The Potential Mechanism of Alpiniae oxyphyllae Fructus Against Hyperuricemia: An Integration of Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments
Abstract
1. Introduction
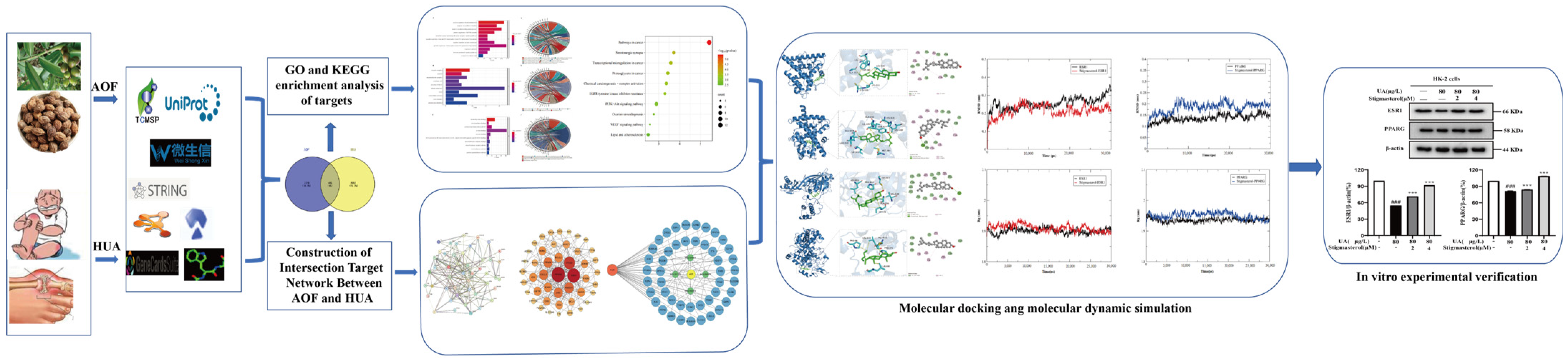
2. Materials and Methods
2.1. Software and Database
2.2. Materials and Instruments
2.3. Determination of Active Ingredients in AOF
2.4. Searching for the Active Ingredients of AOF
2.5. Construction of the Protein–Protein Interaction (PPI) Network
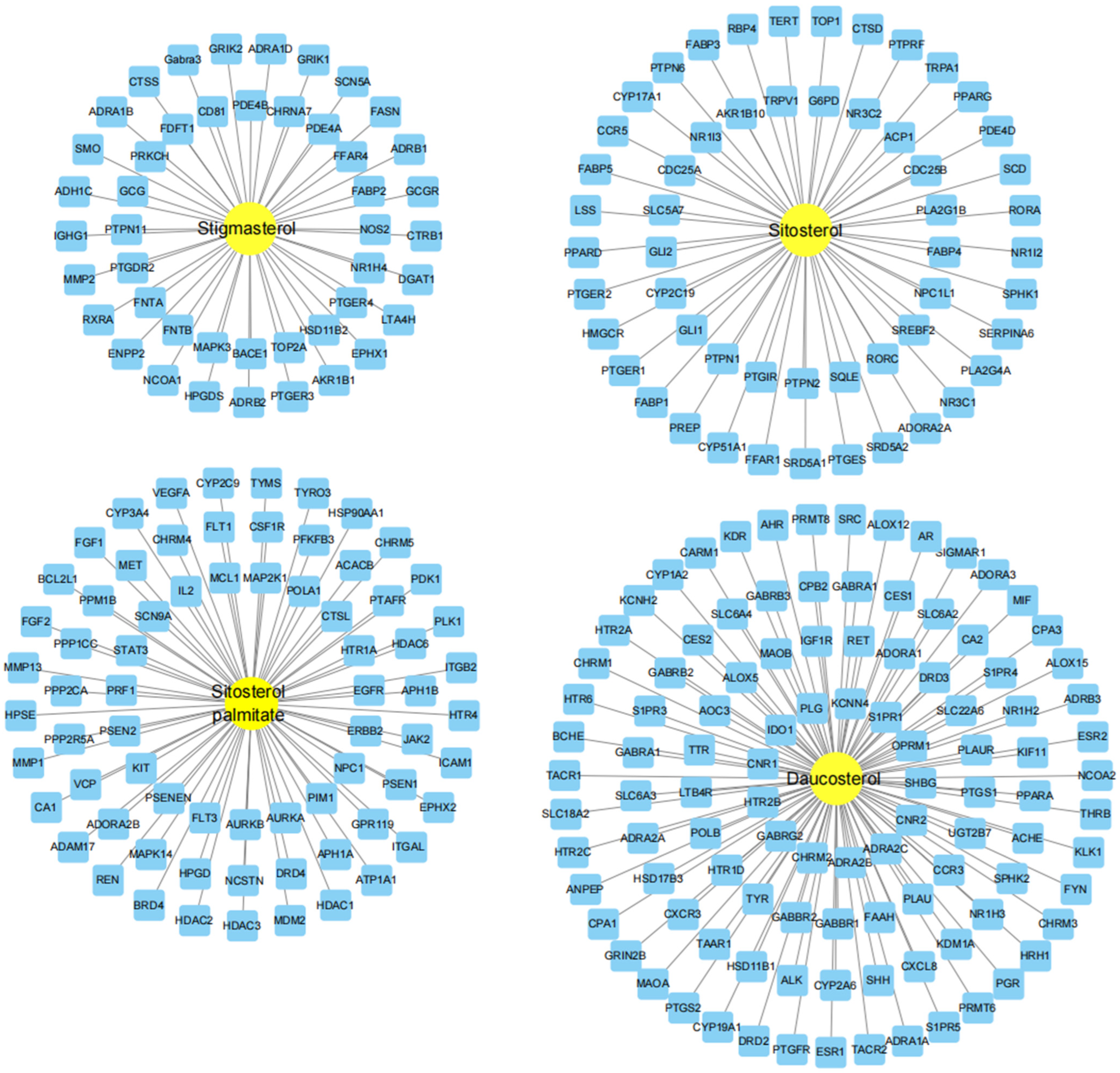
2.6. Construction of the “Drug Ingredient Target Disease Network Diagram”
2.7. GO and KEGG Enrichment Analysis of Targets
2.8. Molecular Docking
2.9. Molecular Dynamic Simulation
2.10. Cell Culture
2.11. Cytotoxicity Experiment
2.12. Western Blotting Asssay
3. Results
3.1. Screening of Active Ingredients and Targets of AOF
3.2. AOF Improved Potential Target Prediction of Hyperuricemia
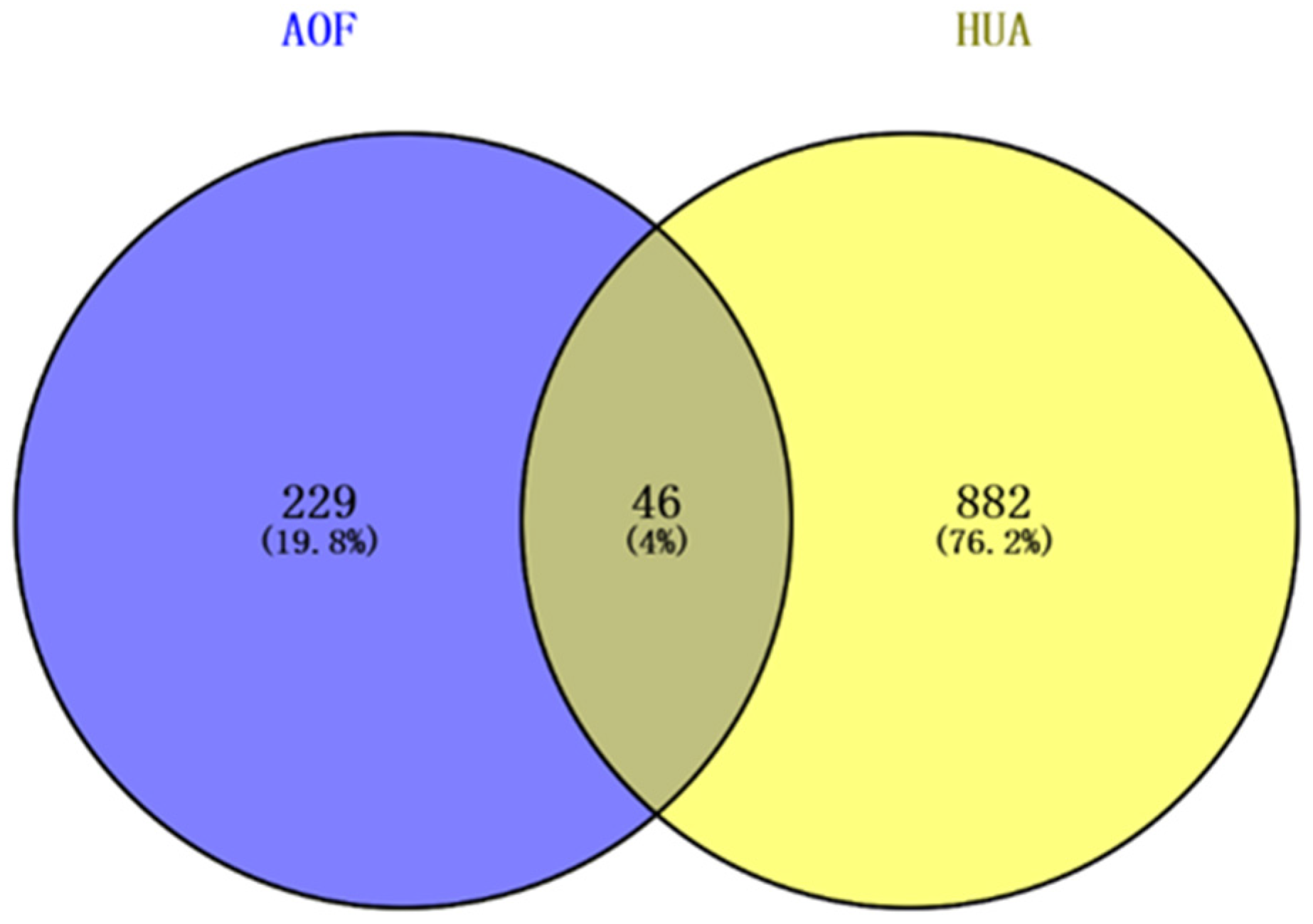
3.3. Construction of Intersection Target Network Between AOF and HUA
3.4. Analysis of PPI Network Construction
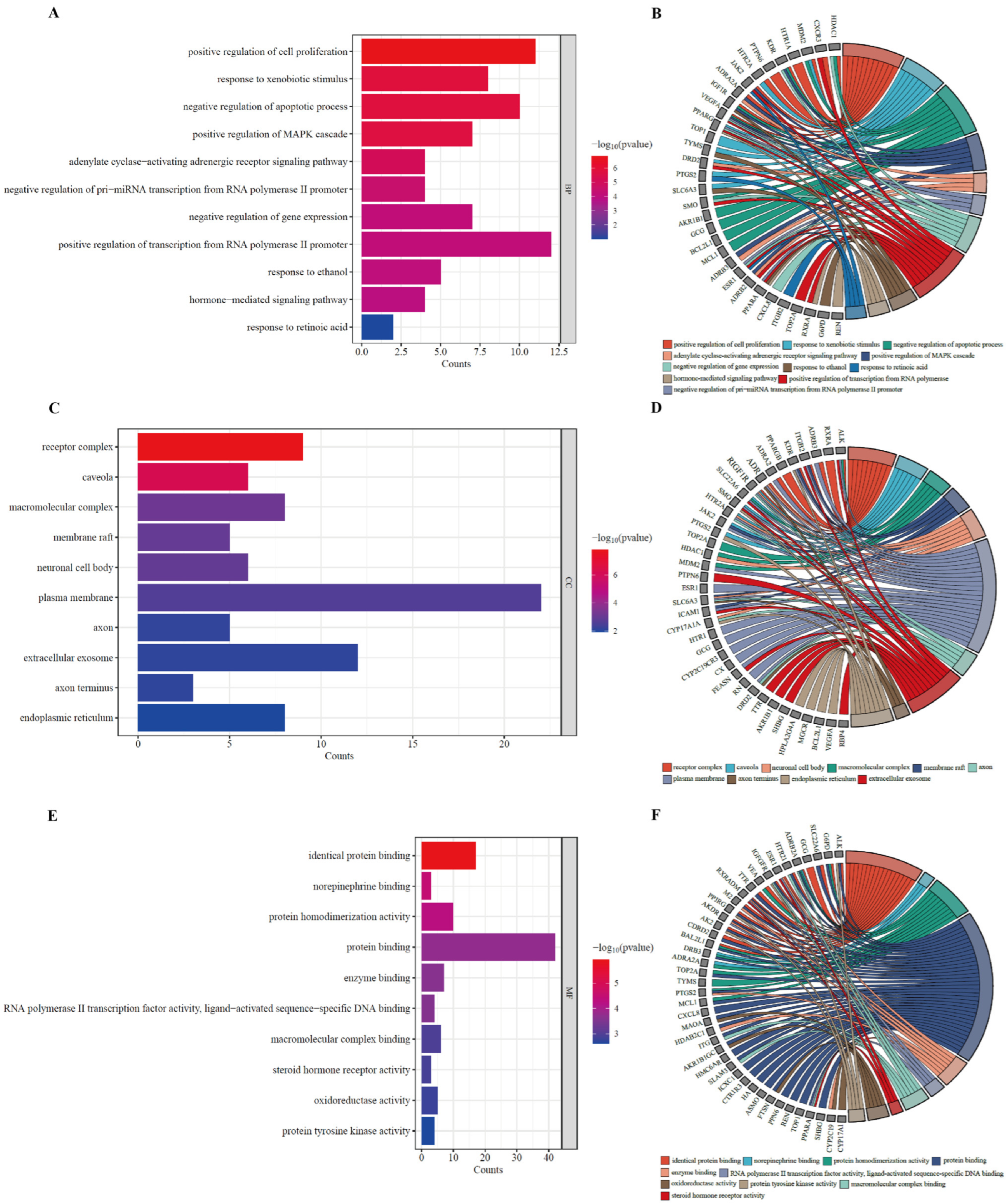
3.5. GO Enrichment Analysis of Potential HUA Targets in AOF
3.6. KEGG Pathway Enrichment Analysis of the Potential Anti-HUA Targets of AOF
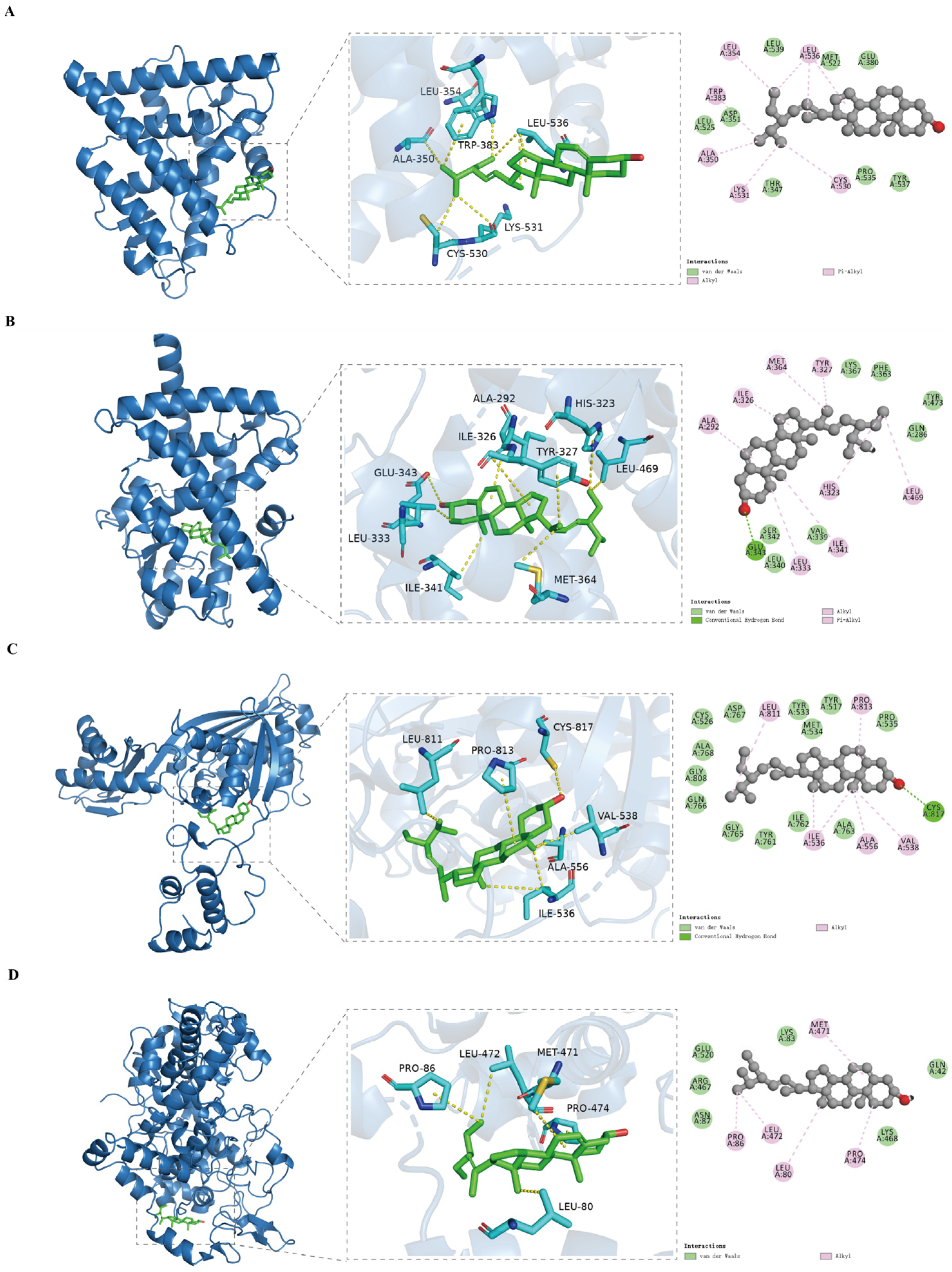
3.7. Analysis of Molecular Docking
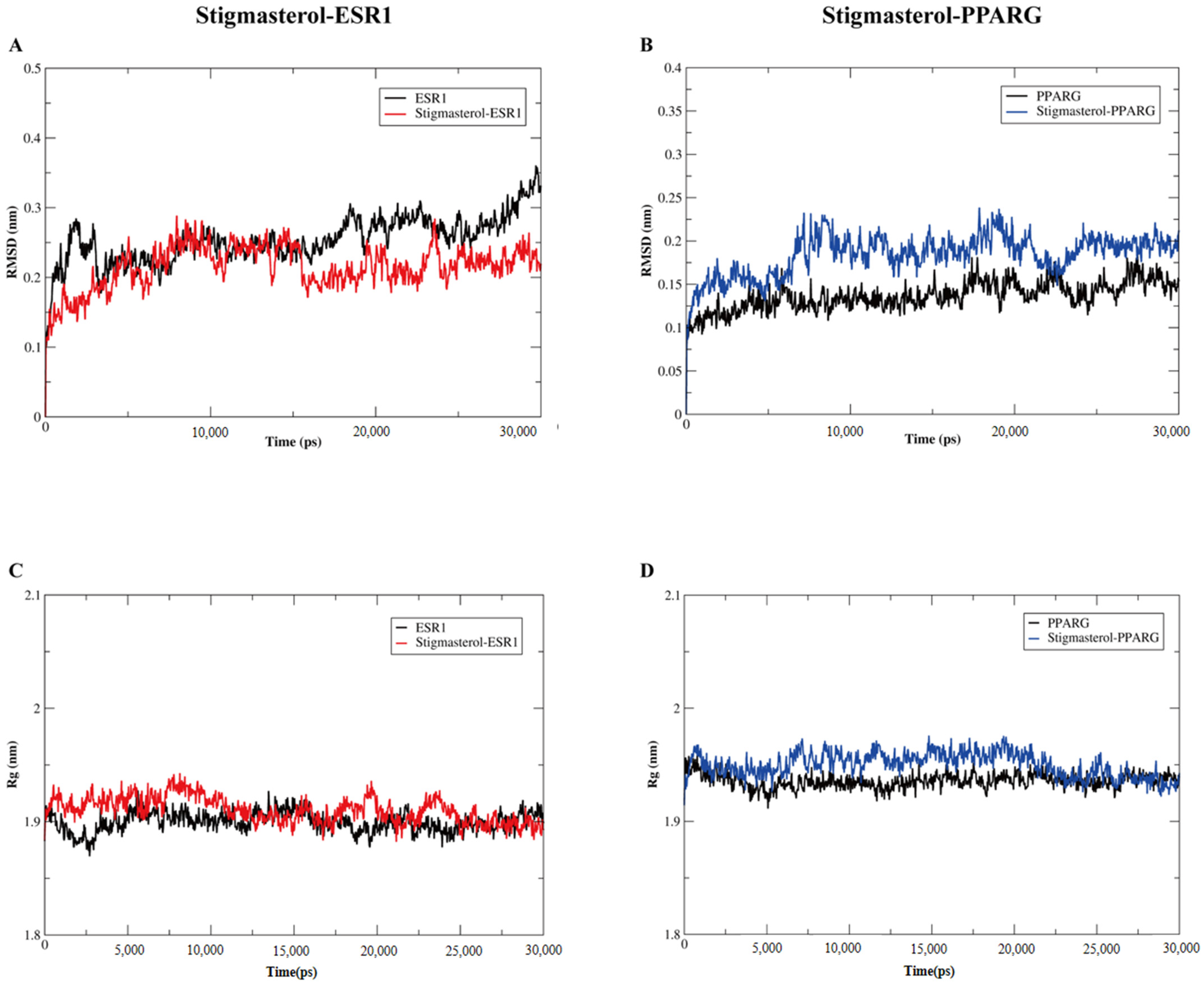
3.8. Verification of the Binding Models by Molecular Dynamic Simulation
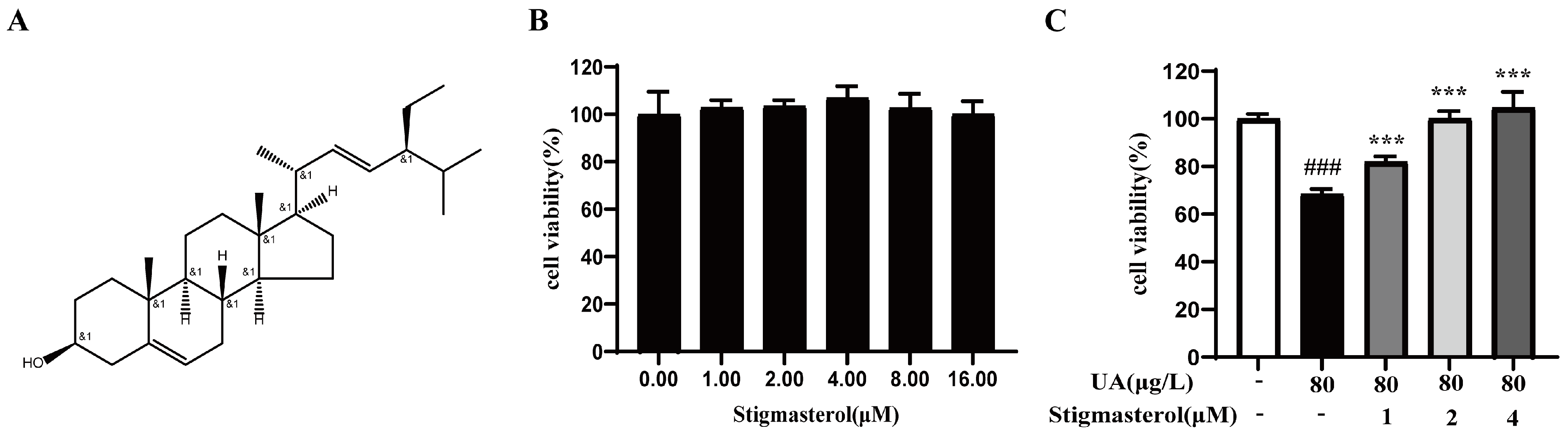
3.9. The Effect of Stigmasterol on UA-Induced Inhibition of HK-2 Cells
