db/db Mice
3.2. LLPK Improved Serum Lipid-Related Indices and DPP-4 Enzyme Activity in db/db Mice
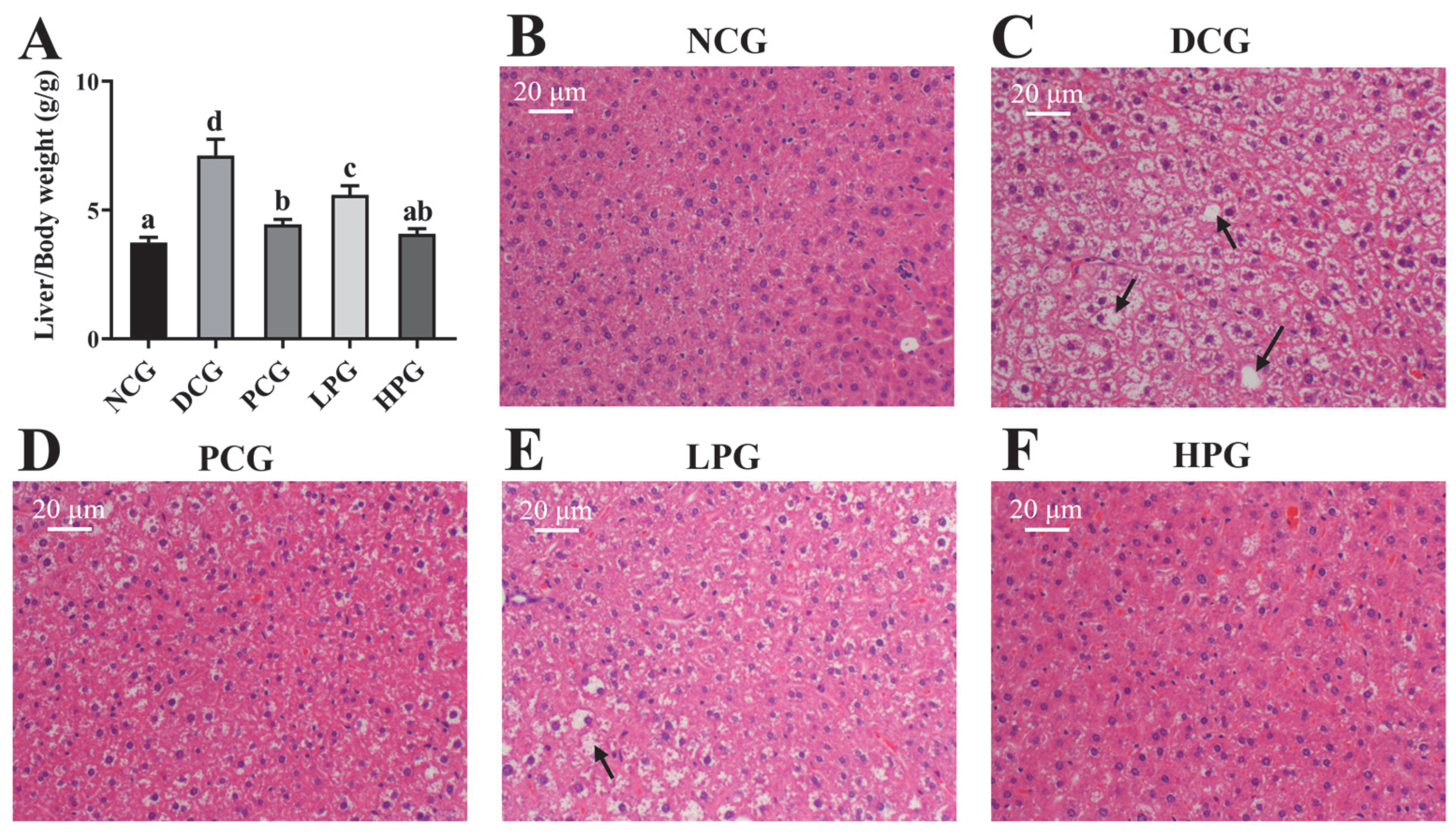
3.3. LLPK Consumption Ameliorated Liver Damage in db/db Mice
3.4. LLPK Altered Liver Proteome Profiles in db/db Mice
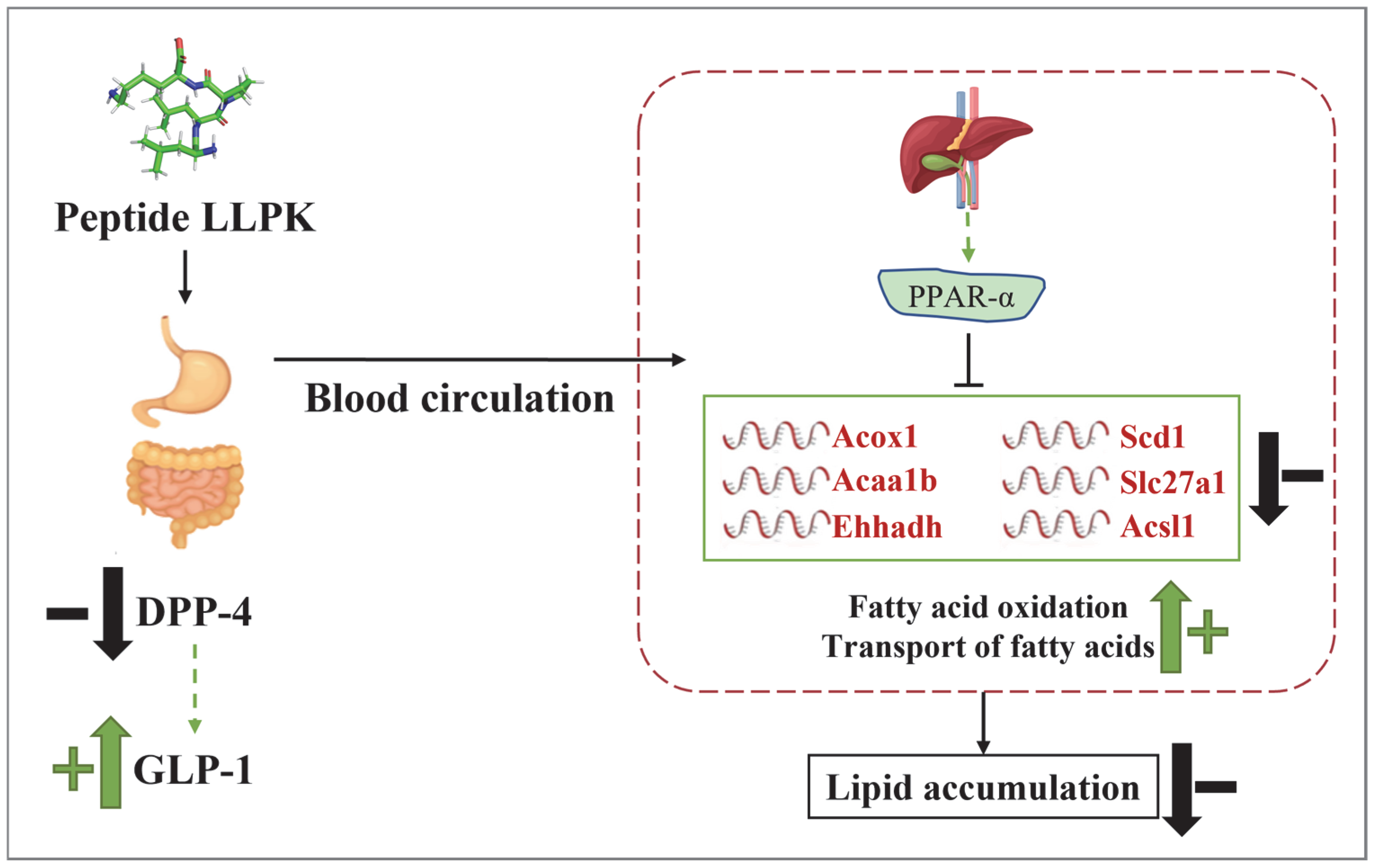
3.5. LLPK Treatment Improves Lipid Metabolism in db/db Mice via PPAR Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acox1 | acyl-coenzyme A oxidase 1 |
| Acaa1b | 3-ketoacyl-CoA thiolase B |
| Acsl1 | long-chain-fatty-acid-CoA ligase 1 |
| DPP-4 | dipeptidyl-peptidase 4 |
| DCG | diabetic control group |
| DEP | different expressed proteins |
| Ehhadh | peroxisomal bifunctional enzyme |
| FBG | fasting blood glucose |
| GLP-1 | glucagon-like peptide 1 |
| HPG | high-dose peptide treatment group |
| ITT | insulin tolerance test |
| LLPK | Leucine-Leucine-Proline-Lysine |
| LPG | low-dose peptide treatment group |
| NCG | normal control group |
| OGTT | oral glucose tolerance tests |
| PCG | metformin control group |
| PCA | principal component analysis |
| PPI | protein–protein Interaction |
| Scd1 | acyl-CoA desaturase 1 |
| Slc27a1 | long-chain fatty acid transport protein 1 |
| T2DM | type 2 diabetes |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Grossman, L.D.; Roscoe, R.; Shack, A.R. Complementary and Alternative Medicine for Diabetes. Can. J. Diabetes 2018, 42, S154–S161. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L.; Wu, G.; Liu, T.; Qi, X.; Zhang, H. Food-derived dipeptidyl peptidase IV inhibitory peptides: Production, identification, structure-activity relationship, and their potential role in glycemic regulation. Crit. Rev. Food Sci. Nutr. 2024, 64, 2053–2075. [Google Scholar] [CrossRef]
- Koirala, P.; Dahal, M.; Rai, S.; Dhakal, M.; Nirmal, N.P.; Maqsood, S.; Al-Asmari, F.; Buranasompob, A. Dairy Milk Protein-Derived Bioactive Peptides: Avengers Against Metabolic Syndrome. Curr. Nutr. Rep. 2023, 12, 308–326. [Google Scholar] [CrossRef]
