Predictors of Vitamin D Status in Religious and Intermittent Fasting: A Comparative Study in Orthodox Nuns and Women from the General Population
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Study Populations
2.3. Dietary Regimens
2.4. Anthropometric and Biochemical Assessment
2.5. Dietary Intake and Sun Exposure
2.6. Statistical Analysis
- Model 1: Demographic and anthropometric parameters (age, BMI, calcium and vitamin D intake, and sun exposure);
- Model 2: Model 1 plus total and visceral fat, as obtained by BIA analysis;
- Model 3: Μodel 2 plus PTH and insulin concentrations.
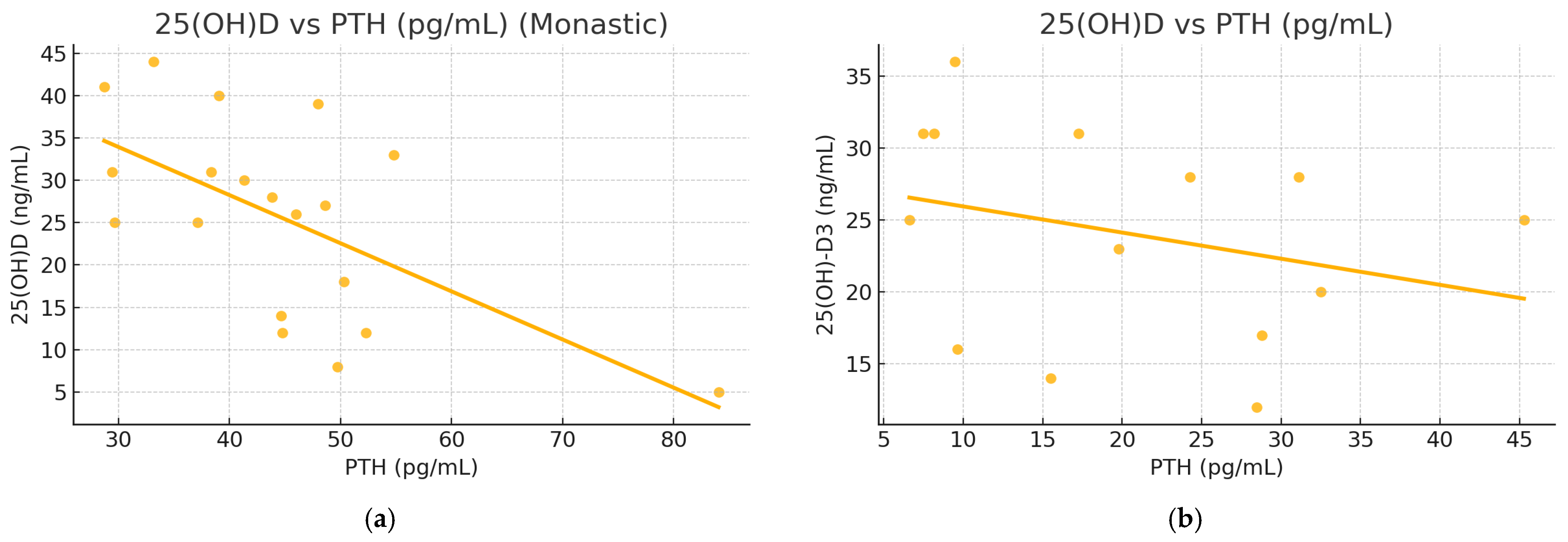
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Management of endocrine disease: Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Antonopoulou, V.; Karalazou, P.; Thisiadou, K.; Mitrofanova, E.; Mulrooney, H.; Petróczi, A.; Zebekakis, P.; et al. Effects of orthodox religious fasting versus combined energy and time restricted eating on body weight, lipid concentrations and glycaemic profile. Int. J. Food Sci. Nutr. 2021, 72, 82–92. [Google Scholar] [CrossRef]
- Michael, A.; Baye, K. Ethiopian orthodox fasting is associated with weight reduction and body composition changes among healthy adults: A prospective cohort study. Sci. Rep. 2023, 13, 7963. [Google Scholar] [CrossRef] [PubMed]
- Georgakouli, K.; Siamata, F.; Draganidis, D.; Tsimeas, P.; Papanikolaou, K.; Batrakoulis, A.; Gatsas, A.; Poulios, A.; Syrou, N.; Deli, C.K.; et al. The Effects of Greek Orthodox Christian Fasting during Holy Week on Body Composition and Cardiometabolic Parameters in Overweight Adults. Diseases 2022, 10, 120. [Google Scholar] [CrossRef]
- Karras, S.N.; Koufakis, T.; Adamidou, L.; Dimakopoulos, G.; Karalazou, P.; Thisiadou, K.; Makedou, K.; Zebekakis, P.; Kotsa, K. Implementation of Christian Orthodox fasting improves plasma adiponectin concentrations compared with time-restricted eating in overweight premenopausal women. Int. J. Food Sci. Nutr. 2022, 73, 210–220. [Google Scholar] [CrossRef]
- Greek National Dietary Guidelines for Adults. Available online: http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/countries/greece/en/ (accessed on 12 December 2024).
