Sarcopenia in MASLD—Eat to Beat Steatosis, Move to Prove Strength
Abstract
1. Introduction
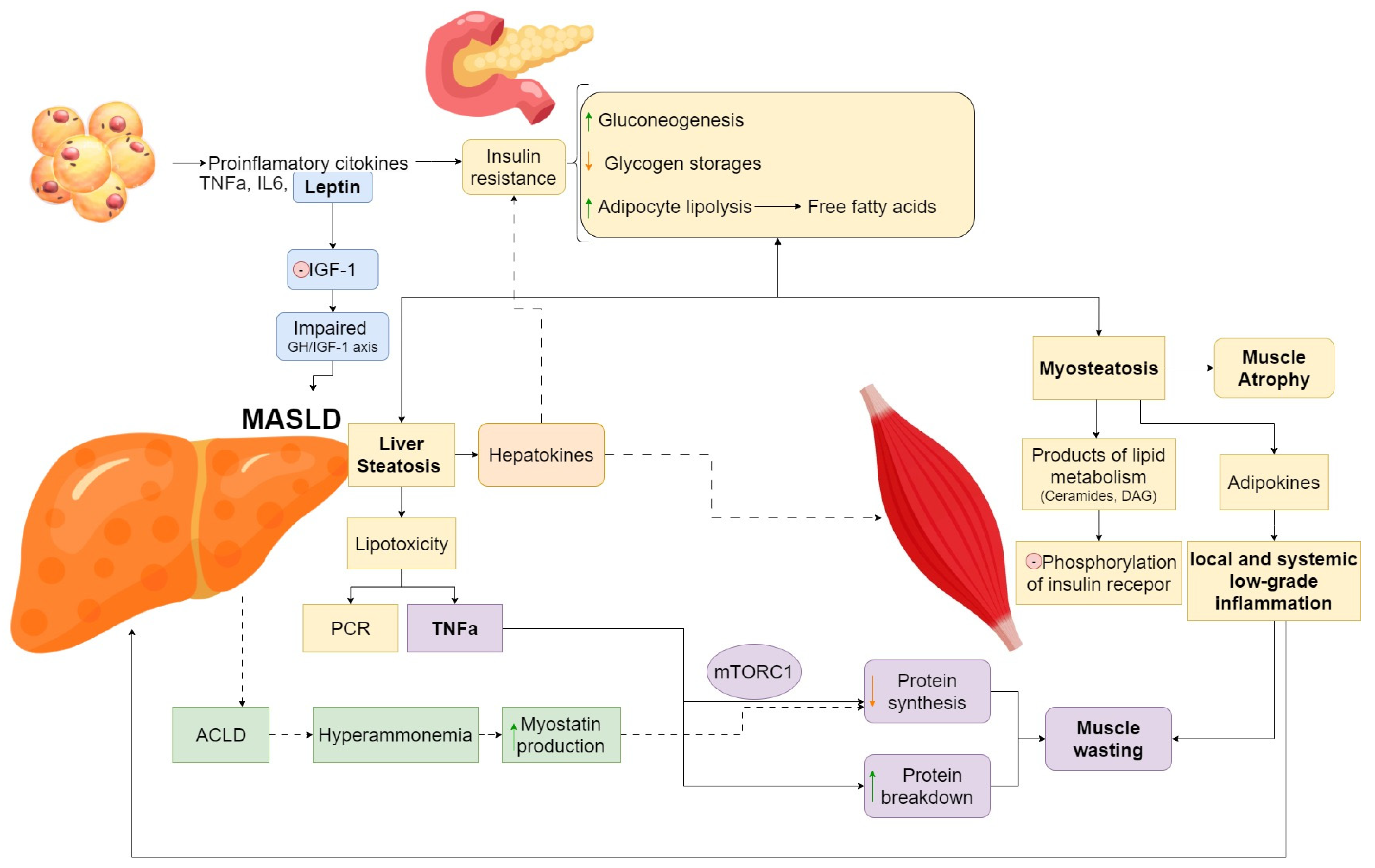
2. Pathophysiology of Sarcopenia in MASLD
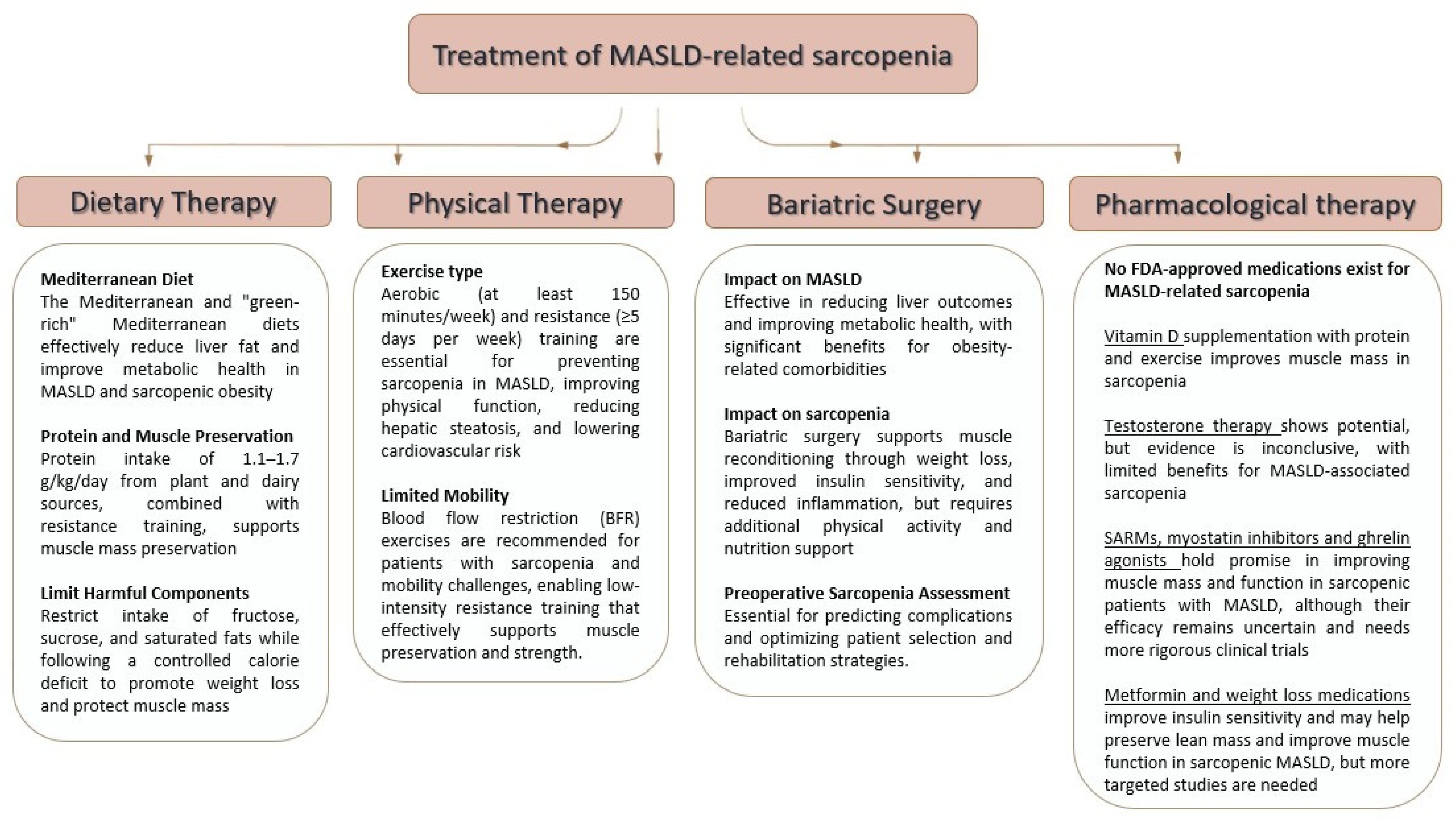
3. Therapeutic Options for Sarcopenia in MASLD
3.1. Pharmacologic Therapies
3.1.1. Vitamin D
3.1.2. Testosterone
3.1.3. Selective Androgen Receptor Modulators (SARMs)
3.1.4. Growth Hormones
3.1.5. Ghrelin Agonists
3.1.6. Drugs Targeting Myostatin and Activin Receptor Pathway
3.1.7. Ammonia-Lowering Treatment
3.1.8. Metformin
3.1.9. Weight Loss Medication
| Medication | Mechanism of Action | Evidence | Sources |
|---|---|---|---|
| Vitamin D | Enhances calcium and phosphate metabolism, improving muscle function. | Improves grip strength and muscle mass when combined with protein and exercise. | Hong et al. (2014) [30], Scott et al. (2010) [31], Barchetta et al. (2011) [32], Badarin et al. (2021) [33] |
| Testosterone | Replenishes testosterone levels to promote muscle growth and reduce fat accumulation. | Limited and conflicting data; ICSFR does not recommend its use due to insufficient evidence. | McKee et al. (2017) [34], Dent et al. (2018) [35], Jaruvongvanich et al. (2017) [36], Lee et al. (2023) [37] |
| SARMs | Selectively activates androgen receptors, reducing adverse effects compared to testosterone. | Enobosarm showed initial promise but failed phase III trials; no approved applications. | Narayanan et al. (2018) [39], Dobs et al. (2013) [40], Crawford et al. (2016) [41] |
| Growth Hormones (GH) | Modulates GH/IGF-1 axis for muscle growth and reduced liver steatosis. | Promising preclinical and clinical studies; improved protein balance and reduced liver damage in mice; human evidence limited. | Koehler et al. (2011) [42], Cristin et al. (2023) [43], Cabrera et al. (2018) [44] |
| Ghrelin Agonists | Increases appetite and serum IGF-1 levels. | Anamorelin approved for cancer cachexia in Japan; potential in sarcopenia associated with NAFLD not yet explored. | Ebner et al. (2020) [47] |
| Myostatin Inhibitors | Blocks myostatin and activin receptor pathway to enhance muscle growth. | Bimagrumab improved lean body mass but not strength in trials; mixed results in older adults. | Kim et al. (2021) [49], Trendelenburg et al. (2009) [50], Rooks et al. (2020) [51] |
| Ammonia-Lowering | Reduces hyperammonemia and myostatin levels, improving muscle protein synthesis. | L-ornithine L-aspartate and L-carnitine linked to improved muscle growth and function in cirrhotic patients; more research needed for MASLD-related sarcopenia. | Kumar et al. (2017) [53], Butterworth (2019) [54], Allen et al. (2021) [55], Zakharova et al. (2023) [56], Savic et al. (2020) [57] |
| Metformin | Activates AMP-activated protein kinase (AMPK), improving mitochondrial function and reducing inflammation. | Shown to enhance muscle quality in preclinical studies; potential protective role in sarcopenia remains under investigation. | Pyrgioti et al. (2024) [58] |