- Khalid, N.; Abdelrahim, D.N.; Hanach, N.; Alkurd, R.; Khan, M.; Mahrous, L.; Radwan, H.; Naja, F.; Madkour, M.; Obaideen, K.; et al. Effect of camel milk on lipid profile among patients with diabetes: A systematic review, meta-analysis, and meta-regression of randomized controlled trials. BMC Complement. Med. Ther. 2023, 23, 438. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Hou, Y.; Zhong, J.; Hettinga, K.; Zhou, P. Phosphoproteomics reveals that camel and goat milk improve glucose homeostasis in HDF/STZ-induced diabetic rats through activation of hepatic AMPK and GSK3-GYS axis. Food Res. Int. 2022, 157, 111254. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ge, W.; Song, Y.; He, R.; Wang, Z.; Zhao, L. Camel milk peptides alleviate hyperglycemia by regulating gut microbiota and metabolites in type 2 diabetic mice. Food Res. Int. 2023, 173, 113278. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Wang, S.; He, R.; Wang, Z.; Zhao, L.; Ge, W. Preparation, characterization, and mechanism of DPP-IV inhibitory peptides derived from Bactrian camel milk. Int. J. Biol. Macromol. 2024, 277, 134232. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Mudgil, P.; Palakkott, A.; Iratni, R.; Gan, C.-Y.; Maqsood, S.; Ayoub, M.A. Molecular basis of the anti-diabetic properties of camel milk through profiling of its bioactive peptides on dipeptidyl peptidase IV (DPP-IV) and insulin receptor activity. J. Dairy Sci. 2021, 104, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Elhadad, N.; de Campos Zani, S.C.; Chan, C.B.; Wu, J. Ovalbumin Hydrolysates Enhance Skeletal Muscle Insulin-Dependent Signaling Pathway in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2024, 72, 15248–15255. [Google Scholar] [CrossRef]
- Luo, Z.; Fu, C.; Li, T.; Gao, Q.; Miao, D.; Xu, J.; Zhao, Y. Hypoglycemic Effects of Licochalcone A on the Streptozotocin-Induced Diabetic Mice and Its Mechanism Study. J. Agric. Food Chem. 2021, 69, 2444–2456. [Google Scholar] [CrossRef]
- Wilson, M.E.; Tzeng, S.-C.; Augustin, M.M.; Meyer, M.; Jiang, X.; Choi, J.H.; Rogers, J.C.; Evans, B.S.; Kutchan, T.M.; Nusinow, D.A. Quantitative Proteomics and Phosphoproteomics Support a Role for Mut9-Like Kinases in Multiple Metabolic and Signaling Pathways in Arabidopsis. Mol. Cell. Proteom. 2021, 20, 100063. [Google Scholar] [CrossRef]
