Long-Term Supplementation of GABA Regulates Growth, Food Intake, Locomotion, and Lipid Metabolism by Increasing Ghrelin and Growth Hormone in Adolescent Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Oral GABA Treatment
2.2. Measurements of Daily Water and Food Consumption
2.3. Anthropometric Measurements
2.4. Metabolic Assessments
2.5. Measurement of Blood Glucose
2.6. Serum Collection and GH Assay
2.7. Tissue Collection
2.8. Immunohistochemistry
2.9. Western Blot
2.10. Statistical Analysis
3. Results
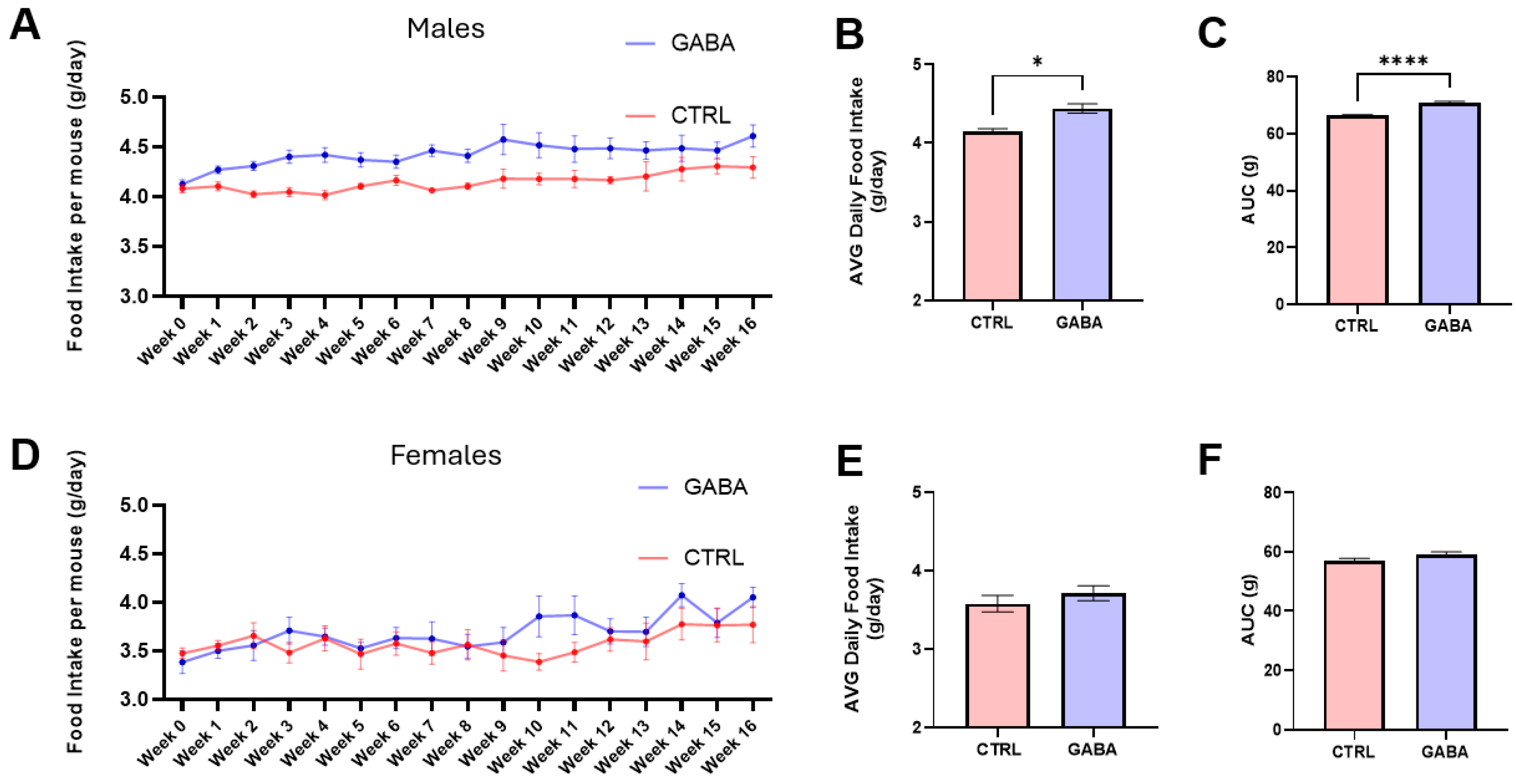
3.1. Effects of GABA on Food and Water Consumption
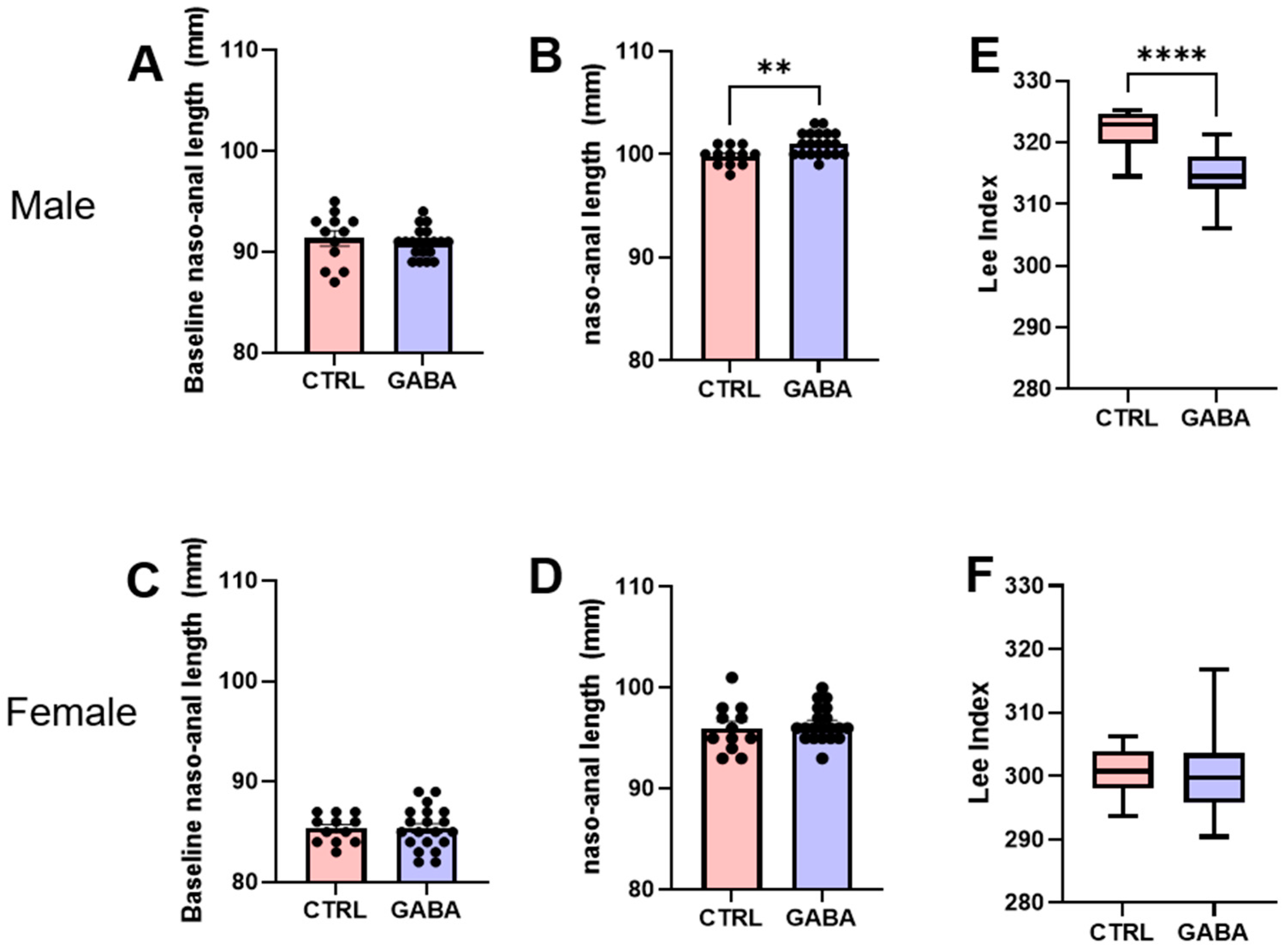
3.2. Effects of GABA on Bodyweight, Nasoanal Length, and Lee Index
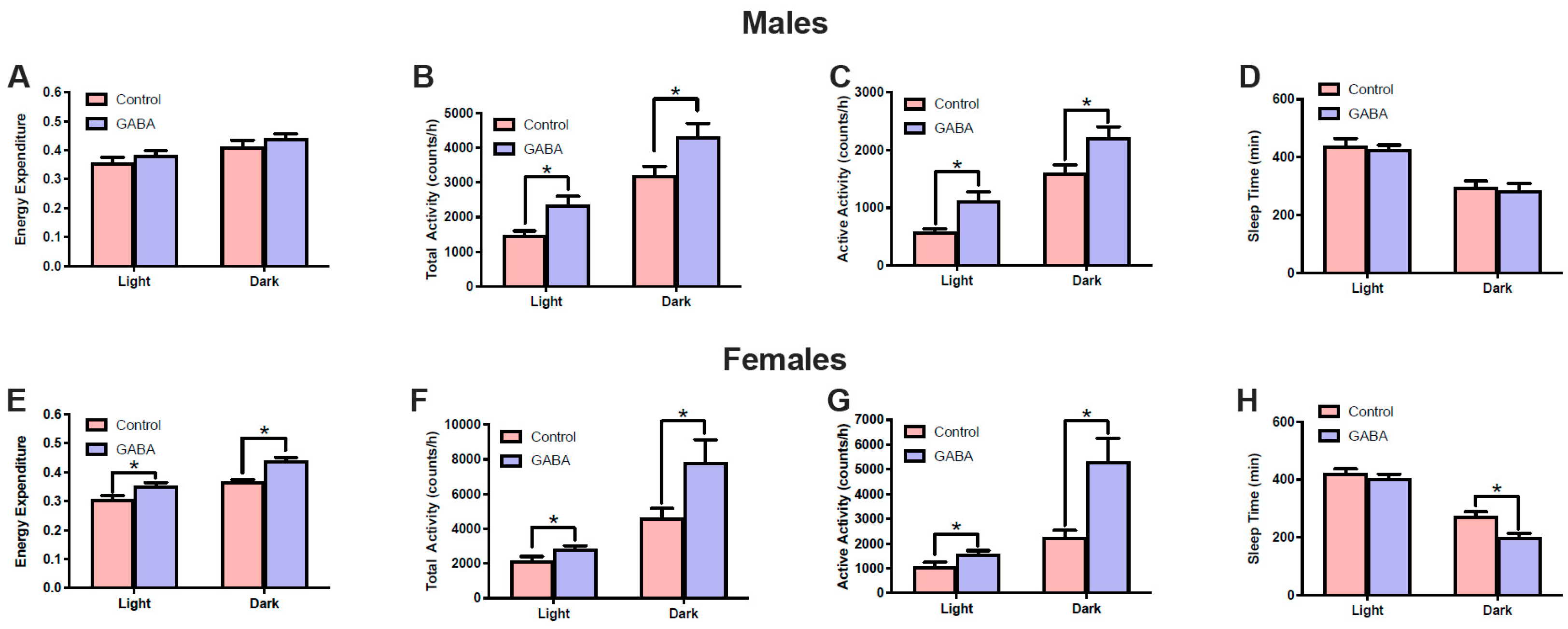
3.3. Effects of GABA on Metabolism and Locomotor Activity
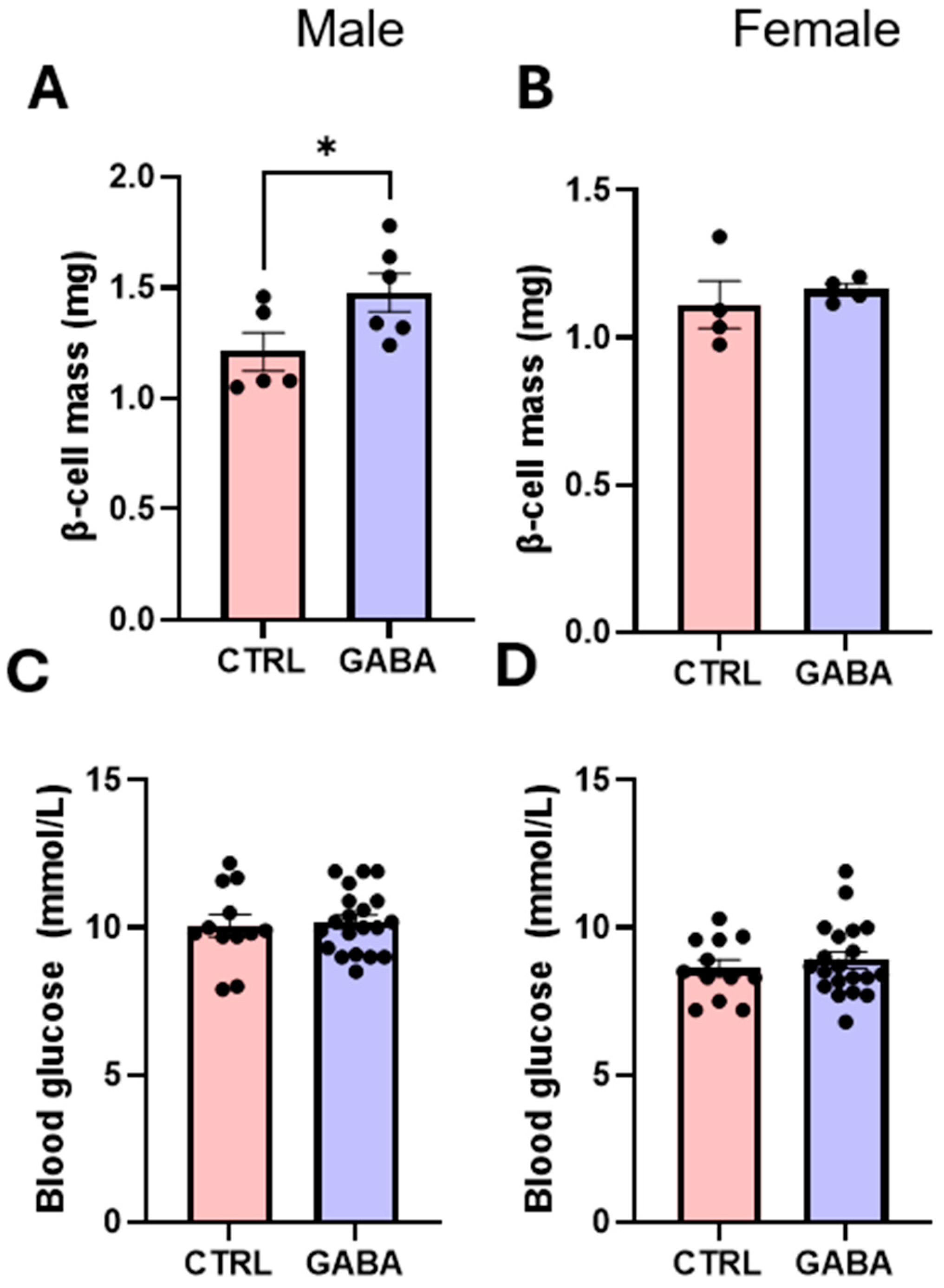
3.4. Effects of GABA on Pancreatic β-Cell Mass and Blood Glucose
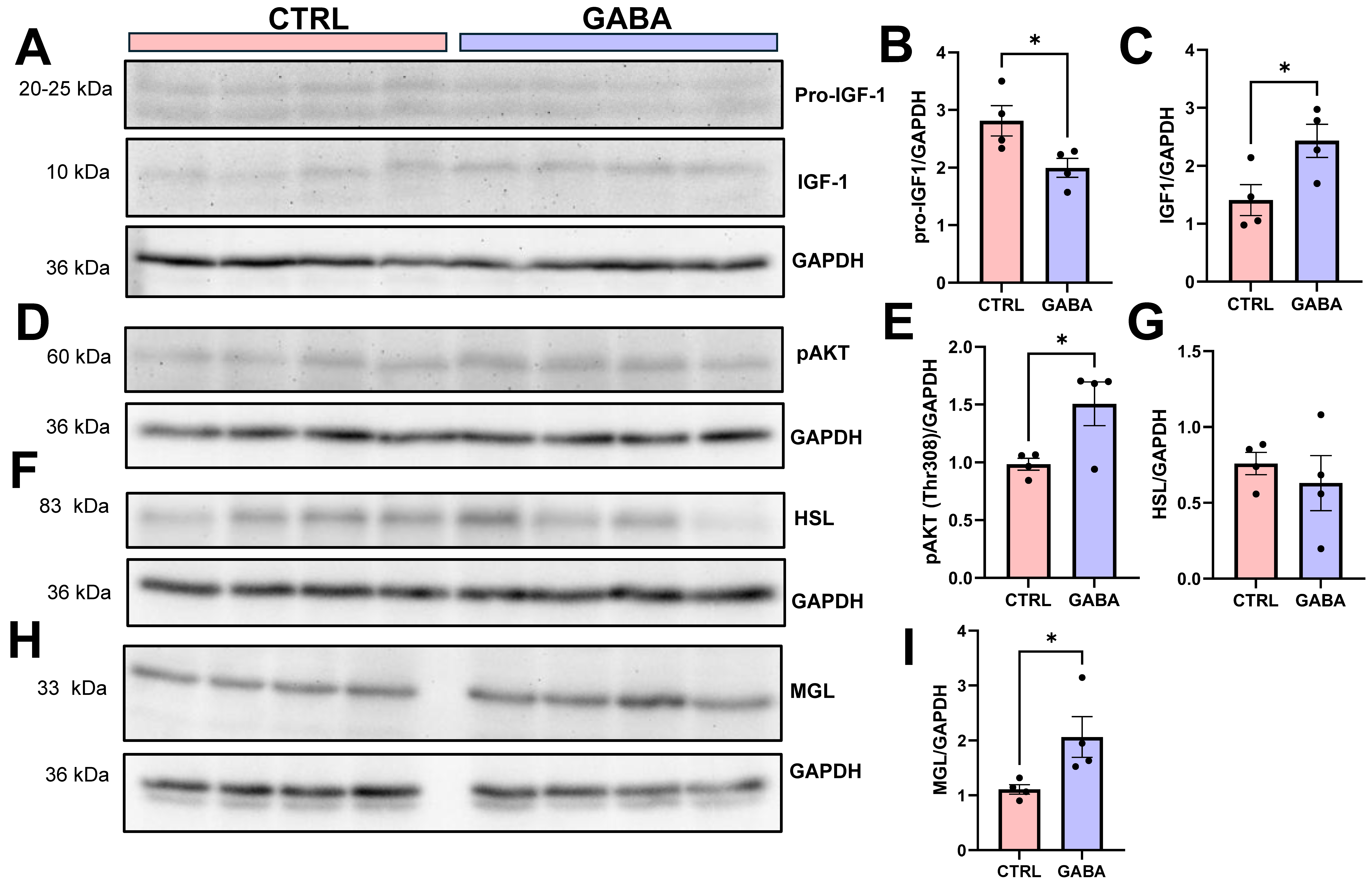
3.5. Effects of GABA on Lipolytic Enzyme Activation in Adipose Tissue
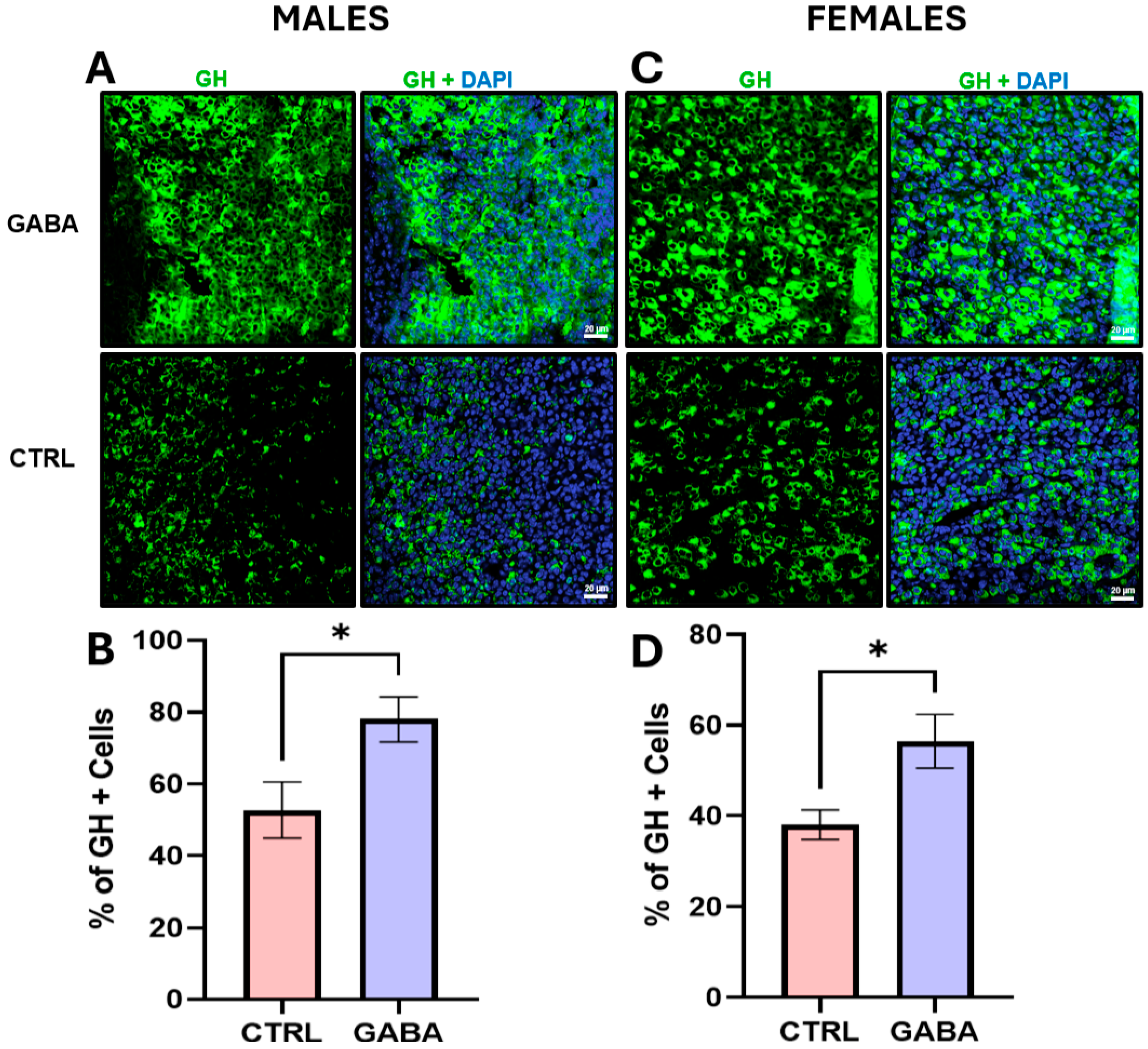
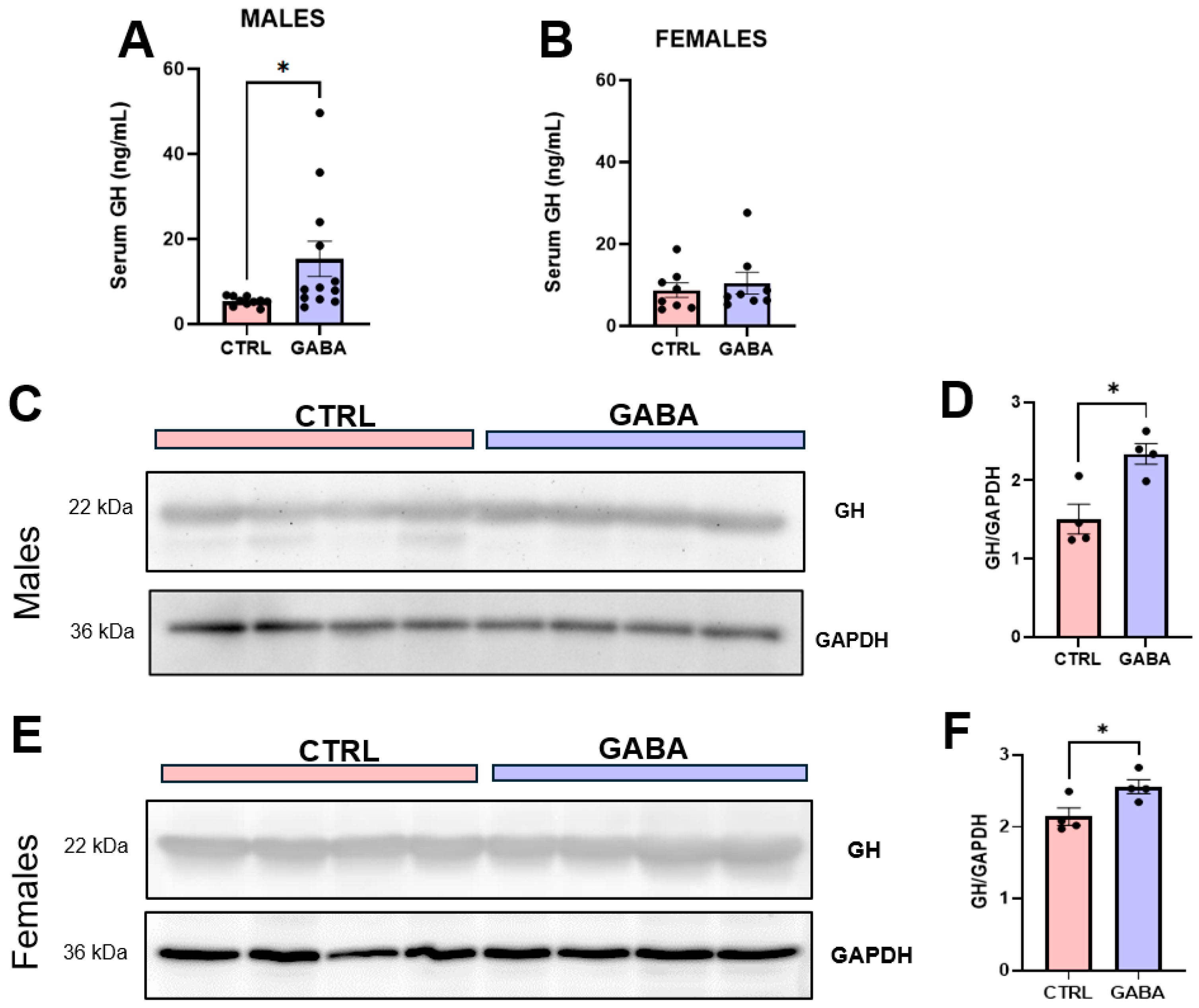
3.6. Effects of GABA on Serum GH, Pituitary GH, and GH-Positive Cells
3.7. Effects of GABA on GH Downstream Effectors in the Liver
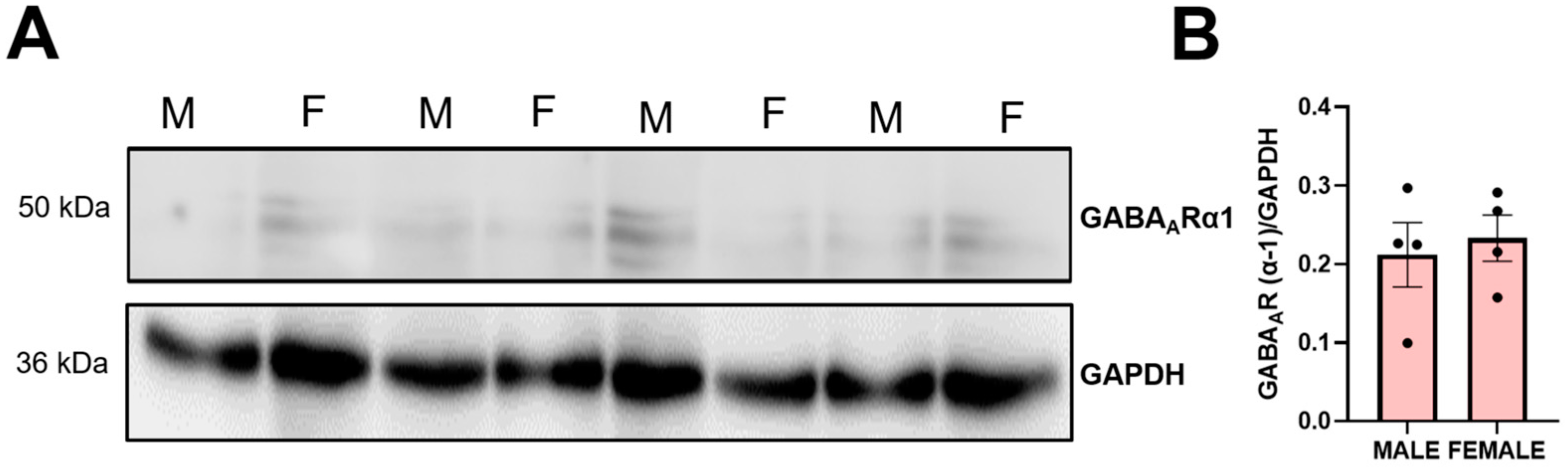
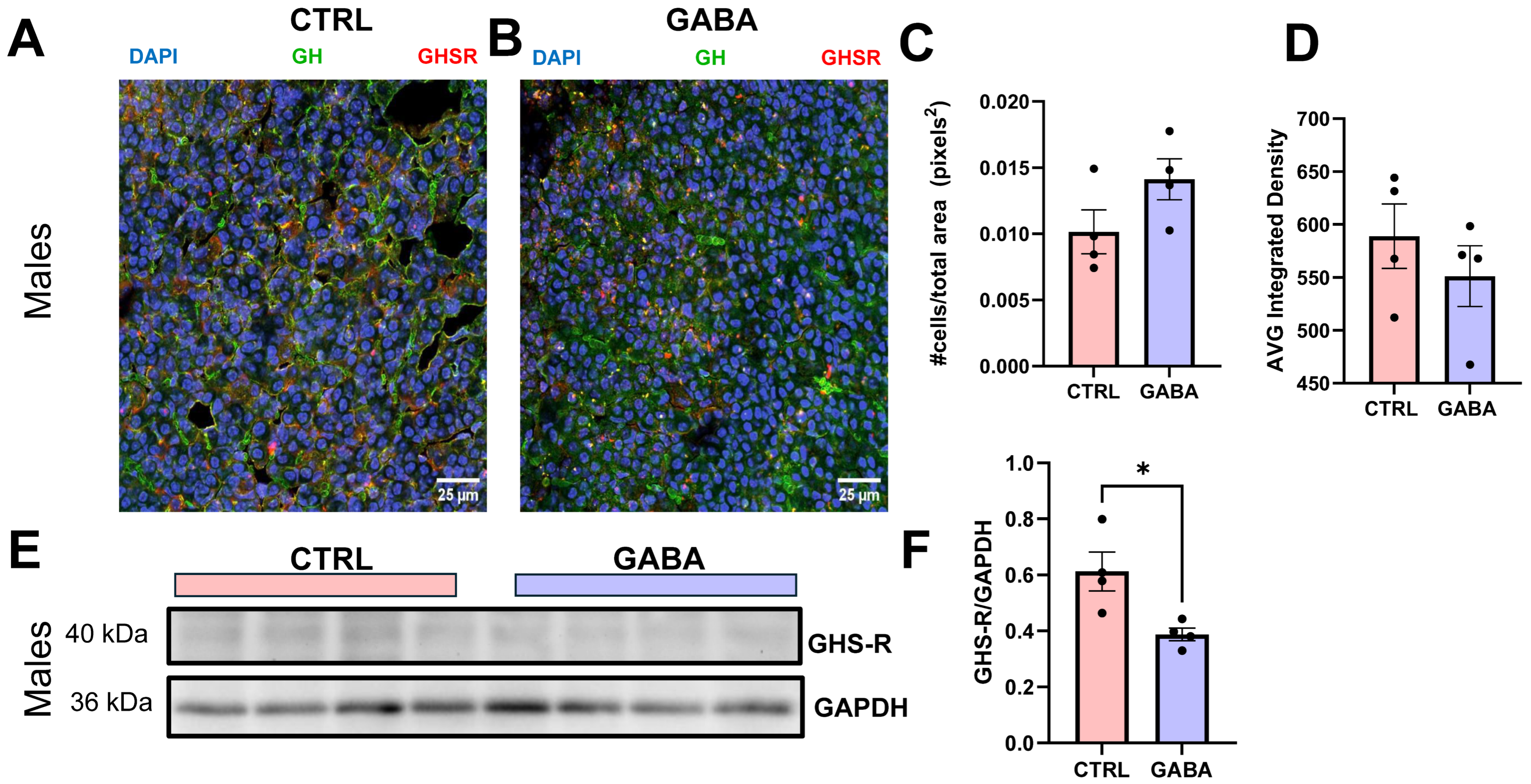
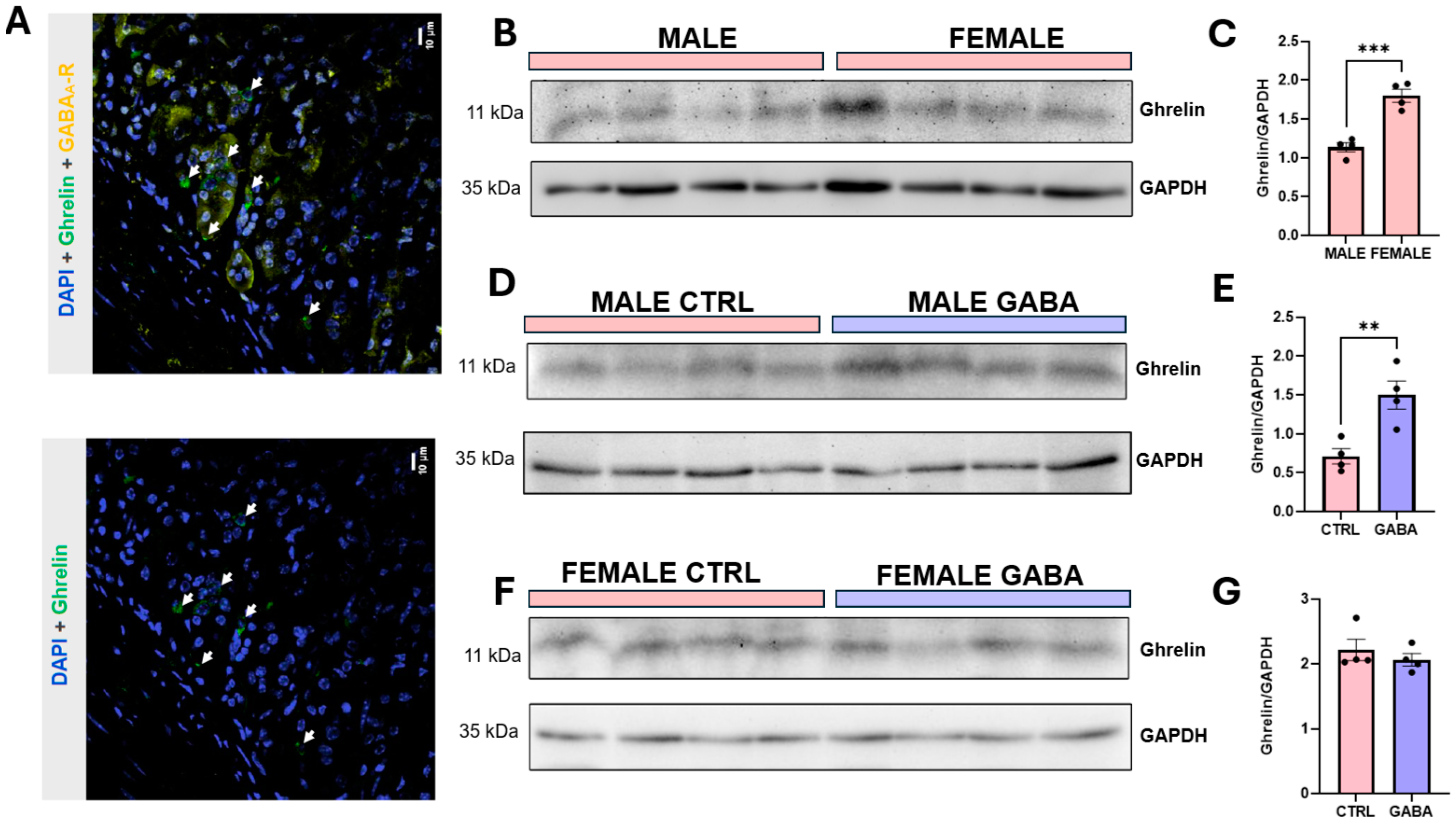
3.8. Effects on Hypothalamic GHRH and Gastric Ghrelin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.F.; Kriegstein, A.R. Is There More to Gaba than Synaptic Inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Mendu, S.K.; Bhandage, A.; Jin, Z.; Birnir, B. Different Subtypes of GABA-A Receptors Are Expressed in Human, Mouse and Rat T Lymphocytes. PLoS ONE 2012, 7, e42959. [Google Scholar] [CrossRef]
- Januzi, L.; Poirier, J.W.; Maksoud, M.J.E.; Xiang, Y.-Y.; Veldhuizen, R.A.W.; Gill, S.E.; Cregan, S.P.; Zhang, H.; Dekaban, G.A.; Lu, W.-Y. Autocrine GABA Signaling Distinctively Regulates Phenotypic Activation of Mouse Pulmonary Macrophages. Cell. Immunol. 2018, 332, 7–23. [Google Scholar] [CrossRef]
- Xiang, Y.-Y.; Wang, S.; Liu, M.; Hirota, J.; Li, J.; Ju, W.; Fan, Y.; Kelly, M.; Ye, B.; Orser, B.; et al. A GABAergic System in Airway Epithelium Is Essential for Mucus Overproduction in Asthma. Nat. Med. 2007, 13, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.-Y.; Chen, X.; Li, J.; Wang, S.; Faclier, G.; Macdonald, J.F.; Hogg, J.C.; Orser, B.A.; Lu, W.-Y. Isoflurane Regulates Atypical Type-A γ-Aminobutyric Acid Receptors in Alveolar Type II Epithelial Cells. Anesthesiology 2013, 118, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiang, Y.-Y.; Zhu, J.; Yi, F.; Li, J.; Liu, C.; Lu, W.-Y. Protective Roles of Hepatic GABA Signaling in Acute Liver Injury of Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G208–G218. [Google Scholar] [CrossRef]