- WHO Global Database on Body Mass Index. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed on 12 December 2024).
- Tanita Academy Understanding Your Measurements. Available online: http://tanita.eu/ (accessed on 12 December 2024).
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Wallace, A.; Gibson, S.; de la Hunty, A.; Lamberg-Allardt, C.; Ashwell, M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: Current procedures, performance characteristics and limitations. Steroid Biochem. Mol. Biol. 2010, 121, 10–17. [Google Scholar] [CrossRef]
- Willett, W.C.; Sampson, L.; Stampfer, M.J.; Rosner, B.; Bain, C.; Witschi, J.; Hennekens, C.H.; Speizer, F.E. Reproducibility and validity of a semi-quantitative food frequency questionnaire. Am. J. Epidemiol. 1985, 122, 51–65. [Google Scholar] [CrossRef]
- Glanz, K.; Yaroch, A.L.; Dancel, M.; Saraiya, M.; Crane, L.A.; Buller, D.B.; Manne, S.; O’riordan, D.L.; Heckman, C.J.; Hay, J.; et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch. Dermatol. 2008, 144, 217–222. [Google Scholar] [CrossRef]
- Galchenko, A.V.; Rizzo, G.; Baroni, L. Nutrient Intakes in Vegans, Lacto-Ovo-Vegetarians, Orthodox Fasters, and Omnivores in Russia: A Cross-Sectional Study. Foods 2025, 14, 1062. [Google Scholar] [CrossRef]
- Leucuta, D.C.; Dumitrascu, D.L.; Bangdiwala, S.I.; Palsson, O.S.; Sperber, A.D. Effect of Vegan Diet During Greek-Orthodox Religious Fasting on Symptoms of Disorders of Gut-Brain Interaction. J. Gastrointest. Liver Dis. 2025, 34, 40–46. [Google Scholar] [CrossRef]
- Gholampoor, N.; Sharif, A.H.; Mellor, D. The effect of observing religious or faith-based fasting on cardiovascular disease risk factors: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1097–1109. [Google Scholar] [CrossRef]
- Rodopaios, N.E.; Poulios, E.; Papadopoulou, S.K.; Alexatou, O.; Koulouri, A.A.; Kafatos, A.G.; Papaliagkas, V.; Psara, E.; Giannakoula, A.; Tsourouflis, G.; et al. Association of Christian Orthodox Fasting with Sociodemographic, Anthropometric and Lifestyle Factors and Serum Biochemical Indices: A Cross-Sectional Study on Patients with Metabolic Diseases. Metabolites 2024, 14, 67. [Google Scholar] [CrossRef]
- Kokkinopoulou, A.; Katsiki, N.; Pagkalos, I.; Rodopaios, N.E.; Koulouri, A.A.; Vasara, E.; Papadopoulou, S.K.; Skepastianos, P.; Hassapidou, M.; Kafatos, A.G. Consumption of Ultra-Processed Food and Drink Products in a Greek Christian Orthodox Church Fasting Population. Nutrients 2023, 15, 4907. [Google Scholar] [CrossRef]
- Karras, S.N.; Michalakis, K.; Katsiki, N.; Kypraiou, M.; Vlastos, A.; Anemoulis, M.; Koukoulis, G.; Mouslech, Z.; Talidis, F.; Tzimagiorgis, G.; et al. Interrelations of Leptin and Interleukin-6 in Vitamin D Deficient and Overweight Orthodox Nuns from Northern Greece: A Pilot Study. Nutrients 2025, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Michalakis, K.; Tekos, F.; Skaperda, Z.; Vardakas, P.; Ziakas, P.D.; Kypraiou, M.; Anemoulis, M.; Vlastos, A.; Tzimagiorgis, G.; et al. Effects of Religious Fasting on Markers of Oxidative Status in Vitamin D-Deficient and Overweight Orthodox Nuns versus Implementation of Time-Restricted Eating in Lay Women from Central and Northern Greece. Nutrients 2024, 16, 3300. [Google Scholar] [CrossRef]