- Althnaibat, R.M.; Bruce, H.L.; Wu, J.; Gänzle, M.G. Bioactive peptides in hydrolysates of bovine and camel milk proteins: A review of studies on peptides that reduce blood pressure, improve glucose homeostasis, and inhibit pathogen adhesion. Food Res. Int. 2024, 175, 113748. [Google Scholar] [CrossRef]
- Pham, T.K.; Nguyen, T.H.T.; Yi, J.M.; Kim, G.S.; Yun, H.R.; Kim, H.K.; Won, J.C. Evogliptin, a DPP-4 inhibitor, prevents diabetic cardiomyopathy by alleviating cardiac lipotoxicity in db/db mice. Exp. Mol. Med. 2023, 55, 767–778. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. Natural compounds with DPP-4 inhibitory effects: Implications for the treatment of diabetes. J. Cell. Biochem. 2019, 120, 10909–10913. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Rai, C.; Priyadarshini, P. Whey protein hydrolysates improve high-fat-diet-induced obesity by modulating the brain-peripheral axis of GLP-1 through inhibition of DPP-4 function in mice. Eur. J. Nutr. 2023, 62, 2489–2507. [Google Scholar] [CrossRef]
- Vergès, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Tibori, K.; Orosz, G.; Zámbó, V.; Szelényi, P.; Sarnyai, F.; Tamási, V.; Rónai, Z.; Mátyási, J.; Tóth, B.; Csala, M.; et al. Molecular Mechanisms Underlying the Elevated Expression of a Potentially Type 2 Diabetes Mellitus Associated SCD1 Variant. Int. J. Mol. Sci. 2022, 23, 6221. [Google Scholar] [CrossRef]
- Görgens, S.W.; Jahn-Hofmann, K.; Bangari, D.; Cummings, S.; Metz-Weidmann, C.; Schwahn, U.; Wohlfart, P.; Schäfer, M.; Bielohuby, M. A siRNA mediated hepatic DPP-4 knockdown affects lipid, but not glucose metabolism in diabetic mice. PLoS ONE 2019, 14, e0225835. [Google Scholar] [CrossRef]
- Zou, J.; Song, Q.; Shaw, P.C.; Zuo, Z. Dendrobium officinale regulate lipid metabolism in diabetic mouse liver via PPAR-RXR signaling pathway: Evidence from an integrated multi-omics analysis. Biomed. Pharmacother. 2024, 173, 116395. [Google Scholar] [CrossRef]