- Dong, H.; Kumar, M.; Zhang, Y.; Gyulkhandanyan, A.; Xiang, Y.-Y.; Ye, B.; Perrella, J.; Hyder, A.; Zhang, N.; Wheeler, M.; et al. Gamma-Aminobutyric Acid up- and Downregulates Insulin Secretion from Beta Cells in Concert with Changes in Glucose Concentration. Diabetologia 2006, 49, 697–705. [Google Scholar] [CrossRef]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA Exerts Protective and Regenerative Effects on Islet Beta Cells and Reverses Diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef]
- Feng, A.L.; Xiang, Y.-Y.; Gui, L.; Kaltsidis, G.; Feng, Q.; Lu, W.-Y. Paracrine GABA and Insulin Regulate Pancreatic Alpha Cell Proliferation in a Mouse Model of Type 1 Diabetes. Diabetologia 2017, 60, 1033–1042. [Google Scholar] [CrossRef]
- Ikegami, R.; Shimizu, I.; Sato, T.; Yoshida, Y.; Hayashi, Y.; Suda, M.; Katsuumi, G.; Li, J.; Wakasugi, T.; Minokoshi, Y.; et al. Gamma-Aminobutyric Acid Signaling in Brown Adipose Tissue Promotes Systemic Metabolic Derangement in Obesity. Cell Rep. 2018, 24, 2827–2837.e5. [Google Scholar] [CrossRef] [PubMed]
- González Solveyra, C.; Estévez, A.G.; Cardinali, D.P. GABA and Its Neural Regulation in Rat Brown Adipose Tissue. J. Neural Transm. Gen. Sect. 1989, 78, 17–28. [Google Scholar] [CrossRef]
- Briguglio, M.; Dell’Osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Zanaboni Dina, C.; Galentino, R.; Porta, M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.A.; Flórez, A.B.; Vázquez, L.; Vasek, O.M.; Mayo, B. Production of γ-Aminobutyric Acid (GABA) by Lactic Acid Bacteria Strains Isolated from Traditional, Starter-Free Dairy Products Made of Raw Milk. Benef. Microbes 2019, 10, 579–587. [Google Scholar] [CrossRef]
- Barido, F.H.; Lee, C.W.; Park, Y.S.; Kim, D.Y.; Lee, S.K. The Effect of a Finishing Diet Supplemented with γ-Aminobutyric Acids on Carcass Characteristics and Meat Quality of Hanwoo Steers. Anim. Biosci. 2021, 34, 621–632. [Google Scholar] [CrossRef]
- Fathi, M.; Saeedyan, S.; Kaoosi, M. Gamma-Amino Butyric Acid (GABA) Supplementation Alleviates Dexamethasone Treatment-Induced Oxidative Stress and Inflammation Response in Broiler Chickens. Stress Amst. Neth. 2023, 26, 2185861. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Pandit, C.; Yu, Y.-H.; Chen, W.-J.; Cheng, Y.-C.; Ali, I.; Cheng, Y.-H. The Impact of Fermented Gamma-Aminobutyric Acid on Poultry Growth Performance Through Insulin-like Growth Factor-1 Activation. Fermentation 2025, 11, 84. [Google Scholar] [CrossRef]
- Ncho, C.M.; Jeong, C.; Gupta, V.; Goel, A. The Effect of Gamma-Aminobutyric Acid Supplementation on Growth Performances, Immune Responses, and Blood Parameters of Chickens Reared under Stressful Environment: A Meta-Analysis. Environ. Sci. Pollut. Res. 2021, 28, 45019–45028. [Google Scholar] [CrossRef]
- Ma, X.; Yan, H.; Hong, S.; Yu, S.; Gong, Y.; Wu, D.; Li, Y.; Xiao, H. Gamma-Aminobutyric Acid Promotes Beige Adipocyte Reconstruction by Modulating the Gut Microbiota in Obese Mice. Nutrients 2023, 15, 456. [Google Scholar] [CrossRef]
- Jin, H.; Han, H.; Song, G.; Oh, H.-J.; Lee, B.-Y. Anti-Obesity Effects of GABA in C57BL/6J Mice with High-Fat Diet-Induced Obesity and 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2024, 25, 995. [Google Scholar] [CrossRef]