3.10. The Effect of Stigmasterol on the Expression of Key Target Proteins in HK-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Luo, Y.Y.; Hu, S.S.; Gan, R.; Zeng, L. The chemistry, processing, and preclinical anti-hyperuricemia potential of tea: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7065–7090. [Google Scholar] [CrossRef]
- Cheng, S.J.; Shan, L.S.; You, Z.Y.; Xia, Y.; Zhao, Y.; Zhang, H.; Zhao, Z. Dietary patterns, uric acid levels, and hyperuricemia: A systematic review and meta-analysis. Food Funct. 2023, 14, 7853–7868. [Google Scholar] [CrossRef]
- Sellmayr, M.; Petzsche, M.R.H.; Ma, Q.; Krüger, N.; Liapis, H.; Brink, A.; Lenz, B.; Angelotti, M.L.; Gnemmi, V.; Kuppe, C.; et al. Only Hyperuricemia with Crystalluria, but not Asymptomatic Hyperuricemia, Drives Progression of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 2773–2792. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liu, N.; Chen, J. The Role of the Intestine in the Development of Hyperuricemia. Front. Immunol. 2022, 13, 845684. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Dai, X.; Liu, T.; Liu, Y.; Shi, H.; Yin, J.; Xu, T.; Zhang, Y.; Zhao, D. Simiao San alleviates hyperuricemia and kidney inflammation by inhibiting NLRP3 inflammasome and JAK2/STAT3 signaling in hyperuricemia mice. J. Ethnopharmacol. 2023, 312, 116530. [Google Scholar] [CrossRef] [PubMed]
- Nutmakul, T. A review on benefits of quercetin in hyperuricemia and gouty arthritis. Saudi Pharm. J. 2022, 30, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, H.X.; Guo, Y.N.; Wei, F.; Yang, X.; Li, D.; Li, M.; Xu, W.; Li, W.; Sun, L.; et al. Comparative efficacy and safety of urate-lowering therapy for the treatment of hyperuricemia: A systematic review and network meta-analysis. Sci. Rep. 2016, 6, 33082. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, M.; Sun, L.; Ding, W.; Cai, S.; Zhao, Q. Co-administration of Colchicine and Allopurinol alleviates vascular calcification induced by hyperuricemia via suppressing inflammation and oxidative stress. Arch. Med. Science. 2021. [Google Scholar] [CrossRef]
- Konishi, M.; Kojima, S.; Uchiyama, K.; Yokota, N.; Tokutake, E.; Wakasa, Y.; Hiramitsu, S.; Waki, M.; Jinnouchi, H.; Kakuda, H.; et al. Effect of febuxostat on clinical outcomes in patients with hyperuricemia and cardiovascular disease. Int. J. Cardiol. 2022, 349, 127–133. [Google Scholar] [CrossRef]
- Yu, X.; Ren, S.; Zhou, J.; Liao, Y.; Huang, Y.; Dong, H. A potential therapeutic agent for the treatment of hyperuricemia and gout: 3,4-Dihydroxy-5-nitrobenzaldehyde phenylthiosemicarbazide. Eur. J. Pharm. Sci. 2024, 198, 106778. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Zhuang, Z.N.; Li, C.X.; Pan, P.; Zhang, C.; Zhang, X.Z. Bio-inspired nanoenzyme for metabolic reprogramming and anti-inflammatory treatment of hyperuricemia and gout. Sci. China Chem. 2021, 64, 616–628. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Ozdemir, Z.; Wimmer, Z. Recent Achievements in Medicinal and Supramolecular Chemistry of Betulinic Acid and Its Derivatives (double dagger). Molecules 2019, 24, 3546. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Chen, H.; Xu, T.; Li, C.; Zhou, R.; Gao, L.; Han, M.; He, X.; Chen, Y. Extraction, isolation, immunoregulatory activity, and characterization of Alpiniae oxyphyllae fructus polysaccharides. Int. J. Biol. Macromol. 2020, 155, 927–937. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Zhang, Q.; Duan, H.; Qian, D.; Yang, F.; Xia, J. Alpiniae oxyphyllae fructus possesses neuroprotective effects on H2O2 stimulated PC12 cells via regulation of the PI3K/Akt signaling Pathway. Front. Pharmacol. 2022, 13, 966348. [Google Scholar] [CrossRef]
- Qi, Y.; Zhou, Q.; Zhang, Y.; Deng, J.; Li, R.; Zhang, X. Exploring the active components and potential mechanisms of Alpiniae oxyphyllae Fructus in treating diabetes mellitus with depression by UPLC-Q-Exactive Orbitrap/MS, network pharmacology and molecular docking. Metab. Brain Dis. 2024, 39, 1065–1084. [Google Scholar] [CrossRef]
- Su, M.-S.; Xu, L.; Gu, S.-G.; Huang, N.; Ren, X.-K.; Cai, X.-H.; Li, C.-C. Therapeutic effects and modulatory mechanism of Alpiniae oxyphyllae Fructus in chronic intermittent hypoxia induced enuresis in rats. Sleep. Breath. 2020, 24, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, L.; Kang, Y.; Liu, S.; Li, X.; Li, L.; Rui, K.; Xiao, M.; Xie, Y. Integrating serum pharmacochemistry and network pharmacology to explore potential compounds and mechanisms of Alpiniae oxyphyllae fructus in the treatment of cellular senescence in diabetic kidney disease. Front. Med. 2024, 11, 1424644. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, D.S. Synergistic Impacts of Alpinia oxyphylla Seed Extract and Allopurinol against Experimental Hyperuricemia. Biomed. Res. Int. 2022, 2022, 2824535. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Q.; Zhang, Z.; Yang, X.; Man, J.; Wang, D.; Li, X. Based on Network Pharmacology and Molecular Docking, the Active Components, Targets, and Mechanisms of Flemingia philippinensis in Improving Inflammation Were Excavated. Nutrients 2024, 16, 1850. [Google Scholar] [CrossRef]
