Lactobacillus plantarum and Galacto-Oligosaccharides Synbiotic Relieve Irritable Bowel Syndrome by Reshaping Gut Microbiota and Attenuating Mast Cell Hyperactivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Probiotic Growth Curve
2.3. Animal Experiment Procedure
2.4. Behavioral Assessments
2.4.1. Open Field Test
2.4.2. Marble Burying Test
2.4.3. Physiological Markers of Colonic Content Transit
2.4.4. Visceral Hypersensitivity
2.5. Real-Time qPCR
2.6. Determination of Biochemical Indicators
2.7. The H&E and Alcian Blue Staining
2.8. Immunofluorescence Staining
2.9. 16S rRNA Sequencing Analysis
2.10. Quantification of SCFAs Levels
2.11. Correlation Analysis
2.12. Statistical Analysis
3. Results
3.1. Establishing a Synbiotic of L. plantarum ZYC501 and GOS
3.2. The Synbiotic Alleviated Colonic Transit Dysfunction, Visceral Hypersensitivity, and Anxiety-like Behaviors in IBS Mice
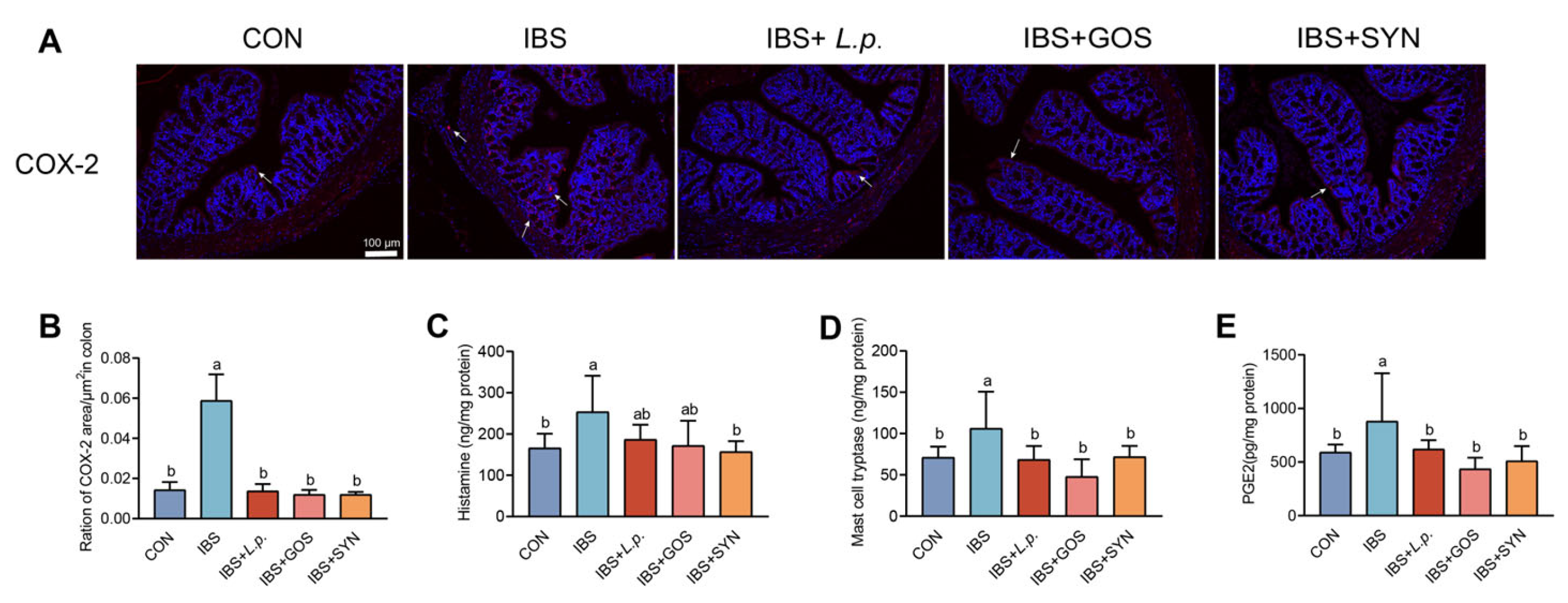
3.3. Effects of Synbiotic Treatment on Modulating Mast Cell Hyperactivation
3.4. Synbiotic Treatment Enhances the Integrity of the Colonic Barrier in IBS Mice
3.5. Synbiotic Treatment Effectively Reduces Colonic Inflammation in the IBS Mice
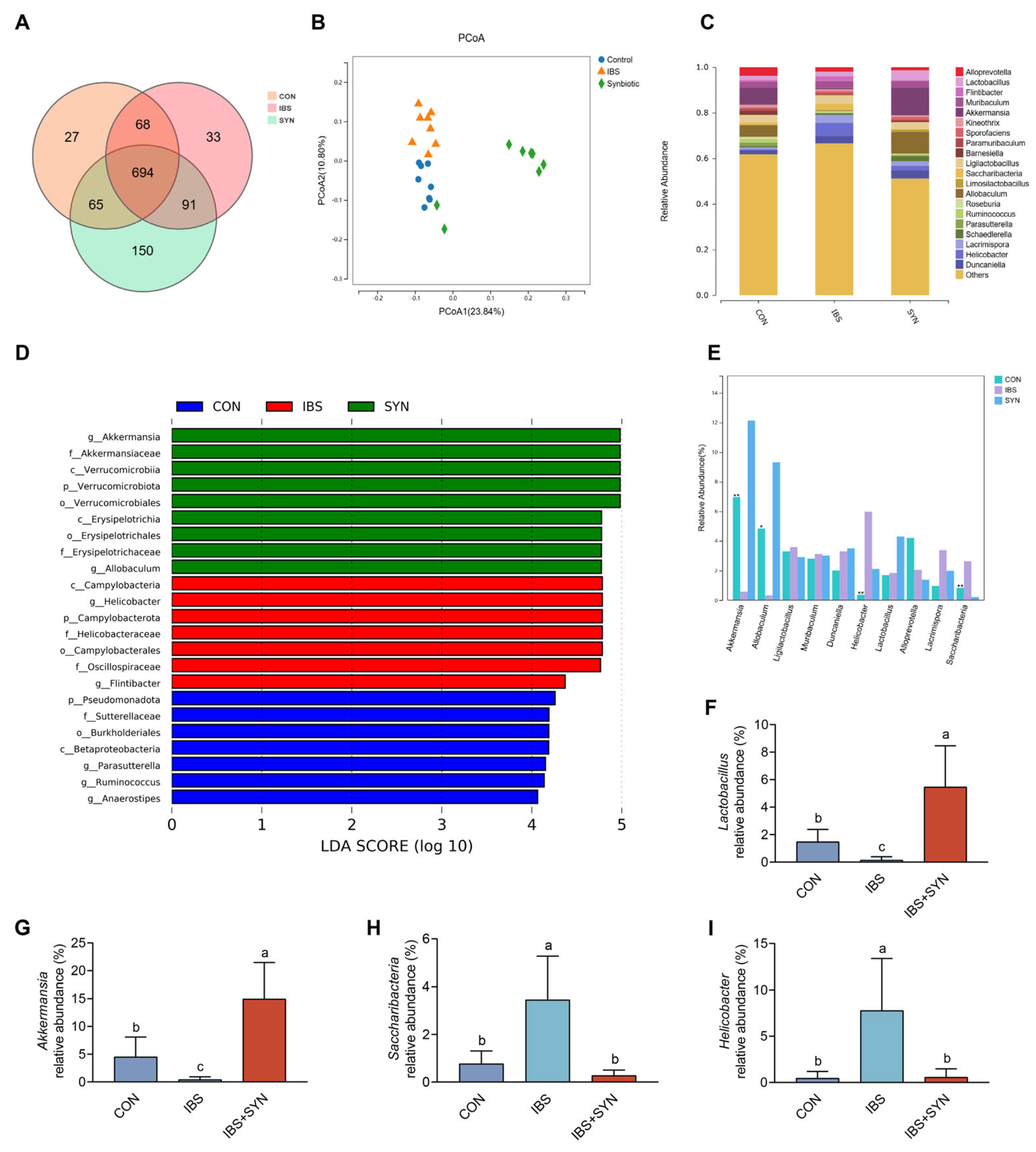
3.6. Effects of Synbiotic Treatment on SCFAs Production and Gut Microbiota Diversity in IBS Mice
3.7. Correlational Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. irritable bowel syndrome. Nat. Rev. Dis. Primers 2016, 2, 16014. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut Microbiota Role in Irritable Bowel Syndrome: New Therapeutic Strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef]
- Kamphuis, J.B.J.; Guiard, B.; Leveque, M.; Olier, M.; Jouanin, I.; Yvon, S.; Tondereau, V.; Rivière, P.; Guéraud, F.; Chevolleau, S.; et al. Lactose and Fructo-Oligosaccharides Increase Visceral Sensitivity in Mice via Glycation Processes, Increasing Mast Cell Density in Colonic Mucosa. Gastroenterology 2020, 158, 652–663.e6. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, J.; Sun, C.; Zhu, S.; Zhai, Y.; Dai, Y.; Wang, X.; Gao, X. Sishen Wan Enhances Intestinal Barrier Function via Regulating Endoplasmic Reticulum Stress to Improve Mice with Diarrheal Irritable Bowel Syndrome. Phytomedicine 2024, 129, 155541. [Google Scholar] [CrossRef]
- Yue, C.; Ma, B.; Zhao, Y.; Li, Q.; Li, J. Lipopolysaccharide-Induced Bacterial Translocation Is Intestine Site-Specific and Associates with Intestinal Mucosal Inflammation. Inflammation 2012, 35, 1880–1888. [Google Scholar] [CrossRef]
- Simpson, C.A.; Mu, A.; Haslam, N.; Schwartz, O.S.; Simmons, J.G. Feeling down? A Systematic Review of the Gut Microbiota in Anxiety/Depression and Irritable Bowel Syndrome. J. Affect. Disord. 2020, 266, 429–446. [Google Scholar] [CrossRef]