| Weight Loss Drugs | Reduces weight by targeting appetite and metabolism; mechanisms vary among drugs. | Liraglutide preserves lean mass during weight loss in non-diabetics; effects in MASLD-related sarcopenia unknown. | Batsis et al. (2018) [59] |
3.1.10. Other Emerging Mechanisms and Therapeutic Potential for Sarcopenic Obesity Are Shown in the Table Below [61] (Table 2)
3.2. Lifestyle Intervention—Dietary Therapy
3.3. Lifestyle Intervention—Physical Therapy
3.4. Additional Treatments—Bariatric Surgery
4. Adherence to Treatment—The Gaps in Therapeutic Interventions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 (2019). Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef]
- Rotundo, L.; Persaud, A.; Feurdean, M.; Ahlawat, S.; Kim, H.S. The Association of leptin with severity of non-alcoholic fatty liver disease: A population-based study. Clin. Mol. Hepatol. 2018, 24, 392–401. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.; Bava, U.; Lin, C.; Naot, D.; Hill, B.; Grey, A.; Broom, N.; Myers, D.; Nicholson, G.; et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J. Endocrinol. 2002, 175, 405–415. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metab. Clin. Exp. 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- De Larichaudy, J.; Zufferli, A.; Serra, F.; Isidori, A.M.; Naro, F.; Dessalle, K.; Desgeorges, M.; Piraud, M.; Cheillan, D.; Vidal, H.; et al. TNF-α- and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet. Muscle 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A. Role of mTORC1 in mechanically induced increases in translation and skeletal muscle mass. J. Appl. Physiol. 2019, 127, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Schrauwen, P.; de Vogel, J. Muscular diacylglycerol metabolism and insulin resistance. Physiol. Behav. 2008, 94, 242–251. [Google Scholar] [CrossRef]
- Watt, M.J.; Hoy, A.J. Lipid metabolism in skeletal muscle: Generation of adaptive and maladaptive intracellular signals for cellular function. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1315–E1328. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Nachit, M.; Kwanten, W.J.; Thissen, J.P.; Op De Beeck, B.; Van Gaal, L.; Vonghia, L.; Verrijken, A.; Driessen, A.; Horsmans, Y.; Francque, S.; et al. Muscle Fat Content Is Strongly Associated with NASH: A Longitudinal Study in Patients with Morbid Obesity. J. Hepatol. 2021, 75, 292–301. [Google Scholar] [CrossRef]
- Pasco, J.A.; Sui, S.X.; West, E.C.; Anderson, K.B.; Rufus-Membere, P.; Tembo, M.C.; Hyde, N.K.; Williams, L.J.; Liu, Z.S.J.; Kotowicz, M.A. Fatty Liver Index and Skeletal Muscle Density. Calcif. Tissue Int. 2022, 110, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, K.L.; Eriksen, P.L.; Kerbert, A.J.; De Chiara, F.; Jalan, R.; Vilstrup, H. Role of ammonia in NAFLD: An unusual suspect. JHEP Rep. 2023, 5, 100780. [Google Scholar] [CrossRef]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef]