- Zhong, H.; Duan, B.H.; Du, F.M.; Wang, W.-M.; Qiao, H. Identification of key genes, biological functions, and pathways of empagliflozin by network pharmacology and its significance in the treatment of type 2 diabetes mellitus. Ann. Transl. Med. 2023, 11, 123. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Q.Y.; Li, C.; Tian, H.; Zhuo, C. Exploring the potential pharmacological mechanism of aripiprazole against hyperprolactinemia based on network pharmacology and molecular docking. Schizophrenia 2024, 10, 105. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.Y.; Zhang, J.H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Aydın, S.G. Review on Molecular Modeling and Docking. SAR J. Sci. Res. 2022, 5, 206–210. [Google Scholar] [CrossRef]
- Dudhat, K. Panoramic Review on Progress and Development of Molecular Docking. Pharm. Sci. Technol. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Tuo, Y.; Lu, X.; Tao, F.; Tukhvatshin, M.; Xiang, F.; Wang, X.; Shi, Y.; Lin, J.; Hu, Y. The Potential Mechanisms of Catechins in Tea for Anti-Hypertension: An Integration of Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. Foods 2024, 13, 2685. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, P.W.; Rose, A.S.; Tiemann, J.K.S. Bringing Molecular Dynamics Simulation Data into View. Trends Biochem. Sci. 2019, 44, 902–913. [Google Scholar] [CrossRef]