- Gao, J.; Xiong, T.; Grabauskas, G.; Owyang, C. Mucosal Serotonin Reuptake Transporter (SERT) Expression in IBS Is Modulated by Gut Microbiota via Mast Cell–Prostaglandin E2. Gastroenterology 2022, 162, 1962–1974.e6. [Google Scholar] [CrossRef]
- Barbara, G. Mucosal Barrier Defects in Irritable Bowel Syndrome. Who Left the Door Open? Am. J. Gastroenterol. 2006, 101, 1295–1298. [Google Scholar] [CrossRef]
- Grabauskas, G.; Wu, X.; Gao, J.; Li, J.-Y.; Turgeon, D.K.; Owyang, C. Prostaglandin E2, Produced by Mast Cells in Colon Tissues from Patients with Irritable Bowel Syndrome, Contributes to Visceral Hypersensitivity in Mice. Gastroenterology 2020, 158, 2195–2207.e6. [Google Scholar] [CrossRef]
- Tap, J.; Derrien, M.; Törnblom, H.; Brazeilles, R.; Cools-Portier, S.; Doré, J.; Störsrud, S.; Le Nevé, B.; Öhman, L.; Simrén, M. Identification of an Intestinal Microbiota Signature Associated with Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123.e8. [Google Scholar] [CrossRef]
- Zhu, X.; Hong, G.; Li, Y.; Yang, P.; Cheng, M.; Zhang, L.; Li, Y.; Ji, L.; Li, G.; Chen, C.; et al. Understanding of the Site-Specific Microbial Patterns towards Accurate Identification for Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Microbiol. Spectr. 2021, 9, e0125521. [Google Scholar] [CrossRef]
- Cheng, P.; Wu, J.; Zong, G.; Wang, F.; Deng, R.; Tao, R.; Qian, C.; Shan, Y.; Wang, A.; Zhao, Y.; et al. Capsaicin Shapes Gut Microbiota and Pre-Metastatic Niche to Facilitate Cancer Metastasis to Liver. Pharmacol. Res. 2023, 188, 106643. [Google Scholar] [CrossRef]
- Chang, L.; Tong, K.; Ameen, V. Ischemic Colitis and Complications of Constipation Associated with the Use of Alosetron under a Risk Management Plan: Clinical Characteristics, Outcomes, and Incidences. Am. J. Gastroenterol. 2010, 105, 866–875. [Google Scholar] [CrossRef]
- Lembo, A.J.; Lacy, B.E.; Zuckerman, M.J.; Schey, R.; Dove, L.S.; Andrae, D.A.; Davenport, J.M.; McIntyre, G.; Lopez, R.; Turner, L.; et al. Eluxadoline for Irritable Bowel Syndrome with Diarrhea. N. Engl. J. Med. 2016, 374, 242–253. [Google Scholar] [CrossRef]
- Andresen, V.; Gschossmann, J.; Layer, P. Heat-Inactivated Bifidobacterium Bifidum MIMBb75 (SYN-HI-001) in the Treatment of Irritable Bowel Syndrome: A Multicentre, Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Lancet Gastroenterol. Hepatol. 2020, 5, 658–666. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient Intake, Diet Quality, and Diet Diversity in Irritable Bowel Syndrome and the Impact of the Low FODMAP Diet. J. Acad. Nutr. Diet. 2020, 120, 535–547. [Google Scholar] [CrossRef]
- Lin, C.-S.; Chang, C.-J.; Lu, C.-C.; Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Young, J.D.; Lai, H.-C. Impact of the Gut Microbiota, Prebiotics, and Probiotics on Human Health and Disease. Biomed. J. 2014, 37, 259–268. [Google Scholar] [CrossRef]