- Lee, S.J. Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 2004, 20, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Cody, S.O.; Potthoff, M.J. Hepatokines and metabolism: Deciphering communication from the liver. Mol. Metab. 2021, 44, 101138. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Schick, F.; Birkenfeld, A.L.; Häring, H.U.; White, M.F. The role of hepatokines in NAFLD. Cell Metab. 2023, 35, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Kim, T.; Papizan, J.B.; Okerberg, C.K.; Kothari, V.M.; Zaid, H.; Bilan, P.J.; Araya-Ramirez, F.; Littlefield, L.A.; Bowers, R.L.; et al. Phosphorylation status of fetuin-A is critical for inhibition of insulin action and is correlated with obesity and insulin resistance. Am. J. Physiol. Endocrinol Metab. 2019, 317, E250–E260. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Misu, H.; Chikamoto, K.; Takayama, H.; Kikuchi, A.; Mohri, K.; Takata, N.; Hayashi, H.; Matsuzawa-Nagata, N.; Takeshita, Y.; et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes 2014, 63, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Montano-Loza, A.J.; Raman, M.; Tandon, P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018, 38, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Kakisaka, K.; Abe, T.; Yusa, K.; Nakaya, I.; Watanabe, T.; Suzuki, A.; Yoshida, Y.; Oikawa, T.; Miyasaka, A.; et al. Positive impact of obesity on the prognosis of liver cirrhosis. J. Gastroenterol. Hepatol. 2024, 39, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Emhmed Ali, S.; Nguyen, M.H. Sarcopenic Obesity in Non-Alcoholic Fatty Liver Disease-The Union of Two Culprits. Life 2021, 11, 119. [Google Scholar] [CrossRef]
- Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between Sarcopenia and Nonalcoholic Fatty Liver Disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Blizzard, L.; Fell, J.; Ding, C.; Winzenberg, T.; Jones, G. A Prospective Study of the Associations between 25-Hydroxy-Vitamin D, Sarcopenia Progression and Physical Activity in Older Adults. Clin. Endocrinol. 2010, 73, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Angelico, F.; Ben, M.D.; Baroni, M.G.; Pozzilli, P.; Morini, S.; Cavallo, M.G. Strong Association between Non Alcoholic Fatty Liver Disease (NAFLD) and Low 25(OH) Vitamin D Levels in an Adult Population with Normal Serum Liver Enzymes. BMC Med. 2011, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Badarin, K.; Hemmingsson, T.; Hillert, L.; Kjellberg, K. Physical Workload and Increased Frequency of Musculoskeletal Pain: A Cohort Study of Employed Men and Women with Baseline Occasional Pain. Occup. Environ. Med. 2021, 78, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Ki, S.-W.; Kim, H.; Kang, S.; Kim, H.; Go, G. Recent Advances in Nutraceuticals for the Treatment of Sarcopenic Obesity. Nutrients 2023, 15, 3854. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.; Morley, J.E.; Matsumoto, A.M.; Vinik, A. Sarcopenia: An Endocrine Disorder? Endocri. Pract. 2017, 23, 1143–1152. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, S.H.; Swerdloff, R.S.; Pak, Y.; Budoff, M.J.; Wang, C.C. OR25-06 the Effect of Testosterone Replacement Therapy on NAFLD in Elderly Hypogonadal Men. J. Endocr. Soc. 2023, 7, bvad114-1705. [Google Scholar]
- Jaruvongvanich, V.; Sanguankeo, A.; Riangwiwat, T.; Upala, S. Testosterone, Sex Hormone-Binding Globulin and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Ann. Hepatol. 2017, 16, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Coss, C.C.; Dalton, J.T. Development of Selective Androgen Receptor Modulators (SARMs). Mol. Cell Endocrinol. 2018, 465, 134–142. [Google Scholar] [CrossRef]
- Dobs, A.S.; Boccia, R.V.; Croot, C.C.; Gabrail, N.Y.; Dalton, J.T.; Hancock, M.L.; Johnston, M.A.; Steiner, M.S. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013, 14, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Prado, C.M.; Johnston, M.A.; Gralla, R.J.; Taylor, R.P.; Hancock, M.L.; Dalton, J.T. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials). Curr. Oncol. Rep. 2016, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Koehler, E.; Swain, J.; Sanderson, S.; Krishnan, A.; Watt, K.; Charlton, M. Growth Hormone, Dehydroepiandrosterone and Adiponectin Levels in Non-Alcoholic Steatohepatitis: An Endocrine Signature for Advanced Fibrosis in Obese Patients. Liver Int. 2011, 32, 279–286. [Google Scholar] [CrossRef]
- Cristin, L.; Montini, A.; Martinino, A.; Scarano Pereira, J.P.; Giovinazzo, F.; Agnes, S. The Role of Growth Hormone and Insulin Growth Factor 1 in the Development of Non-Alcoholic Steato-Hepatitis: A Systematic Review. Cells 2023, 12, 517. [Google Scholar] [CrossRef]
- Cabrera, D.; Cabello-Verrugio, C.; Solís, N.; San Martín, D.; Cofre, C.; Pizarro, M.; Arab, J.P.; Abrigo, J.; Campos, F.; Irigoyen, B.; et al. Somatotropic Axis Dysfunction in Non-Alcoholic Fatty Liver Disease: Beneficial Hepatic and Systemic Effects of Hormone Supplementation. Int. J. Mol. Sci. 2018, 19, 1339. [Google Scholar] [CrossRef] [PubMed]
- Brioche, T.; Kireev, R.A.; Cuesta, S.; Gratas-Delamarche, A.; Tresguerres, J.A.; Gomez-Cabrera, M.C.; Vina, J. Growth hormone replacement therapy prevents sarcopenia by a dual mechanism: Improvement of protein balance and of antioxidant defenses. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef]
- Ebner, N.; Anker, S.D.; Haehling, S. Recent Developments in the Field of Cachexia, Sarcopenia, and Muscle Wasting: Highlights from the 12th Cachexia Conference. J. Cachexia Sarcopenia Muscle 2020, 11, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.; Temel, J.S.; Abernethy, A.; Milanowski, J.; Friend, J.; Fearon, K.C. ROMANA 3: A phase 3 safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann. Oncol. 2012, 28, 1949–1956. [Google Scholar] [CrossRef]