- Dong, D.; Xu, Z.; Zhong, W.; Peng, S. Parallelization of Molecular Docking: A Review. Curr. Top. Med. Chem. 2018, 18, 1015–1028. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xu, P.; Liu, J.; Ma, J.; Wang, Y.; Liu, Z.; Lv, M.; Wang, F.; Tang, X. Molecular Mechanism of the Effect of Zhizhu Pill on Gastroesophageal Reflux Disease Based on Network Pharmacology and Molecular Docking. Evid. Based Complement. Altern. Med. 2022, 2022, 13. [Google Scholar] [CrossRef]
- Su, J.; Huo, M.M.; Xu, F.N.; Ding, L. Molecular mechanism of lycorine in the treatment of glioblastoma based on network pharmacology and molecular docking. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.M.; Liu, Y.Z.; Feng, A.; Zhang, S. Sex-Specific Differences in the Association of Metabolically Healthy Obesity With Hyperuricemia and a Network Perspective in Analyzing Factors Related to Hyperuricemia. Front. Endocrinol. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef]
- Lou, Y.; Gao, Q.; Fan, M.C.; Waleed, A.-A.; Wang, L.; Li, Y.; Qian, H. Ferulic acid ameliorates hyperuricemia by regulating xanthine oxidase. Int. J. Biol. Macromol. 2023, 253, 126542. [Google Scholar] [CrossRef]
- Zhang, N.X.; Zhang, B.C.; Chen, X.J.; Zhang, Y.; Wang, Y.; Lu, S.; Zhang, H.; Chen, Y.; Jiang, H.; Zhou, H. Effects and mechanisms of Polygonati Rhizoma polysaccharide on potassium oxonate and hypoxanthine-induced hyperuricemia in mice. Int. J. Biol. Macromol. 2024, 280, 135550. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Antwi, A.O.; Obiri, D.D.; Osafo, N.; Forkuo, A.D.; Essel, L.B. Stigmasterol inhibits lipopolysaccharide-induced innate immune responses in murine models. Int. Immunopharmacol. 2017, 53, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Li, R.C.; Guo, S.W.; Liang, C. Stigmasterol exerts antiglioma effects by regulating lipid metabolism. Mol. Med. Rep. 2024, 30, 227. [Google Scholar] [CrossRef]
