The Association Between Maternal Urinary Iodine Concentration and Neonatal Anthropometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Settings, and Design
2.2. Study Protocol
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. UIC Values and Their Relation to the Anthropometric Measures
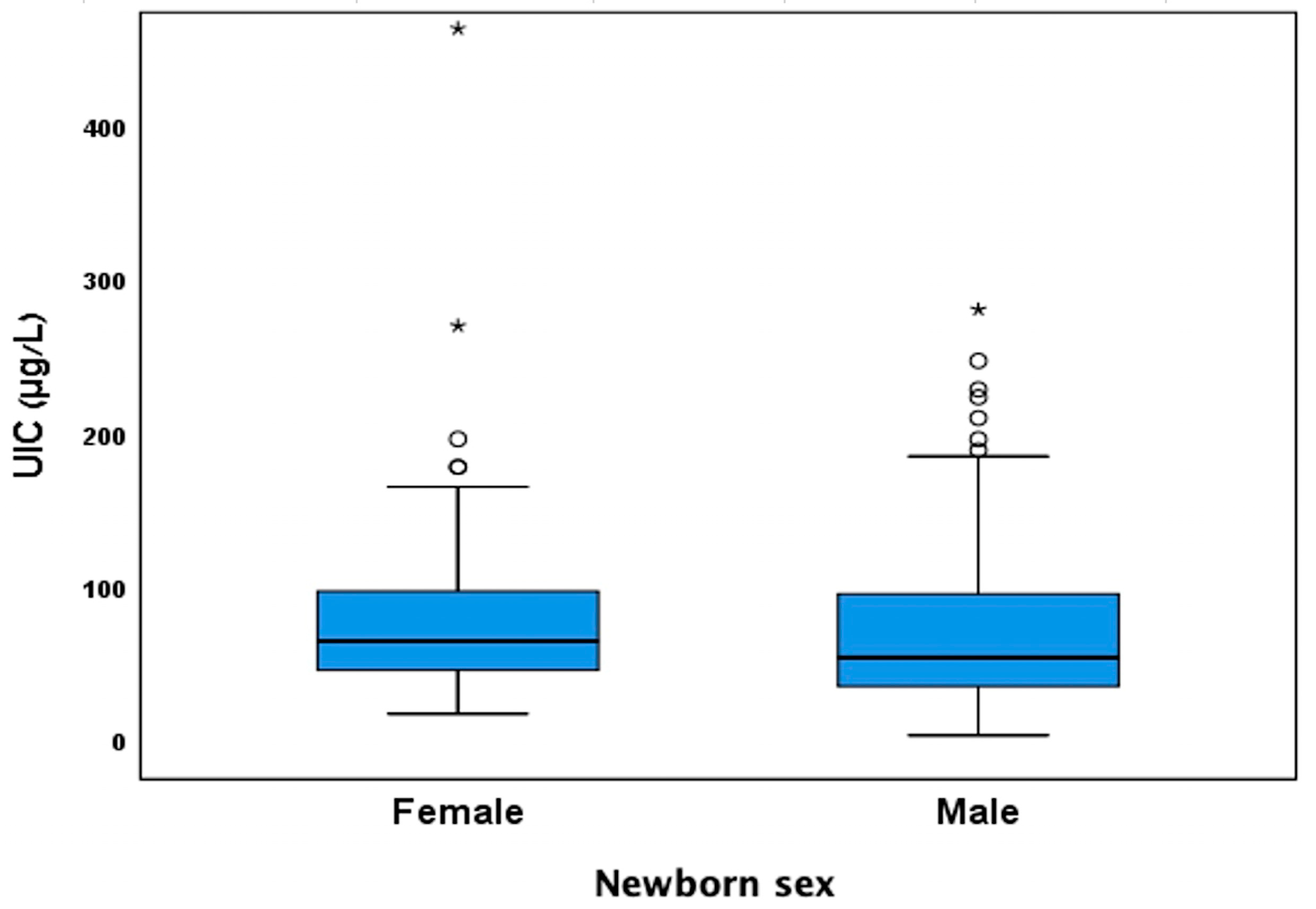
3.3. Effect of Stratification by Newborn Sex on the Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ID | Iodine deficiency |
| ATA | American Thyroid Association |
| WHO | World Health Organization |
| UIC | Urinary Iodine Concentration |
| BUMCA | Barzilai University Medical Center, Ashkelon |
References
- De Benoist, B. Iodine Status Worldwide: WHO Global Database on Iodine Deficiency; Department of Nutrition for Health and Development, World Health Organization: Geneva, Switzerland, 2004; ISBN 978-92-4-159200-0. [Google Scholar]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; WHO Library Cataloguing-in-Publication Data: Lyon, France, 2007. [Google Scholar]
- Jansen, T.A.; Korevaar, T.I.M.; Mulder, T.A.; White, T.; Muetzel, R.L.; Peeters, R.P.; Tiemeier, H. Maternal Thyroid Function during Pregnancy and Child Brain Morphology: A Time Window-Specific Analysis of a Prospective Cohort. Lancet Diabetes Endocrinol. 2019, 7, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Editorial: Consequences of Iodine Deficiency in Pregnancy. Front. Endocrinol. 2021, 12, 740239. [Google Scholar] [CrossRef]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of Iodine Deficiency and Excess in Pregnant Women: An Overview of Current Knowns and Unknowns. Am. J. Clin. Nutr. 2016, 104, 918S–923S. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The Effects of Iodine Deficiency in Pregnancy and Infancy. Paediatr. Perinat. Epidemiol. 2012, 26, 108–117. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Urinary iodine concentrations for determining iodine status in populations. In VMNIS—Vitamin and Mineral Nutrition Information System; WHO/NMH/NHD/EPG/13.1; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Ovadia, Y.S.; Arbelle, J.E.; Gefel, D.; Brik, H.; Wolf, T.; Nadler, V.; Hunziker, S.; Zimmermann, M.B.; Troen, A.M. First Israeli National Iodine Survey Demonstrates Iodine Deficiency Among School-Aged Children and Pregnant Women. Thyroid 2017, 27, 1083–1091. [Google Scholar] [CrossRef]
- Sahu, M.T.; Das, V.; Mittal, S.; Agarwal, A.; Sahu, M. Overt and Subclinical Thyroid Dysfunction among Indian Pregnant Women and Its Effect on Maternal and Fetal Outcome. Arch. Gynecol. Obstet. 2010, 281, 215–220. [Google Scholar] [CrossRef]