- Lu, D.L.; He, A.Y.; Tan, M.; Mrad, M.; El Daibani, A.; Hu, D.H.; Liu, X.J.; Kleiboeker, B.; Che, T.; Hsu, F.F.; et al. Liver ACOX1 regulates levels of circulating lipids that promote metabolic health through adipose remodeling. Nat. Commun. 2024, 15, 4214. [Google Scholar] [CrossRef]
- Houten, S.M.; Denis, S.; Argmann, C.A.; Jia, Y.; Ferdinandusse, S.; Reddy, J.K.; Wanders, R.J.A. Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. J. Lipid Res. 2012, 53, 1296–1303. [Google Scholar] [CrossRef]
- Dave, A.; Park, E.-J.; Kumar, A.; Parande, F.; Idle, J.R.; Pezzuto, J.M.; Beyoglu, D. Consumption of Grapes Modulates Gene Expression, Reduces Non-Alcoholic Fatty Liver Disease, and Extends Longevity in Female C57BL/6J Mice Provided with a High-Fat Western-Pattern Diet. Foods 2022, 11, 1984. [Google Scholar] [CrossRef]
- He, A.; Chen, X.; Tan, M.; Chen, Y.; Lu, D.; Zhang, X.; Dean, J.M.; Razani, B.; Lodhi, I.J. Acetyl-CoA Derived from Hepatic Peroxisomal β-Oxidation Inhibits Autophagy and Promotes Steatosis via mTORC1 Activation. Mol. Cell 2020, 79, 30–42.e34. [Google Scholar] [CrossRef]
- Wang, C.Q.; Hu, M.H.; Yi, Y.H.; Wen, X.N.; Lv, C.H.; Shi, M.; Zeng, C.X. Multiomic analysis of dark tea extract on glycolipid metabolic disorders in db/db mice. Front. Nutr. 2022, 9, 1006517. [Google Scholar] [CrossRef]
- Anderson, C.M.; Stahl, A. SLC27 fatty acid transport proteins. Mol. Asp. Med. 2013, 34, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Reilly, S.M.; Abu-Odeh, M.; Murphy, A.N.; Mahata, S.K.; Zhang, J.Y.; Cho, Y.; Seo, J.B.; Hung, C.W.; Green, C.R.; et al. TANK-Binding Kinase 1 Regulates the Localization of Acyl-CoA Synthetase ACSL1 to Control Hepatic Fatty Acid Oxidation. Cell Metab. 2020, 32, 1012–1027.e7. [Google Scholar] [CrossRef] [PubMed]
- Pfohl, M.; DaSilva, N.A.; Marques, E.; Agudelo, J.; Liu, C.; Goedken, M.; Slitt, A.L.; Seeram, N.P.; Ma, H. Hepatoprotective and anti-inflammatory effects of a standardized pomegranate (Punica granatum) fruit extract in high fat diet-induced obese C57BL/6 mice. Int. J. Food Sci. Nutr. 2021, 72, 499–510. [Google Scholar] [CrossRef] [PubMed]