- He, M.; Shi, B. Gut Microbiota as a Potential Target of Metabolic Syndrome: The Role of Probiotics and Prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef]
- De Andrés, J.; Manzano, S.; García, C.; Rodríguez, J.M.; Espinosa-Martos, I.; Jiménez, E. Modulatory Effect of Three Probiotic Strains on Infants’ Gut Microbial Composition and Immunological Parameters on a Placebo-Controlled, Double-Blind, Randomised Study. Benef. Microbes. 2018, 9, 573–584. [Google Scholar] [CrossRef]
- Ma, T.; Jin, H.; Kwok, L.-Y.; Sun, Z.; Liong, M.-T.; Zhang, H. Probiotic Consumption Relieved Human Stress and Anxiety Symptoms Possibly via Modulating the Neuroactive Potential of the Gut Microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Hojsak, I. Health Benefits of Lactobacillus Rhamnosus GG and Bifidobacterium Animalis Subspecies Lactis BB-12 in Children. Postgrad. Med. 2020, 132, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Ducrotté, P.; Sawant, P.; Jayanthi, V. Clinical Trial: Lactobacillus Plantarum 299v (DSM 9843) Improves Symptoms of Irritable Bowel Syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, W.; Yu, L.; Tian, F.; Wang, G.; Lu, W.; Narbad, A.; Chen, W.; Zhai, Q. Evidence from Comparative Genomic Analyses Indicating That Lactobacillus-Mediated Irritable Bowel Syndrome Alleviation Is Mediated by Conjugated Linoleic Acid Synthesis. Food Funct. 2021, 12, 1121–1134. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Xue, Z.; Zhai, Q.; et al. Lactobacillus Plantarum CCFM8610 Alleviates Irritable Bowel Syndrome and Prevents Gut Microbiota Dysbiosis: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. Engineering 2021, 7, 376–385. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Wang, G.; Xiong, Z.; Wei, G.; Liao, Z.; Qian, Y.; Cai, Z.; Ai, L. Lactobacillus Plantarum AR495 Improves Colonic Transport Hyperactivity in Irritable Bowel Syndrome through Tryptophan Metabolism. Food Funct. 2024, 15, 7416–7429. [Google Scholar] [CrossRef]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical Trial: The Effects of a Trans-Galactooligosaccharide Prebiotic on Faecal Microbiota and Symptoms in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kosikowski, W.; Szczerbiński, M.; Gantzel, J.; Cukrowska, B. The Effectiveness of Synbiotic Preparation Containing Lactobacillus and Bifidobacterium Probiotic Strains and Short Chain Fructooligosaccharides in Patients with Diarrhea Predominant Irritable Bowel Syndrome-A Randomized Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 1999. [Google Scholar] [CrossRef]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a Diet Based on Inulin-Rich Vegetables on Gut Health and Nutritional Behavior in Healthy Humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Pham, V.T.; Calatayud, M.; Rotsaert, C.; Seifert, N.; Richard, N.; Van den Abbeele, P.; Marzorati, M.; Steinert, R.E. Antioxidant Vitamins and Prebiotic FOS and XOS Differentially Shift Microbiota Composition and Function and Improve Intestinal Epithelial Barrier In Vitro. Nutrients 2021, 13, 1125. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The Pros, Cons, and Many Unknowns of Probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Görke, B.; Stülke, J. Carbon Catabolite Repression in Bacteria: Many Ways to Make the Most out of Nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Niu, Z.; Zou, M.; Liu, S.; Wang, M.; Gu, X.; Lu, H.; Tian, H.; Jha, R. Probiotics, Prebiotics, and Synbiotics Regulate the Intestinal Microbiota Differentially and Restore the Relative Abundance of Specific Gut Microorganisms. J. Dairy Sci. 2020, 103, 5816–5829. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Beale, D.J.; Karpe, A.V.; Shastri, S.; Basheer, W.; Southam, B.; Eri, R.; et al. Synbiotic Supplementation with Prebiotic Green Banana Resistant Starch and Probiotic Bacillus Coagulans Spores Ameliorates Gut Inflammation in Mouse Model of Inflammatory Bowel Diseases. Eur. J. Nutr. 2020, 59, 3669–3689. [Google Scholar] [CrossRef]
- Cai, W.; Pierzynowska, K.; Stiernborg, M.; Xu, J.; Nilsson, I.A.; Svensson, U.; Melas, P.A.; Lavebratt, C. Multispecies Synbiotics Alleviate Dextran Sulfate Sodium (DSS)-Induced Colitis: Effects on Clinical Scores, Intestinal Pathology, and Plasma Biomarkers in Male and Female Mice. Clin. Nutr. ESPEN 2024, 63, 74–83. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academic Press: Washington, DC, USA, 2011; ISBN 10: 0-309-15396-4. [Google Scholar]
- He, Y.; Zhang, B.; Xin, Y.; Wang, W.; Wang, X.; Liu, Z.; She, Y.; Guo, R.; Jia, G.; Wu, S.; et al. Synbiotic Combination of 2′-Fucosyllactose and Bifidobacterium Mitigates Neurodevelopmental Disorders and ASD-like Behaviors Induced by Valproic Acid. Food Funct. 2025, 16, 2703–2717. [Google Scholar] [CrossRef]
- Ibeakanma, C.; Ochoa-Cortes, F.; Miranda-Morales, M.; McDonald, T.; Spreadbury, I.; Cenac, N.; Cattaruzza, F.; Hurlbut, D.; Vanner, S.; Bunnett, N.; et al. Brain-Gut Interactions Increase Peripheral Nociceptive Signaling in Mice with Postinfectious Irritable Bowel Syndrome. Gastroenterology 2011, 141, 2098–2108.e5. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, T.; Zhou, S.; Yuan, Y.; Chen, W.; Zheng, J.; Liu, X.; Yuan, T.; Lu, Y.; Liu, Z. Effects of the ApoE Genotype on Cognitive Function in Aging Mice Fed with a High-Fat Diet and the Protective Potential of n-3 Polyunsaturated Fatty Acids. Food Funct. 2024, 15, 2249–2264. [Google Scholar] [CrossRef]