- Nagao, T.; Braga, J.D.; Chen, S.; Thongngam, M.; Chartkul, M.; Yanaka, N.; Kumrungsee, T. Synergistic Effects of Peripheral GABA and GABA-Transaminase Inhibitory Drugs on Food Intake Control and Weight Loss in High-Fat Diet-Induced Obese Mice. Front. Pharmacol. 2024, 15, 1487585. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Xuan, L.; Li, A.; Yang, Y.; Zhang, W.; Zhang, J.; Zhang, Y.; An, Z. Gamma-Aminobutyric Acid Supplementation Improves Olanzapine-Induced Insulin Resistance by Inhibiting Macrophage Infiltration in Mice Subcutaneous Adipose Tissue. Diabetes Obes. Metab. 2024, 26, 2695–2705. [Google Scholar] [CrossRef]
- Geisler, C.E.; Ghimire, S.; Bruggink, S.M.; Miller, K.E.; Weninger, S.N.; Kronenfeld, J.M.; Yoshino, J.; Klein, S.; Duca, F.A.; Renquist, B.J. A Critical Role of Hepatic GABA in the Metabolic Dysfunction and Hyperphagia of Obesity. Cell Rep. 2021, 35, 109301. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.B.; Faraguna, U.; Zoltan, J.T.; Tononi, G.; Cirelli, C. Sleep Patterns and Homeostatic Mechanisms in Adolescent Mice. Brain Sci. 2013, 3, 318–343. [Google Scholar] [CrossRef]
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in Adolescent Growth and Development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef] [PubMed]
- King, V.; Norman, J.E.; Seckl, J.R.; Drake, A.J. Post-Weaning Diet Determines Metabolic Risk in Mice Exposed to Overnutrition in Early Life. Reprod. Biol. Endocrinol. 2014, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, W.; Zhang, Y.; Di, L.; Shan, J. A Review of Saponin Intervention in Metabolic Syndrome Suggests Further Study on Intestinal Microbiota. Pharmacol. Res. 2020, 160, 105088. [Google Scholar] [CrossRef]
- Rogers, P.; Webb, G.P. Estimation of Body Fat in Normal and Obese Mice. Br. J. Nutr. 1980, 43, 83–86. [Google Scholar] [CrossRef]
- Guzman, M.S.; De Jaeger, X.; Drangova, M.; Prado, M.A.M.; Gros, R.; Prado, V.F. Mice with Selective Elimination of Striatal Acetylcholine Release Are Lean, Show Altered Energy Homeostasis and Changed Sleep/Wake Cycle. J. Neurochem. 2013, 124, 658–669. [Google Scholar] [CrossRef]
- Cao, D.; Ma, X.; Zhang, W.J.; Xie, Z. Dissection and Coronal Slice Preparation of Developing Mouse Pituitary Gland. J. Vis. Exp. 2017, 129, 56356. [Google Scholar] [CrossRef]
- Basil, P.; Li, Q.; McAlonan, G.M.; Sham, P.-C. Genome-Wide DNA Methylation Data from Adult Brain Following Prenatal Immune Activation and Dietary Intervention. Data Brief 2019, 26, 104561. [Google Scholar] [CrossRef]
- Maksoud, M.J.E.; Tellios, V.; An, D.; Xiang, Y.-Y.; Lu, W.-Y. Nitric Oxide Upregulates Microglia Phagocytosis and Increases Transient Receptor Potential Vanilloid Type 2 Channel Expression on the Plasma Membrane. Glia 2019, 67, 2294–2311. [Google Scholar] [CrossRef]
- Tamura, K.; Minami, K.; Kudo, M.; Iemoto, K.; Takahashi, H.; Seino, S. Liraglutide Improves Pancreatic Beta Cell Mass and Function in Alloxan-Induced Diabetic Mice. PLoS ONE 2015, 10, e0126003. [Google Scholar] [CrossRef]
- An, Y.A.; Scherer, P.E. Mouse Adipose Tissue Protein Extraction. Bio-Protoc. 2020, 10, e3631. [Google Scholar] [CrossRef] [PubMed]