- El Omari, N.; Jaouadi, I.; Lahyaoui, M.; Benali, T.; Taha, D.; Bakrim, S.; El Menyiy, N.; El Kamari, F.; Zengin, G.; Bangar, S.P.; et al. Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Appl. Sci. 2022, 12, 5779. [Google Scholar] [CrossRef]
- He, H.D.; Sun, S.H.; Xu, W.H.; Zhang, M. Network Pharmacology Followed by Experimental Validation to Explore the Mechanism of Stigmasterol in Sangbaipi Decoction Regulating PI3K/Akt Signaling to Alleviate Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obs. Pulmon Dis. 2024, 19, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, Z.; Lei, Y.; Han, X.; Tian, D.; Gong, J.; Liu, M. Celastrol Alleviates Autoimmune Hepatitis Through the PI3K/AKT Signaling Pathway Based on Network Pharmacology and Experiments. Front. Pharmacol. 2022, 13, 816350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on its anti-tumor effect and mechanism of action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef] [PubMed]




| Mol ID | Ingredient Name | OB/% | DL |
|---|---|---|---|
| MOL000449 | Stigmasterol | 43.83 | 0.76 |
| MOL000359 | Sitosterol | 36.91 | 0.75 |
| MOL001525 | Daucosterol | 36.91 | 0.75 |
| MOL000355 | Sitosterol palmitate | 30.91 | 0.44 |
| No. | Key Core Targets | Neighborhood Connectivity | Closeness Centrality | Betweenness Centrality |
|---|---|---|---|---|
| 1 | PPARG | 11.08 | 0.6618 | 0.1813 |
| 2 | ESR1 | 11.70 | 0.6618 | 0.1834 |
| 3 | PTGS2 | 12.55 | 0.6081 | 0.0757 |
| 4 | HMGCR | 11.75 | 0.5625 | 0.0512 |