- Zou, Q.; Han, S.; Liang, J.; Yan, G.; Wang, Q.; Wang, Y.; Zhang, Z.; Hu, J.; Li, J.; Yuan, T.; et al. Alleviating Effect of Vagus Nerve Cutting in Salmonella-Induced Gut Infections and Anxiety-like Behavior via Enhancing Microbiota-Derived GABA. Brain Behav. Immun. 2024, 119, 607–620. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, J.; Zou, Q.; Wang, W.; Yan, G.; Guo, R.; Yuan, T.; Wang, Y.; Liu, X.; Liu, Z. Tryptophan Metabolism-Regulating Probiotics Alleviate Hyperuricemia by Protecting the Gut Barrier Integrity and Enhancing Colonic Uric Acid Excretion. J. Agric. Food Chem. 2024, 72, 26746–26761. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Pan, F.; Li, Y.; Wang, D.; Pang, J.; Sang, H.; Xi, Y.; Shi, L.; Liu, Z. Dietary Methionine Restriction Alleviates Cognitive Impairment in Alzheimer’s Disease Mice via Sex-Dependent Modulation on Gut Microbiota and Tryptophan Metabolism: A Multiomics Analysis. J. Agric. Food Chem. 2025, 73, 1356–1372. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, C.; Jia, M.; Wang, Y.; Zhao, Y.; Li, Q.; Gong, J.; He, Y.; Xu, K.; Liu, X.; et al. Synbiotic Therapy with Clostridium Sporogenes and Xylan Promotes Gut-Derived Indole-3-Propionic Acid and Improves Cognitive Impairments in an Alzheimer’s Disease Mouse Model. Food Funct. 2024, 15, 7865–7882. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, M.; Ding, C.; Bao, B.; Li, H.; Ma, J.; Dong, W.; Gao, R.; Chen, X.; Chen, J.; et al. Time-Restricted Feeding Mitigates Alzheimer’s Disease-Associated Cognitive Impairments via a B. Pseudolongum-Propionic Acid-FFAR3 Axis. iMeta 2025, 4, e70006. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Ning, F.; Wen, J.; Wang, X.; Chen, J.; Hu, J.; Chen, X.; Liu, Z. Secoisolariciresinol Diglucoside Attenuates Neuroinflammation and Cognitive Impairment in Female Alzheimer’s Disease Mice via Modulating Gut Microbiota Metabolism and GPER/CREB/BDNF Pathway. J. Neuroinflamm. 2024, 21, 201. [Google Scholar] [CrossRef]
- De Palma, G.; Lynch, M.D.J.; Lu, J.; Dang, V.T.; Deng, Y.; Jury, J.; Umeh, G.; Miranda, P.M.; Pigrau Pastor, M.; Sidani, S.; et al. Transplantation of Fecal Microbiota from Patients with Irritable Bowel Syndrome Alters Gut Function and Behavior in Recipient Mice. Sci. Transl. Med. 2017, 9, eaaf6397. [Google Scholar] [CrossRef]
- Mujagic, Z.; Jonkers, D.M.A.E.; Ludidi, S.; Keszthelyi, D.; Hesselink, M.A.; Weerts, Z.Z.R.M.; Kievit, R.N.; Althof, J.F.; Leue, C.; Kruimel, J.W.; et al. Biomarkers for Visceral Hypersensitivity in Patients with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2017, 29, e13137. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Irritable Bowel Syndrome: Treatment Based on Pathophysiology and Biomarkers. Gut 2023, 72, 590–599. [Google Scholar] [CrossRef]
- Mertz, H.; Naliboff, B.; Munakata, J.; Niazi, N.; Mayer, E.A. Altered Rectal Perception Is a Biological Marker of Patients with Irritable Bowel Syndrome. Gastroenterology 1995, 109, 40–52. [Google Scholar] [CrossRef]