- Heeley, N.; Blouet, C. Central Amino Acid Sensing in the Control of Feeding Behavior. Front. Endocrinol. 2016, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, A.; Zylińska, L.; Boczek, T.; Rębas, E. GABA-Shunt Enzymes Activity in GH3 Cells with Reduced Level of PMCA2 or PMCA3 Isoform. Biochem. Biophys. Res. Commun. 2011, 411, 815–820. [Google Scholar] [CrossRef]
- Sadler, D.G.; Treas, L.; Sikes, J.D.; Porter, C. A Modest Change in Housing Temperature Alters Whole Body Energy Expenditure and Adipocyte Thermogenic Capacity in Mice. Am. J. Physiol.Endocrinol. Metab. 2022, 323, E517–E528. [Google Scholar] [CrossRef]
- Oraha, J.; Enriquez, R.F.; Herzog, H.; Lee, N.J. Sex-Specific Changes in Metabolism during the Transition from Chow to High-Fat Diet Feeding Are Abolished in Response to Dieting in C57BL/6J Mice. Int. J. Obes. 2022, 46, 1749–1758. [Google Scholar] [CrossRef]
- Cavalcanti-de-Albuquerque, J.P.; de-Souza-Ferreira, E.; de Carvalho, D.P.; Galina, A. Coupling of GABA Metabolism to Mitochondrial Glucose Phosphorylation. Neurochem. Res. 2022, 47, 470–480. [Google Scholar] [CrossRef]
- Untereiner, A.; Abdo, S.; Bhattacharjee, A.; Gohil, H.; Pourasgari, F.; Ibeh, N.; Lai, M.; Batchuluun, B.; Wong, A.; Khuu, N.; et al. GABA Promotes β-Cell Proliferation, but Does Not Overcome Impaired Glucose Homeostasis Associated with Diet-Induced Obesity. FASEB J. 2019, 33, 3968–3984. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Liu, X.; Wang, Y.; Mao, F.; Mao, J.; Lu, X.; Jiang, D.; Wan, Y.; Lv, J.-Y.; et al. Study of GABA in Healthy Volunteers: Pharmacokinetics and Pharmacodynamics. Front. Pharmacol. 2015, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- Blissett, J.; Harris, G.; Kirk, J. Effect of Growth Hormone Therapy on Feeding Problems and Food Intake in Children with Growth Disorders. Acta Paediatr. 2000, 89, 644–649. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Novosyadlyy, R.; Wu, Y.; Yakar, S.; LeRoith, D. Biological Effects of Growth Hormone on Carbohydrate and Lipid Metabolism. Growth Horm. IGF Res. 2010, 20, 1–7. [Google Scholar] [CrossRef]
- Donato, J.; Wasinski, F.; Furigo, I.C.; Metzger, M.; Frazão, R. Central Regulation of Metabolism by Growth Hormone. Cells 2021, 10, 129. [Google Scholar] [CrossRef]
- Gertner, J.M. Growth Hormone Actions on Fat Distribution and Metabolism. Horm. Res. 1992, 38, 41–43. [Google Scholar] [CrossRef]
- Zemkova, H.W.; Bjelobaba, I.; Tomic, M.; Zemkova, H.; Stojilkovic, S.S. Molecular Pharmacological and Functional Properties of GABAA Receptors in Anterior Pituitary Cells. J. Physiol. 2008, 586, 3097–3111. [Google Scholar] [CrossRef]
- Tardelli, M. Monoacylglycerol Lipase Reprograms Lipid Precursors Signaling in Liver Disease. World J. Gastroenterol. 2020, 26, 3577–3585. [Google Scholar] [CrossRef]
- Vance, M.L. Growth-Hormone-Releasing Hormone. Clin. Chem. 1990, 36, 415–420. [Google Scholar] [CrossRef]
- Mizutani, M.; Atsuchi, K.; Asakawa, A.; Matsuda, N.; Fujimura, M.; Inui, A.; Kato, I.; Fujimiya, M. Localization of Acyl Ghrelin- and Des-Acyl Ghrelin-Immunoreactive Cells in the Rat Stomach and Their Responses to Intragastric pH. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G974–G980. [Google Scholar] [CrossRef]