- Farebrother, J.; Naude, C.E.; Nicol, L.; Sang, Z.; Yang, Z.; Jooste, P.L.; Andersson, M.; Zimmermann, M.B. Effects of Iodized Salt and Iodine Supplements on Prenatal and Postnatal Growth: A Systematic Review. Adv. Nutr. 2018, 9, 219–237. [Google Scholar] [CrossRef]
- Rydbeck, F.; Rahman, A.; Grandér, M.; Ekström, E.-C.; Vahter, M.; Kippler, M. Maternal Urinary Iodine Concentration up to 1.0 Mg/L Is Positively Associated with Birth Weight, Length, and Head Circumference of Male Offspring. J. Nutr. 2014, 144, 1438–1444. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Webster, J.; Keeble, C.; Taylor, E.; Hardie, L.J. Maternal Iodine Status and Birth Outcomes: A Systematic Literature Review and Meta-Analysis. Nutrients 2023, 15, 387. [Google Scholar] [CrossRef]
- Nazarpour, S.; Ramezani Tehrani, F.; Behboudi-Gandevani, S.; Bidhendi Yarandi, R.; Azizi, F. Maternal Urinary Iodine Concentration and Pregnancy Outcomes in Euthyroid Pregnant Women: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2020, 197, 411–420. [Google Scholar] [CrossRef]
- Ma, Z.F.; Skeaff, S.A. Thyroglobulin as a Biomarker of Iodine Deficiency: A Review. Thyroid 2014, 24, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- CDC. Laboratory Procedure Manual: Iodine in Urine. In NHANES 2003–2004; Inorganic Toxicology and Nutrition Branch, Division of Laboratory Sciences, National Center for Environmental Health: Salt Lake City, UT, USA, 2007. [Google Scholar]
- Dollberg, S.; Haklai, Z.; Mimouni, F.B.; Gorfein, I.; Gordon, E.-S. Birth Weight Standards in the Live-Born Population in Israel. Isr. Med. Assoc. J. IMAJ 2005, 7, 311–314. [Google Scholar]
- Davidson, S.; Sokolover, N.; Erlich, A.; Litwin, A.; Linder, N.; Sirota, L. New and Improved Israeli Reference of Birth Weight, Birth Length, and Head Circumference by Gestational Age: A Hospital-Based Study. Isr. Med. Assoc. J. IMAJ 2008, 10, 130–134. [Google Scholar] [PubMed]
- Rosen, S.R.; Ovadia, Y.S.; Anteby, E.Y.; Fytlovich, S.; Aharoni, D.; Zamir, D.; Gefel, D.; Shenhav, S. Low Intake of Iodized Salt and Iodine Containing Supplements among Pregnant Women with Apparently Insufficient Iodine Status—Time to Change Policy? Isr. J. Health Policy Res. 2020, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Snart, C.J.P.; Threapleton, D.E.; Keeble, C.; Taylor, E.; Waiblinger, D.; Reid, S.; Alwan, N.A.; Mason, D.; Azad, R.; Cade, J.E.; et al. Maternal Iodine Status, Intrauterine Growth, Birth Outcomes and Congenital Anomalies in a UK Birth Cohort. BMC Med. 2020, 18, 132. [Google Scholar] [CrossRef]