- Kim, Y. Emerging Treatment Options for Sarcopenia in Chronic Liver Disease. Life 2021, 11, 250. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef] [PubMed]
- Rooks, D.; Petricoul, O.; Praestgaard, J.; Bartlett, M.; Laurent, D.; Roubenoff, R. Safety and Pharmacokinetics of Bimagrumab in Healthy Older and Obese Adults with Body Composition Changes in the Older Cohort. J. Cachexia Sarcopenia Muscle 2020, 11, 1525–1534. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Coleman, L.A.; Miller, R.; Rooks, D.S.; Laurent, D.; Petricoul, O.; Praestgaard, J.; Swan, T.; Wade, T.; Perry, R.G.; et al. Effect of Bimagrumab vs Placebo on Body Fat Mass Among Adults With Type 2 Diabetes and Obesity: A Phase 2 Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2033457. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Davuluri, G.; Silva, R.N.E.; Engelen, M.P.K.J.; Ten Have, G.A.M.; Prayson, R.; Deutz, N.E.P.; Dasarathy, S. Ammonia Lowering Reverses Sarcopenia of Cirrhosis by Restoring Skeletal Muscle Proteostasis. Hepatology 2017, 65, 2045–2058. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. L-Ornithine L-Aspartate for the Treatment of Sarcopenia in Chronic Liver Disease: The Taming of a Vicious Cycle. Can. J. Gastroenterol. Hepatol. 2019, 1, 8182195. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.L.; Quinlan, J.I.; Dhaliwal, A.; Armstrong, M.J.; Elsharkawy, A.M.; Greig, C.A.; Lord, J.M.; Lavery, G.G.; Breen, L. Sarcopenia in chronic liver disease: Mechanisms and countermeasures. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G241–G257. [Google Scholar] [CrossRef]
- Zakharova, N.; Luo, C.; Aringazina, R.; Samusenkov, V. The Efficacy of L-Carnitine in Patients with Nonalcoholic Steatohepatitis and Concomitant Obesity. Lipids Health Dis. 2023, 22, 101. [Google Scholar] [CrossRef]
- Savic, D.; Hodson, L.; Neubauer, S.; Pavlides, M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Pyrgioti, E.E.; Karakousis, N.D.; Georgakopoulos, P.N.; Papanas, N. Metformin in Type 2 Diabetes: Evidence for Its Beneficial Effects on Frailty and Sarcopenia. Curr. Diabetes Rev. 2024, 20, e270723219177. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic Obesity in Older Adults: Aetiology, Epidemiology and Treatment Strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Manigault, K.R.; Thurston, M.M. Liraglutide: A Glucagon-Like Peptide-1 Agonist for Chronic Weight Management. Consult. Pharm. 2016, 31, 685–697. [Google Scholar] [CrossRef]
- Axelrod, C.L.; Dantas, W.S.; Kirwan, J.P. Sarcopenic Obesity: Emerging Mechanisms and Therapeutic Potential. Metabolism 2023, 146, 155639. [Google Scholar]
- Chen, S.-Y.; Beretta, M.; Alexopoulos, S.J.; Shah, D.P.; Olzomer, E.M.; Hargett, S.R.; Childress, E.S.; Salamoun, J.M.; Aleksovska, I.; Roseblade, A.; et al. Mitochondrial Uncoupler SHC517 Reverses Obesity in Mice without Affecting Food Intake. Metabolism 2021, 117, 154724. [Google Scholar] [CrossRef]