- Kakee, A.; Takanaga, H.; Terasaki, T.; Naito, M.; Tsuruo, T.; Sugiyama, Y. Efflux of a Suppressive Neurotransmitter, GABA, across the Blood-Brain Barrier. J. Neurochem. 2001, 79, 110–118. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.A.; Madden, E.F.; Roe, A.L.; Betz, J.M. United States Pharmacopeia (USP) Safety Review of Gamma-Aminobutyric Acid (GABA). Nutrients 2021, 13, 2742. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.T.; Basterfield, L.; McCleave, P.M.J.; Carter, S.M.; Simmons, N.L. Gamma-Aminobutyric Acid (GABA) Transport across Human Intestinal Epithelial (Caco-2) Cell Monolayers. Br. J. Pharmacol. 2000, 129, 457–464. [Google Scholar] [CrossRef]
- Minuk, G.Y. Gamma-Aminobutyric Acid and the Liver. Dig. Dis. 1993, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin Enhances Appetite and Increases Food Intake in Humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992. [Google Scholar] [CrossRef] [PubMed]
- Furigo, I.C.; Teixeira, P.D.S.; de Souza, G.O.; Couto, G.C.L.; Romero, G.G.; Perelló, M.; Frazão, R.; Elias, L.L.; Metzger, M.; List, E.O.; et al. Growth Hormone Regulates Neuroendocrine Responses to Weight Loss via AgRP Neurons. Nat. Commun. 2019, 10, 662. [Google Scholar] [CrossRef]
- Wang, D.M.; Chacher, B.; Liu, H.Y.; Wang, J.K.; Lin, J.; Liu, J.X. Effects of γ-Aminobutyric Acid on Feed Intake, Growth Performance and Expression of Related Genes in Growing Lambs. Animal 2015, 9, 445–448. [Google Scholar] [CrossRef]
- Wang, D.M.; Wang, C.; Liu, H.Y.; Liu, J.X.; Ferguson, J.D. Effects of Rumen-Protected γ-Aminobutyric Acid on Feed Intake, Lactation Performance, and Antioxidative Status in Early Lactating Dairy Cows. J. Dairy Sci. 2013, 96, 3222–3227. [Google Scholar] [CrossRef]
- El-Naggar, K.; El-Kassas, S.; Abdo, S.E.; Kirrella, A.A.K.; Al Wakeel, R.A. Role of Gamma-Aminobutyric Acid in Regulating Feed Intake in Commercial Broilers Reared under Normal and Heat Stress Conditions. J. Therm. Biol. 2019, 84, 164–175. [Google Scholar] [CrossRef]
- Sato, K.; Komaru, T.; Arima, T.; Jardson, C.; Yanaka, N.; Kumrungsee, T. Dietary GABA and Its Combination with Vigabatrin Mimic Calorie Restriction and Induce Antiobesity-like Effects in Lean Mice. J. Funct. Foods 2021, 78, 104367. [Google Scholar] [CrossRef]
- De Bie, T.H.; Witkamp, R.F.; Balvers, M.G.J.; Jongsma, M.A. Effects of γ-Aminobutyric Acid Supplementation on Glucose Control in Adults with Prediabetes: A Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2023, 118, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Börchers, S.; Krieger, J.-P.; Maric, I.; Carl, J.; Abraham, M.; Longo, F.; Asker, M.; Richard, J.E.; Skibicka, K.P. From an Empty Stomach to Anxiolysis: Molecular and Behavioral Assessment of Sex Differences in the Ghrelin Axis of Rats. Front. Endocrinol. 2022, 13, 901669. [Google Scholar] [CrossRef]
- Jessup, S.K.; Dimaraki, E.V.; Symons, K.V.; Barkan, A.L. Sexual Dimorphism of Growth Hormone (GH) Regulation in Humans: Endogenous GH-Releasing Hormone Maintains Basal GH in Women but Not in Men. J. Clin. Endocrinol. Metab. 2003, 88, 4776–4780. [Google Scholar] [CrossRef]