| Chemical Compound | CAS | Average Binding Energy (kcal/mol) | |||
|---|---|---|---|---|---|
| PPARG | ESR1 | PTGS2 | HMGCR | ||
| Stigmasterol | 83-48-7 | −9.38 | −8.73 | −6.43 | −7.49 |
| Sitosterol | 83-46-5 | −8.94 | −8.50 | −5.93 | −5.01 |
| Daucosterol | 474-58-8 | −4.01 | −3.05 | −5.13 | −2.52 |
| Sitosteryl palmitate | 2308-85-2 | −2.59 | −2.56 | −3.06 | −0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Yang, Y.; Zhang, R.; Gao, J.; Wu, M.; Wang, J.; Sheng, J.; Sun, P. The Potential Mechanism of Alpiniae oxyphyllae Fructus Against Hyperuricemia: An Integration of Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments. Nutrients 2025, 17, 71. https://doi.org/10.3390/nu17010071
Zhang S, Yang Y, Zhang R, Gao J, Wu M, Wang J, Sheng J, Sun P. The Potential Mechanism of Alpiniae oxyphyllae Fructus Against Hyperuricemia: An Integration of Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments. Nutrients. 2025; 17(1):71. https://doi.org/10.3390/nu17010071
Chicago/Turabian StyleZhang, Shuanggou, Yuanfei Yang, Ruohan Zhang, Jian Gao, Mengyun Wu, Jing Wang, Jun Sheng, and Peiyuan Sun. 2025. "The Potential Mechanism of Alpiniae oxyphyllae Fructus Against Hyperuricemia: An Integration of Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments" Nutrients 17, no. 1: 71. https://doi.org/10.3390/nu17010071
APA StyleZhang, S., Yang, Y., Zhang, R., Gao, J., Wu, M., Wang, J., Sheng, J., & Sun, P. (2025). The Potential Mechanism of Alpiniae oxyphyllae Fructus Against Hyperuricemia: An Integration of Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments. Nutrients, 17(1), 71. https://doi.org/10.3390/nu17010071