- Alexopoulos, S.J.; Chen, S.-Y.; Brandon, A.E.; Salamoun, J.M.; Byrne, F.L.; Garcia, C.J.; Beretta, M.; Olzomer, E.M.; Shah, D.P.; Philp, A.M.; et al. Mitochondrial Uncoupler BAM15 Reverses Diet-Induced Obesity and Insulin Resistance in Mice. Nat. Commun. 2020, 11, 2397. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, C.L.; King, W.T.; Davuluri, G.; Noland, R.C.; Hall, J.; Hull, M.; Dantas, W.S.; Zunica, E.R.; Alexopoulos, S.J.; Hoehn, K.L.; et al. BAM15-Mediated Mitochondrial Uncoupling Protects against Obesity and Improves Glycemic Control. EMBO Mol. Med. 2020, 12, e12088. [Google Scholar] [CrossRef] [PubMed]
- Rivas, D.A.; Rice, N.P.; Ezzyat, Y.; McDonald, D.J.; Cooper, B.E.; Fielding, R.A. Sphingosine-1-Phosphate Analog FTY720 Reverses Obesity but Not Age-Induced Anabolic Resistance to Muscle Contraction. AJP Cell Physiol. 2019, 317, C502–C512. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol Prevents Sarcopenic Obesity by Reversing Mitochondrial Dysfunction and Oxidative Stress via the PKA/LKB1/AMPK Pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, C.; Suliburk, J.; Hsu, J.W.; Muthupillai, R.; Jahoor, F.; Minard, C.G.; Taffet, G.E.; Sekhar, R.V. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 75–89. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of Current Treatments on Liver Disease, Glucose Metabolism and Cardiovascular Risk in Non-Alcoholic Fatty Liver Disease (NAFLD): A Systematic Review and Meta-Analysis of Randomised Trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic Obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Baek, Y.; Jeong, K.; Lee, S. Association of Dietary Factors with Grip Strength, Body Fat, and Prevalence of Sarcopenic Obesity in Rural Korean Elderly with Cardiometabolic Multimorbidity. Front. Nutr. 2022, 9, 910481. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xu, S.; Cao, L.; Wang, Y.; Chen, F.; Tian, H.; Hu, J.; Wang, Z.; Wang, D. A Lacto-Ovo-Vegetarian Dietary Pattern Is Protective against Sarcopenic Obesity: A Cross-Sectional Study of Elderly Chinese People. Nutrition 2021, 91, 111386. [Google Scholar] [CrossRef] [PubMed]
- Rasaei, N.; Kashavarz, S.A.; Yekaninejad, M.S.; Mirzaei, K. The Association between Sarcopenic Obesity (SO) and Major Dietary Patterns in Overweight and Obese Adult Women. Diabetes Metab. Syndr. 2019, 13, 2519–2524. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary Carbohydrates and Fats in Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Kamada, Y.; Takahashi, H.; Shimizu, M.; Kawaguchi, T.; Sumida, Y.; Fujii, H.; Seko, Y.; Fukunishi, S.; Tokushige, K.; Nakajima, A.; et al. Clinical Practice Advice on Lifestyle Modification in the Management of Nonalcoholic Fatty Liver Disease in Japan: An Expert Review. J. Gastroenterol. 2021, 56, 1045–1061. [Google Scholar] [CrossRef] [PubMed]