- Gamel-Didelon, K.; Kunz, L.; Fohr, K.J.; Gratzl, M.; Mayerhofer, A. Molecular and Physiological Evidence for Functional Gamma-Aminobutyric Acid (GABA)-C Receptors in Growth Hormone-Secreting Cells. J. Biol. Chem. 2003, 278, 20192–20195. [Google Scholar] [CrossRef]
- Acs, Z.; Szabó, B.; Kapócs, G.; Makara, G.B. Gamma-Aminobutyric Acid Stimulates Pituitary Growth Hormone Secretion in the Neonatal Rat. A Superfusion Study. Endocrinology 1987, 120, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Yarrow, J.F.; McCoy, S.C.; Borst, S.E. Growth Hormone Isoform Responses to GABA Ingestion at Rest and after Exercise. Med. Sci. Sports Exerc. 2008, 40, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Khatib, N.; Gaidhane, S.; Gaidhane, A.M.; Khatib, M.; Simkhada, P.; Gode, D.; Zahiruddin, Q.S. Ghrelin: Ghrelin as a Regulatory Peptide in Growth Hormone Secretion. J. Clin. Diagn. Res. 2014, 8, MC13–MC17. [Google Scholar] [CrossRef]
- Strasser, F.; Lutz, T.A.; Maeder, M.T.; Thuerlimann, B.; Bueche, D.; Tschöp, M.; Kaufmann, K.; Holst, B.; Brändle, M.; von Moos, R.; et al. Safety, Tolerability and Pharmacokinetics of Intravenous Ghrelin for Cancer-Related Anorexia/Cachexia: A Randomised, Placebo-Controlled, Double-Blind, Double-Crossover Study. Br. J. Cancer 2008, 98, 300–308. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- García, A.; Alvarez, C.V.; Smith, R.G.; Diéguez, C. Regulation of Pit-1 Expression by Ghrelin and GHRP-6 through the GH Secretagogue Receptor. Mol. Endocrinol. 2001, 15, 1484–1495. [Google Scholar] [CrossRef][Green Version]
- Pfaffle, R.W.; Kim, C.; Blankenstein, O.; Kentrup, H. GH Transcription Factors. J. Pediatr. Endocrinol. Metab. 1999, 12, 311–317. [Google Scholar] [PubMed]
- Jerlhag, E.; Egecioglu, E.; Dickson, S.L.; Andersson, M.; Svensson, L.; Engel, J.A. Ghrelin Stimulates Locomotor Activity and Accumbal Dopamine-Overflow via Central Cholinergic Systems in Mice: Implications for Its Involvement in Brain Reward. Addict. Biol. 2006, 11, 45–54. [Google Scholar] [CrossRef]
- Jerlhag, E.; Egecioglu, E.; Dickson, S.L.; Douhan, A.; Svensson, L.; Engel, J.A. Ghrelin Administration into Tegmental Areas Stimulates Locomotor Activity and Increases Extracellular Concentration of Dopamine in the Nucleus Accumbens. Addict. Biol. 2007, 12, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Prins, K.; Huisman, M.; McLuskey, A.; Mies, R.; Karels, B.; Delhanty, P.J.D.; Visser, J.A. Ghrelin Deficiency Sex-Dependently Affects Food Intake, Locomotor Activity, and Adipose and Hepatic Gene Expression in a Binge-Eating Mouse Model. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E494–E507. [Google Scholar] [CrossRef] [PubMed]
- Kineman, R.D.; Rio-Moreno, M.; del Sarmento-Cabral, A. 40 YEARS of IGF1: Understanding the Tissue-Specific Roles of IGF1/IGF1R in Regulating Metabolism Using the Cre/loxP System. J. Mol. Endocrinol. 2018, 61, T187–T198. [Google Scholar] [CrossRef]