| NCG | DCG | PCG | LPG | HPG | |
|---|---|---|---|---|---|
| TC (mmol/L) | 6.72 ± 0.16 a | 15.63 ± 0.81 d | 7.63 ± 0.57 b | 9.77 ± 1.00 c | 10.53 ± 1.09 c |
| TG (mmol/L) | 5.14 ± 0.39 b | 7.5 ± 0.36 d | 5.51 ± 0.22 c | 5.44 ± 0.3 bc | 4.79 ± 0.19 a |
| HDL-C (mmol/L) | 3.17 ± 0.45 c | 1.44 ± 0.32 a | 2.82 ± 0.91 c | 1.78 ± 0.68 b | 1.26 ± 0.53 b |
| LDL-C (mmol/L) | 3.40 ± 0.43 a | 12.89 ± 0.65 d | 4.96 ± 0.79 b | 7.53 ± 1.06 c | 7.72 ± 1.13 c |
| Insulin (mIU/L) | 8.35 ± 0.24 c | 9.96 ± 0.29 d | 7.63 ± 0.47 ab | 7.75 ± 0.45 ab | 7.93 ± 0.23 b |
| HOMA-IR | 2.44 ± 0.27 a | 11.64 ± 0.48 d | 3.53 ± 0.77 b | 6.5 ± 1.39 c | 4.29 ± 1.38 b |
| HOMA-IS | 0.0192 ± 0.0016 d | 0.0038 ± 0.0001 a | 0.0142 ± 0.0022 c | 0.0085 ± 0.0010 b | 0.0112 ± 0.0031 bc |
| GLP-1 (pmol/L) | 0.87 ± 0.04 bc | 0.80 ± 0.03 a | 1.10 ± 0.07 d | 0.83 ± 0.05 ab | 0.90 ± 0.06 c |
| DPP-4 activity (mU/mL) | 5.72 ± 0.46 a | 29.95 ± 0.93 d | 6.45 ± 0.35 a | 23.33 ± 1.82 c | 13.51 ± 0.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Ye, Y.; Zhang, C.; Zhang, L.; Zhou, P. A Novel Camel Milk-Derived Peptide LLPK Improves Glucose-Lipid Metabolism in db/db Mice via PPAR Signaling Pathway. Nutrients 2025, 17, 1693. https://doi.org/10.3390/nu17101693
Han B, Ye Y, Zhang C, Zhang L, Zhou P. A Novel Camel Milk-Derived Peptide LLPK Improves Glucose-Lipid Metabolism in db/db Mice via PPAR Signaling Pathway. Nutrients. 2025; 17(10):1693. https://doi.org/10.3390/nu17101693
Chicago/Turabian StyleHan, Binsong, Yuhui Ye, Cunzheng Zhang, Lina Zhang, and Peng Zhou. 2025. "A Novel Camel Milk-Derived Peptide LLPK Improves Glucose-Lipid Metabolism in db/db Mice via PPAR Signaling Pathway" Nutrients 17, no. 10: 1693. https://doi.org/10.3390/nu17101693
APA StyleHan, B., Ye, Y., Zhang, C., Zhang, L., & Zhou, P. (2025). A Novel Camel Milk-Derived Peptide LLPK Improves Glucose-Lipid Metabolism in db/db Mice via PPAR Signaling Pathway. Nutrients, 17(10), 1693. https://doi.org/10.3390/nu17101693