- Volynets, V.; Machann, J.; Küper, M.A.; Maier, I.B.; Spruss, A.; Königsrainer, A.; Bischoff, S.C.; Bergheim, I. A Moderate Weight Reduction through Dietary Intervention Decreases Hepatic Fat Content in Patients with Non-Alcoholic Fatty Liver Disease (NAFLD): A Pilot Study. Eur. J. Nutr. 2012, 52, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Charlton, M.; Kawaguchi, A.; Yamamura, S.; Nakano, D.; Tsutsumi, T.; Zafer, M.; Torimura, T. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin. Liver Dis. 2021, 41, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.Y.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of Green-Mediterranean Diet on Intrahepatic Fat: The DIRECT plus Randomised Controlled Trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Kirk, E.; Reeds, D.N.; Finck, B.N.; Mayurranjan, M.S.; Patterson, B.W.; Klein, S. Dietary Fat and Carbohydrates Differentially Alter Insulin Sensitivity during Caloric Restriction. Gastroenterology 2009, 136, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Baker, J.A.; Rogers, T.; Davis, J.; Satapati, S.; Burgess, S.C. Short-Term Weight Loss and Hepatic Triglyceride Reduction: Evidence of a Metabolic Advantage with Dietary Carbohydrate Restriction. Am. J. Clin. Nutr. 2011, 93, 1048–1052. [Google Scholar] [CrossRef]
- Nunes, E.A.; Colenso-Semple, L.; McKellar, S.R.; Yau, T.; Ali, M.U.; Fitzpatrick-Lewis, D.; Sherifali, D.; Gaudichon, C.; Tomé, D.; Atherton, P.J.; et al. Systematic Review and Meta-Analysis of Protein Intake to Support Muscle Mass and Function in Healthy Adults. J. Cachexia Sarcopenia Muscle 2022, 13, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Eglseer, D.; Traxler, M.; Embacher, S.; Reiter, L.; Schoufour, J.D.; Weijs, P.J.M.; Voortman, T.; Boirie, Y.; Cruz-Jentoft, A.; Bauer, S. Nutrition and Exercise Interventions to Improve Body Composition for Persons with Overweight or Obesity near Retirement Age: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 516–538. [Google Scholar] [CrossRef]
- Khazaei, Y.; Dehghanseresht, N.; Ebrahimi Mousavi, S.; Nazari, M.; Salamat, S.; Asbaghi, O.; Mansoori, A. Association between Protein Intake from Different Animal and Plant Origins and the Risk of Non-Alcoholic Fatty Liver Disease: A Case-Control Study. Clin. Nutr. Res. 2023, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, H.S.; Ahn, S.B.; Kwon, Y.-J. Dairy Protein Intake Is Inversely Related to Development of Non-Alcoholic Fatty Liver Disease. Clin. Nutr. 2021, 40, 5252–5260. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High Red and Processed Meat Consumption Is Associated with Non-Alcoholic Fatty Liver Disease and Insulin Resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Nascimbeni, F.; Pais, R.; Bellentani, S.; Day, C.P.; Ratziu, V.; Loria, P.; Lonardo, A. From NAFLD in clinical practice to answers from guidelines. J. Hepatol. 