- Chen, Q.; Ren, Y.; Lu, J.; Bartlett, M.; Chen, L.; Zhang, Y.; Guo, X.; Liu, C. A Novel Prebiotic Blend Product Prevents Irritable Bowel Syndrome in Mice by Improving Gut Microbiota and Modulating Immune Response. Nutrients 2017, 9, 1341. [Google Scholar] [CrossRef]
- Bashashati, M.; Moradi, M.; Sarosiek, I. Interleukin-6 in irritable bowel syndrome: A Systematic Review and Meta-Analysis of IL-6 (-G174C) and Circulating IL-6 Levels. Cytokine 2017, 99, 132–138. [Google Scholar] [CrossRef]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients with Irritable Bowel Syndrome. Gastroenterology 2016, 150, 875–887.e9. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.R.; Cremon, C.; Marasco, G.; Savarino, E.; Guglielmetti, S.; Bonomini, F.; Palombo, M.; Fuschi, D.; Rotondo, L.; Mantegazza, G.; et al. Molecular Mechanisms Underlying Loss of Vascular and Epithelial Integrity in Irritable Bowel Syndrome. Gastroenterology 2024, 167, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Fan, Y.; Huang, S.; Lv, J.; Zhang, Y.; Hao, Z. Baizhu Shaoyao Decoction Restores the Intestinal Barrier and Brain-Gut Axis Balance to Alleviate Diarrhea-Predominant Irritable Bowel Syndrome via FoxO1/FoxO3a. Phytomedicine 2024, 122, 155163. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.S.; Hansson, G.C. Intestinal Mucus and Their Glycans: A Habitat for Thriving Microbiota. Cell Host Microbe 2023, 31, 1087–1100. [Google Scholar] [CrossRef]
- Gallego, P.; Garcia-Bonete, M.-J.; Trillo-Muyo, S.; Recktenwald, C.V.; Johansson, M.E.V.; Hansson, G.C. The Intestinal MUC2 Mucin C-Terminus Is Stabilized by an Extra Disulfide Bond in Comparison to von Willebrand Factor and Other Gel-Forming Mucins. Nat. Commun. 2023, 14, 1969. [Google Scholar] [CrossRef]
- Camilleri, M. Intestinal Secretory Mechanisms in Irritable Bowel Syndrome-Diarrhea. Clin. Gastroenterol. Hepatol. 2015, 13, 1051–1057, quiz e61–e62. [Google Scholar] [CrossRef]
- Li, C.; Shuai, Y.; Zhou, X.; Chen, H. Association between Helicobacter Pylori Infection and Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Medicine 2020, 99, e22975. [Google Scholar] [CrossRef]
- Liang, C.-M.; Hsu, C.-H.; Chung, C.-H.; Chen, C.-Y.; Wang, L.-Y.; Hsu, S.-D.; Chang, P.-K.; Hong, Z.-J.; Chien, W.-C.; Hu, J.-M. Risk for Irritable Bowel Syndrome in Patients with Helicobacter Pylori Infection: A Nationwide Population-Based Study Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 3737. [Google Scholar] [CrossRef]
- Moran, A.P. The Role of Lipopolysaccharide in Helicobacter Pylori Pathogenesis. Aliment. Pharmacol. Ther. 1996, 10 (Suppl. 1), 39–50. [Google Scholar] [CrossRef]