- Montazeri, M.; Zarkesh, M.; Zadeh-Vakili, A.; Khalili, D.; Movahedi, M.; Khalaj, A. Association of Physical Activity with Increased PI3K and Akt mRNA Levels in Adipose Tissues of Obese and Non-Obese Adults. Sci. Rep. 2023, 13, 9291. [Google Scholar] [CrossRef]
- Vujic, N.; Korbelius, M.; Leopold, C.; Duta-Mare, M.; Rainer, S.; Schlager, S.; Goeritzer, M.; Kolb, D.; Eichmann, T.O.; Diwoky, C.; et al. Monoglyceride Lipase Deficiency Affects Hepatic Cholesterol Metabolism and Lipid-Dependent Gut Transit in ApoE−/− Mice. Oncotarget 2017, 8, 33122–33136. [Google Scholar] [CrossRef]
- Vahl, N.; Møller, N.; Lauritzen, T.; Christiansen, J.S.; Jørgensen, J.O. Metabolic Effects and Pharmacokinetics of a Growth Hormone Pulse in Healthy Adults: Relation to Age, Sex, and Body Composition. J. Clin. Endocrinol. Metab. 1997, 82, 3612–3618. [Google Scholar] [CrossRef]
- Span, J.P.; Pieters, G.F.; Sweep, F.G.; Hermus, A.R.; Smals, A.G. Gender Differences in rhGH-Induced Changes in Body Composition in GH-Deficient Adults. J. Clin. Endocrinol. Metab. 2001, 86, 4161–4165. [Google Scholar] [CrossRef][Green Version]
- Mauras, N.; Bishop, K.; Welch, S. Growth Hormone Action in Puberty: Effects by Gender. Growth Horm. IGF Res. 2007, 17, 463–471. [Google Scholar] [CrossRef]
- Hobbs, C.J.; Plymate, S.R.; Rosen, C.J.; Adler, R.A. Testosterone Administration Increases Insulin-like Growth Factor-I Levels in Normal Men. J. Clin. Endocrinol. Metab. 1993, 77, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Bellantoni, M.F.; Vittone, J.; Campfield, A.T.; Bass, K.M.; Harman, S.M.; Blackman, M.R. Effects of Oral versus Transdermal Estrogen on the Growth Hormone/Insulin-like Growth Factor I Axis in Younger and Older Postmenopausal Women: A Clinical Research Center Study. J. Clin. Endocrinol. Metab. 1996, 81, 2848–2853. [Google Scholar] [CrossRef]
- Smith, A.; Woodside, B.; Abizaid, A. Ghrelin and the Control of Energy Balance in Females. Front. Endocrinol. 2022, 13, 904754. [Google Scholar] [CrossRef]
- Clegg, D.J.; Brown, L.M.; Zigman, J.M.; Kemp, C.J.; Strader, A.D.; Benoit, S.C.; Woods, S.C.; Mangiaracina, M.; Geary, N. Estradiol-Dependent Decrease in the Orexigenic Potency of Ghrelin in Female Rats. Diabetes 2007, 56, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and Immunity Enhancement Effects of Gamma-Aminobutyric Acid (GABA) Administration in Humans. BioFactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Leonte, A.; Colzato, L.S.; Steenbergen, L.; Hommel, B.; Akyürek, E.G. Supplementation of Gamma-Aminobutyric Acid (GABA) Affects Temporal, but Not Spatial Visual Attention. Brain Cogn. 2018, 120, 8–16. [Google Scholar] [CrossRef]
- Kuriyama, K.; Sze, P.Y. Blood-Brain Barrier to H3-γ-Aminobutyric Acid in Normal and Amino Oxyacetic Acid-Treated Animals. Neuropharmacology 1971, 10, 103–108. [Google Scholar] [CrossRef]
- Takanaga, H.; Ohtsuki, S.; Hosoya, K.-I.; Terasaki, T. GAT2/BGT-1 as a System Responsible for the Transport of γ-Aminobutyric Acid at the Mouse Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2001, 21, 1232–1239. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Xiao, W.; Huang, C.; Li, H.; Sun, M. BBB: Permeable Conjugate of Exogenic GABA. ACS Omega 2017, 2, 4108–4111. [Google Scholar] [CrossRef]