- Koppold, D.A.; Breinlinger, C.; Hanslian, E.; Kessler, C.; Cramer, H.; Khokhar, A.R.; Peterson, C.M.; Tinsley, G.; Vernieri, C.; Bloomer, R.J.; et al. International consensus on fasting terminology. Cell Metab. 2024, 36, 1779–1794.e4. [Google Scholar] [CrossRef]
- Xia, J.; Tu, W.; Manson, J.E.; Nan, H.; Shadyab, A.H.; Bea, J.W.; Cheng, T.D.; Hou, L.; Song, Y. Race-specific associations of 25-hydroxyvitamin D and parathyroid hormone with cardiometabolic biomarkers among US white and black postmenopausal women. Am. J. Clin. Nutr. 2020, 112, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Yalla, N.; Bobba, G.; Guo, G.; Stankiewicz, A.; Ostlund, R. Parathyroid hormone reference ranges in healthy individuals classified by vitamin D status. J. Endocrinol. Investig. 2019, 42, 1353–1360. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Yakout, S.; Bukhari, I.; Khattak, M.N.K.; Al-Saleh, Y.; Aljohani, N.; Al-Attas, O.S.; Alokail, M. Parathyroid hormone in relation to various vitamin D metabolites in adult females. Medicine 2017, 96, e8071. [Google Scholar] [CrossRef]
- Callegari, E.T.; Garland, S.M.; Gorelik, A.; Reavley, N.J.; Wark, J.D. Predictors and correlates of serum 25-hydroxyvitamin D concentrations in young women: Results from the Safe-D study. Br. J. Nutr. 2017, 118, 263–272. [Google Scholar] [CrossRef]
- Sempos, C.T.; Lindhout, E.; Heureux, N.; Hars, M.; Parkington, D.A.; Dennison, E.; Durazo-Arvizu, R.; Jones, K.S.; Wise, S.A. Towards harmonization of directly measured free 25-hydroxyvitamin D using an enzyme-linked immunosorbent assay. Anal. Bioanal. Chem. 2022, 414, 7793–7803. [Google Scholar] [CrossRef] [PubMed]
- Tsuprykov, O.; Elitok, S.; Buse, C.; Chu, C.; Krämer, B.K.; Hocher, B. Opposite correlation of 25-hydroxy-vitamin D- and 1,25-dihydroxy-vitamin D-metabolites with gestational age, bone- and lipid-biomarkers in pregnant women. Sci. Rep. 2021, 11, 1923. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.N.; Chactoura, J.; Nohra, F.; Diogenes, M.E.L.; Bezerra, F.F. Free and Bioavailable Fractions of Vitamin D: Association with Maternal Characteristics in Brazilian Pregnant Women. J. Nutr. Metab. 2020, 2020, 1408659. [Google Scholar] [CrossRef] [PubMed]
- Preka, E.; Wan, M.; Price, K.L.; Long, D.A.; Aitkenhead, H.; Shroff, R. Free 25-hydroxyvitamin-D concentrations are lower in children with renal transplant compared with chronic kidney disease. Pediatr. Nephrol. 