- Gargari, S.S.; Fateh, R.; Bakhshali-Bakhtiari, M.; Saleh, M.; Mirzamoradi, M.; Bakhtiyari, M. Maternal and Neonatal Outcomes and Determinants of Iodine Deficiency in Third Trimester of Pregnancy in an Iodine Sufficient Area. BMC Pregnancy Childbirth 2020, 20, 174. [Google Scholar] [CrossRef]
- Maymon, R.; Pekar-Zlotin, M.; Meiri, H.; Haklai, Z.; Gordon, E.-S.; Shlichkov, G.; Cuckle, H. Change in Prevalence of Preterm Birth in Israel Following Publication of National Guidelines Recommending Routine Sonographic Cervical-Length Measurement at 19-25 Weeks’ Gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2023, 61, 610–616. [Google Scholar] [CrossRef]
- Nazeri, P.; Shab-Bidar, S.; Pearce, E.N.; Shariat, M. Do Maternal Urinary Iodine Concentration or Thyroid Hormones within the Normal Range during Pregnancy Affect Growth Parameters at Birth? A Systematic Review and Meta-Analysis. Nutr. Rev. 2020, 78, 747–763. [Google Scholar] [CrossRef]
- Li, C.; Peng, S.; Zhang, X.; Xie, X.; Wang, D.; Mao, J.; Teng, X.; Shan, Z.; Teng, W. The Urine Iodine to Creatinine as an Optimal Index of Iodine During Pregnancy in an Iodine Adequate Area in China. J. Clin. Endocrinol. Metab. 2016, 101, 1290–1298. [Google Scholar] [CrossRef]
- Montenegro-Bethancourt, G.; Johner, S.A.; Stehle, P.; Neubert, A.; Remer, T. Iodine Status Assessment in Children: Spot Urine Iodine Concentration Reasonably Reflects True Twenty-Four–Hour Iodine Excretion Only When Scaled to Creatinine. Thyroid 2015, 25, 688–697. [Google Scholar] [CrossRef]
- Bienertová-Vašků, J.; Grulichová, M.; Mikeš, O.; Zlámal, F.; Pruša, T.; Pohořalá, A.; Andrýsková, L.; Pikhart, H. Estimated Dietary Iodine Intake as a Predictor of Placental Size: Evidence from the ELSPAC Study. Nutr. Metab. 2018, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Neven, K.Y.; Marien, C.B.D.; Janssen, B.G.; Roels, H.A.; Waegeneers, N.; Nawrot, T.S.; Ruttens, A. Variability of Iodine Concentrations in the Human Placenta. Sci. Rep. 2020, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed]
- Fantz, C.R.; Dagogo-Jack, S.; Ladenson, J.H.; Gronowski, A.M. Thyroid Function during Pregnancy. Clin. Chem. 1999, 45, 2250–2258. [Google Scholar] [CrossRef]




| N | ||
|---|---|---|
| Maternal Age (y) | 171 | 30.88 ± 5.24 |
| Gestational week at recruitment (weeks) | 162 | 32.45 ± 6.21 |
| Gravidity | 171 | 3 (1–10) |
| Parity | 172 | 2 (1–10) |
| Country of birth | 167 | |