- Ben-Othman, N.; Vieira, A.; Courtney, M.; Record, F.; Gjernes, E.; Avolio, F.; Hadzic, B.; Druelle, N.; Napolitano, T.; Navarro-Sanz, S.; et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell 2017, 168, 73–85.e11. [Google Scholar] [CrossRef]
- Jin, Z.; Korol, S.V. GABA Signalling in Human Pancreatic Islets. Front. Endocrinol. 2023, 14, 1059110. [Google Scholar] [CrossRef]
- Zaffanello, M.; Pietrobelli, A.; Cavarzere, P.; Guzzo, A.; Antoniazzi, F. Complex Relationship between Growth Hormone and Sleep in Children: Insights, Discrepancies, and Implications. Front. Endocrinol. 2023, 14, 1332114. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, F.-H.; Karavanaki, K.; Simatou, A.; Tsintzou, E.; Skarakis, N.S.; Kanaka-Gatenbein, C. Association of Growth Hormone Deficiency (GHD) with Anxiety and Depression: Experimental Data and Evidence from GHD Children and Adolescents. Hormones 2021, 20, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, J.E.; Kriström, B.; Jonsson, B.; Tuvemo, T.; Albertsson-Wikland, K. Growth Hormone Treatment Improves Cognitive Function in Short Children with Growth Hormone Deficiency. Horm. Res. Paediatr. 2015, 83, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Athapaththu, A.M.G.K.; Molagoda, I.M.N.; Jayasooriya, R.G.P.T.; Choi, Y.H.; Jeon, Y.-J.; Park, J.-H.; Lee, B.-J.; Kim, G.-Y. Gamma-Aminobutyric Acid (GABA) Promotes Growth in Zebrafish Larvae by Inducing IGF-1 Expression via GABAA and GABAB Receptors. Int. J. Mol. Sci. 2021, 22, 11254. [Google Scholar] [CrossRef]
- Sakashita, M.; Nakamura, U.; Horie, N.; Yokoyama, Y.; Kim, M.; Fujita, S. Oral Supplementation Using Gamma-Aminobutyric Acid and Whey Protein Improves Whole Body Fat-Free Mass in Men After Resistance Training. J. Clin. Med. Res. 2019, 11, 428–434. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begazo-Jimenez, R.; Yu, A.; Gros, R.; Lu, W.-Y. Long-Term Supplementation of GABA Regulates Growth, Food Intake, Locomotion, and Lipid Metabolism by Increasing Ghrelin and Growth Hormone in Adolescent Mice. Nutrients 2025, 17, 1634. https://doi.org/10.3390/nu17101634
Begazo-Jimenez R, Yu A, Gros R, Lu W-Y. Long-Term Supplementation of GABA Regulates Growth, Food Intake, Locomotion, and Lipid Metabolism by Increasing Ghrelin and Growth Hormone in Adolescent Mice. Nutrients. 2025; 17(10):1634. https://doi.org/10.3390/nu17101634
Chicago/Turabian StyleBegazo-Jimenez, Rafael, Amelia Yu, Robert Gros, and Wei-Yang Lu. 2025. "Long-Term Supplementation of GABA Regulates Growth, Food Intake, Locomotion, and Lipid Metabolism by Increasing Ghrelin and Growth Hormone in Adolescent Mice" Nutrients 17, no. 10: 1634. https://doi.org/10.3390/nu17101634
APA StyleBegazo-Jimenez, R., Yu, A., Gros, R., & Lu, W.-Y. (2025). Long-Term Supplementation of GABA Regulates Growth, Food Intake, Locomotion, and Lipid Metabolism by Increasing Ghrelin and Growth Hormone in Adolescent Mice. Nutrients, 17(10), 1634. https://doi.org/10.3390/nu17101634