2020, 35, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Toldy, E.; Salamon, A.; Kálmán, B.; Ágota, K.; Horváth, D.; Lőcsei, Z. Prognostic Relevance of Circulating 25OHD Fractions for Early Recovery and Survival in Patients with Hip Fracture. J. Clin. Med. 2018, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Sauer, C.G.; Loop, M.S.; Venkateswaran, S.; Tangpricha, V.; Ziegler, T.R.; Dhawan, A.; McCall, C.; Bonkowski, E.; Mack, D.R.; Boyle, B.; et al. Free and Bioavailable 25-Hydroxyvitamin D Concentrations are Associated With Disease Activity in Pediatric Patients With Newly Diagnosed Treatment Naive Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Battista, C.; Guarnieri, V.; Carnevale, V.; Baorda, F.; Pileri, M.; Garrubba, M.; Salcuni, A.S.; Chiodini, I.; Minisola, S.; Romagnoli, E.; et al. Vitamin D status in primary hyperparathyroidism: Effect of genetic background. Endocrine 2017, 55, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Zagrodna, A.; Lombardi, G.; Słowińska-Lisowska, M. Seasonal changes in free 25-(OH)D and vitamin D metabolite ratios and their relationship with psychophysical stress markers in male professional football players. Front. Physiol. 2023, 14, 1258678. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M.; Vitamin, D. Metabolites: Analytical Challenges and Clinical Relevance. Calcif. Tissue Int. 2023, 112, 158–177. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, S.; Wang, J.; Zheng, D.; Zhang, H.; Yu, W.; Zhu, L.; Liu, Z.; Yang, X.; Yang, L. Threshold for Relationship between Vitamin D and Parathyroid Hormone in Chinese Women of Childbearing Age. Int. J. Environ. Res. Public Health 2021, 18, 13060. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Cao, Y.; Zhang, H.; Zhao, X.; Li, S.; Hu, Y.; Yang, L. Free and bioavailable 25-hydroxyvitamin D thresholds for bone metabolism and their associations with metabolic syndrome in Chinese women of childbearing age. Front. Nutr. 2023, 10, 1131140. [Google Scholar] [CrossRef]
- Gong, M.; Wang, K.; Sun, H.; Wang, K.; Zhou, Y.; Cong, Y.; Deng, X.; Mao, Y. Threshold of 25(OH)D and consequently adjusted parathyroid hormone reference intervals: Data mining for relationship between vitamin D and parathyroid hormone. J. Endocrinol. Investig. 2023, 46, 2067–2077. [Google Scholar] [CrossRef]
- Povaliaeva, A.; Zhukov, A.; Bogdanov, V.; Bondarenko, A.; Senko, O.; Kuznetsova, A.; Kodryan, M.; Ioutsi, V.; Pigarova, E.; Rozhinskaya, L.; et al. Evaluation of the age-specific relationship between PTH and vitamin D metabolites. Bone Rep. 2024, 22, 101800. [Google Scholar] [CrossRef]


| Variable | Monastic Group (n = 40) | General Population (n = 45) | p-Value |
|---|---|---|---|
| Age (years) | 52.4 ± 17.5 | 49.6 ± 14.3 | 0.314 |
| Years in monastery (years) | 18.6 ± 14.1 | — | — |
| Calcium intake (mg/day) | 467.5 ± 34 | 678 ± 69 | 0.034 |
| Vitamin D intake (IU/day) | 245 ± 11 | 267 ± 18 | 0.23 |
| Sunshine exposure (hours/week) | 4.23 ± 1.2 | 11.2 ± 3.8 | <0.01 |
| BMI | 27.3 ± 4.9 | 25.9 ± 3.8 | 0.151 |
| Body fat (%) | 36.0 ± 7.8 | 34.1 ± 6.1 | 0.220 |
| Visceral fat | 7.7 ± 3.9 | 6.1 ± 2.7 | 0.031 |
| Insulin (μIU/mL) | 11.3 ± 10.6 | 8.4 ± 6.9 | 0.081 |
| PTH (pg/mL) | 42.0 ± 15.1 | 24.6 ± 8.2 | <0.01 |
| 25(OH)D (ng/mL) | 22.5 ± 8.7 | 26.4 ± 9.1 | 0.062 |
| Glucose (mg/dL) | 92.4 ± 9.5 | 89.8 ± 8.7 | 0.129 |
| Calcium (mg/dL) | 9.1 ± 0.3 | 9.4 ± 0.3 | 0.914 |