- Ghaffari, S.; Abbasi, A.; Somi, M.H.; Moaddab, S.Y.; Nikniaz, L.; Kafil, H.S.; Ebrahimzadeh Leylabadlo, H. Akkermansia Muciniphila: From Its Critical Role in Human Health to Strategies for Promoting Its Abundance in Human Gut Microbiome. Crit. Rev. Food Sci. Nutr. 2023, 63, 7357–7377. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated with Higher Relative Abundance of Mucin-Degrading Akkermansia Muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Biruete, A.; Cross, T.-W.L.; Allen, J.M.; Kistler, B.M.; de Loor, H.; Evenepoel, P.; Fahey, G.C.; Bauer, L.; Swanson, K.S.; Wilund, K.R. Effect of Dietary Inulin Supplementation on the Gut Microbiota Composition and Derived Metabolites of Individuals Undergoing Hemodialysis: A Pilot Study. J. Ren. Nutr. 2021, 31, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Li, H.; Ding, X.; Li, X.; Jing, X.; Chen, J.; Liu, G.; Lin, Y.; Jiang, C.; et al. Inulin Supplementation Ameliorates Hyperuricemia and Modulates Gut Microbiota in Uox-Knockout Mice. Eur. J. Nutr. 2021, 60, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short Chain Fatty Acids in Human Large Intestine, Portal, Hepatic and Venous Blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary Fiber and SCFAs in the Regulation of Mucosal Immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- van Paassen, N.B.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The Regulation of Intestinal Mucin MUC2 Expression by Short-Chain Fatty Acids: Implications for Epithelial Protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Q.; Zhang, W.; Wang, Y.; Shi, L.; Zhao, Y.; Liang, J.; Zhao, Y.; Kang, J.; Zheng, X.; Guo, R.; et al. Lactobacillus plantarum and Galacto-Oligosaccharides Synbiotic Relieve Irritable Bowel Syndrome by Reshaping Gut Microbiota and Attenuating Mast Cell Hyperactivation. Nutrients 2025, 17, 1670. https://doi.org/10.3390/nu17101670
Yao Q, Zhang W, Wang Y, Shi L, Zhao Y, Liang J, Zhao Y, Kang J, Zheng X, Guo R, et al. Lactobacillus plantarum and Galacto-Oligosaccharides Synbiotic Relieve Irritable Bowel Syndrome by Reshaping Gut Microbiota and Attenuating Mast Cell Hyperactivation. Nutrients. 2025; 17(10):1670. https://doi.org/10.3390/nu17101670
Chicago/Turabian StyleYao, Qi, Wenbo Zhang, Yuze Wang, Le Shi, Yixiao Zhao, Jiarui Liang, Yu Zhao, Jiawei Kang, Xudong Zheng, Rui Guo, and et al. 2025. "Lactobacillus plantarum and Galacto-Oligosaccharides Synbiotic Relieve Irritable Bowel Syndrome by Reshaping Gut Microbiota and Attenuating Mast Cell Hyperactivation" Nutrients 17, no. 10: 1670. https://doi.org/10.3390/nu17101670
APA StyleYao, Q., Zhang, W., Wang, Y., Shi, L., Zhao, Y., Liang, J., Zhao, Y., Kang, J., Zheng, X., Guo, R., Yuan, T., She, Y., & Liu, Z. (2025). Lactobacillus plantarum and Galacto-Oligosaccharides Synbiotic Relieve Irritable Bowel Syndrome by Reshaping Gut Microbiota and Attenuating Mast Cell Hyperactivation. Nutrients, 17(10), 1670. https://doi.org/10.3390/nu17101670