| Israel | 118 (70.7%) | |
| Other | 49 (29.3%) | |
| Current smoking habits | 170 | |
| Not smoking | 144 (84.7%) | |
| Smoking | 26 (15.3%) | |
| BMI (kg/m2) | ||
| BMI before pregnancy | 125 | 24.25 ± 4.75 |
| BMI (in recruitment) | 171 | 28.62 ± 5.10 |
| BMI change | 126 | 4.44 ± 2.29 |
| GDM diagnosis in current pregnancy | 171 | |
| No | 158 (92.4%) | |
| Yes | 13 (7.6%) |
| N | Mean ± SD | |
|---|---|---|
| Birth week | 172 | 38.48 ± 2.16 |
| Birth week < 37 | 24 (14%) | |
| Birth week > 37 | 148 (86%) | |
| Newborn sex | 172 | |
| Male | 94 (54.7%) | |
| Female | 78 (45.3%) | |
| Apgar score | 170 | |
| Apgar 5 min < 7 | 0 (0%) | |
| Apgar 5 min ≥ 7 | 170 (100%) | |
| Newborn birth weight (g) | 172 | 3087.97 ± 606.15 |
| Percentile NBW by Dollberg et al. (2005) [16] | 171 | 52.34 ± 27.27 |
| >90th percentile | 14 (8.2%) | |
| <10th percentile | 15 (8.8%) | |
| Length (cm) | 156 | 50.42 ± 2.90 |
| Percentile NBL by Davidson et al. (2008) [17] | 156 | 72.77 ± 26.06 |
| >90th percentile | 66 (42.3%) | |
| <10th percentile | 3 (1.9%) | |
| Head Circumference (cm) | 157 | 34.19 ± 1.88 |
| UIC (μg/L) | BW < 10th Percentile by Dollberg | BW > 90th Percentile by Dollberg | ||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| N | 137 | 14 | 138 | 13 |
| Mean BT ± SD BT | 62.48 ± 2.08 | 59.90 ± 2.52 | 64.12 ± 2.12 | 48.74 ± 1.91 |
| Median | 61.93 | 44.58 | 60.02 | 56.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shenhav, S.; Tsur Shenhav, L.; Gefel, D.; Rosen, S.R.; Shenhav, A.; Shapin, R.; Anteby, E.Y.; Ovadia, Y.S. The Association Between Maternal Urinary Iodine Concentration and Neonatal Anthropometry. Nutrients 2025, 17, 1624. https://doi.org/10.3390/nu17101624
Shenhav S, Tsur Shenhav L, Gefel D, Rosen SR, Shenhav A, Shapin R, Anteby EY, Ovadia YS. The Association Between Maternal Urinary Iodine Concentration and Neonatal Anthropometry. Nutrients. 2025; 17(10):1624. https://doi.org/10.3390/nu17101624
Chicago/Turabian StyleShenhav, Simon, Leah Tsur Shenhav, Dov Gefel, Shani R. Rosen, Amit Shenhav, Rachel Shapin, Eyal Y. Anteby, and Yaniv S. Ovadia. 2025. "The Association Between Maternal Urinary Iodine Concentration and Neonatal Anthropometry" Nutrients 17, no. 10: 1624. https://doi.org/10.3390/nu17101624
APA StyleShenhav, S., Tsur Shenhav, L., Gefel, D., Rosen, S. R., Shenhav, A., Shapin, R., Anteby, E. Y., & Ovadia, Y. S. (2025). The Association Between Maternal Urinary Iodine Concentration and Neonatal Anthropometry. Nutrients, 17(10), 1624. https://doi.org/10.3390/nu17101624