| Phosphate (mg/dL) | 3.7 ± 0.5 | 3.9 ± 0.4 | 0.087 |
| Predictor | Coefficient | p-Value |
|---|---|---|
| Age (years) | 0.064 | 0.665 |
| BMI | 3.015 | 0.210 |
| Calcium intake (mg/d) | 0.980 | 0.435 |
| Vitamin D intake (IU/d) | 0.142 | 0.123 |
| Sunshine exposure (h/w) | 1.381 | 0.171 |
| Calcium (mg/dL) | 0.054 | 0.779 |
| Phosphate (mg/dL) | −0.217 | 0.528 |
| Predictor | Coefficient | p-Value |
|---|---|---|
| Age (years) | −0.726 | 0.215 |
| BMI | −0.055 | 0.985 |
| Calcium intake (mg/d) | 0.812 | 0.061 |
| Vitamin D intake (IU/d) | 0.143 | 0.056 |
| Sunshine exposure (h/w) | 0.109 | 0.064 |
| Calcium (mg/dL) | 0.123 | 0.708 |
| Phosphate (mg/dL) | −0.278 | 0.412 |
| Body fat (%) | −1.571 | 0.101 |
| Visceral fat | 7.495 | 0.140 |
| Predictor | Orthodox Nuns β (p-Value) | General Population β (p-Value) |
|---|---|---|
| Age (years) | −0.032 (0.784) | −0.017 (0.852) |
| BMI | −0.044 (0.725) | −0.039 (0.791) |
| Calcium intake (mg/day) | 0.129 (0.214) | 0.119 (0.178) |
| Vitamin D intake (IU/day) | 0.086 (0.341) | 0.101 (0.229) |
| Sun exposure (h/week) | 0.121 (0.261) | 0.144 (0.191) |
| Calcium (mg/dL) | 0.045 (0.737) | 0.061 (0.698) |
| Phosphate (mg/dL) | −0.084 (0.611) | −0.112 (0.493) |
| Body fat (%) | −0.091 (0.438) | −0.073 (0.502) |
| Visceral fat | 0.115 (0.301) | 0.132 (0.227) |
| Insulin (μIU/mL) | 0.398 (0.422) | 0.790 (0.231) |
| PTH (pg/mL) | −0.489 (0.013) | −0.549 (0.038) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karras, S.N.; Michalakis, K.; Kypraiou, M.; Vlastos, A.; Anemoulis, M.; Koukoulis, G.; Mouslech, Z.; Talidis, F.; Haitoglou, C.; Michos, G.; et al. Predictors of Vitamin D Status in Religious and Intermittent Fasting: A Comparative Study in Orthodox Nuns and Women from the General Population. Nutrients 2025, 17, 1656. https://doi.org/10.3390/nu17101656
Karras SN, Michalakis K, Kypraiou M, Vlastos A, Anemoulis M, Koukoulis G, Mouslech Z, Talidis F, Haitoglou C, Michos G, et al. Predictors of Vitamin D Status in Religious and Intermittent Fasting: A Comparative Study in Orthodox Nuns and Women from the General Population. Nutrients. 2025; 17(10):1656. https://doi.org/10.3390/nu17101656
Chicago/Turabian StyleKarras, Spyridon N., Konstantinos Michalakis, Maria Kypraiou, Antonios Vlastos, Marios Anemoulis, Georgios Koukoulis, Zadalla Mouslech, Filotas Talidis, Costas Haitoglou, Georgios Michos, and et al. 2025. "Predictors of Vitamin D Status in Religious and Intermittent Fasting: A Comparative Study in Orthodox Nuns and Women from the General Population" Nutrients 17, no. 10: 1656. https://doi.org/10.3390/nu17101656
APA StyleKarras, S. N., Michalakis, K., Kypraiou, M., Vlastos, A., Anemoulis, M., Koukoulis, G., Mouslech, Z., Talidis, F., Haitoglou, C., Michos, G., Papanikolaou, E. G., Skoutas, D., Georgopoulos, N., & Tzimagiorgis, G. (2025). Predictors of Vitamin D Status in Religious and Intermittent Fasting: A Comparative Study in Orthodox Nuns and Women from the General Population. Nutrients, 17(10), 1656. https://doi.org/10.3390/nu17101656