2013, 59, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Annibalini, G.; Lucertini, F.; Agostini, D.; Vallorani, L.; Gioacchini, A.; Barbieri, E.; Guescini, M.; Casadei, L.; Passalia, A.; Del Sal, M.; et al. Concurrent Aerobic and Resistance Training Has Anti-Inflammatory Effects and Increases Both Plasma and Leukocyte Levels of IGF-1 in Late Middle-Aged Type 2 Diabetic Patients. Oxid. Med. Cell. Longev. 2017, 2017, 3937842. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Lopes, K.G.; Bottino, D.A.; Farinatti, P.; de Souza, M.; Maranhao, P.A.; de Araujo, C.M.S.; Bouskela, E.; Lourenco, R.A.; de Oliveira, R.B. Strength training with blood flow restriction—A novel therapeutic approach for older adults with sarcopenia? A case report. Clin. Interv. Aging 2019, 14, 1461–1469. [Google Scholar] [CrossRef]
- Golabi, P.; Gerber, L.; Paik, J.M.; Deshpande, R.; de Avila, L.; Younossi, Z.M. Contribution of Sarcopenia and Physical Inactivity to Mortality in People with Non-Alcoholic Fatty Liver Disease. JHEP Rep. 2020, 2, 100171. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Raman, M.; Mourtzakis, M.; Merli, M. A Practical Approach to Nutritional Screening and Assessment in Cirrhosis. Hepatology 2017, 65, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Rojo, A.; Ruiz-Margáin, A.; Montaño-Loza, A.J.; Macías-Rodríguez, R.U.; Ferrando, A.; Kim, W.R. Exercise and Physical Activity for Patients with End-Stage Liver Disease: Improving Functional Status and Sarcopenia While on the Transplant Waiting List. Liver Transplant. 2018, 24, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Valero-Breton, M.; Huerta-Salgado, C.; Achiardi, O.; Simon, F.; Cabello-Verrugio, C. Impact of Exercise Training on the Sarcopenia Criteria in Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Eur. J. Transl. Myol. 2021, 31, 9630. [Google Scholar] [PubMed]
- Yang, H.J.; Hong, Y.P.; Yoon, T.-Y.; Ryoo, J.-H.; Choi, J.-M.; Oh, C.-M. Independent and Synergistic Associations of Aerobic Physical Activity and Resistance Exercise with Nonalcoholic Fatty Liver Disease. Gut Liver 2023, 17, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.S. Aerobic and Resistance Exercise: Synergistic Influence for Nonalcoholic Fatty Liver Disease. Gut Liver 2023, 17, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Narbro, K.; Sjöström, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Gummesson, A.; Sjöström, C.D.; Narbro, K.; Peltonen, M.; Wedel, H.; Bengtsson, C.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; et al. Effects of Bariatric Surgery on Cancer Incidence in Obese Patients in Sweden (Swedish Obese Subjects Study): A Prospective, Controlled Intervention Trial. Lancet Oncol. 2009, 10, 653–662. [Google Scholar] [CrossRef]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Sjöström, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, Å.; Bengtsson, C.; Bergmark, G.; et al. Bariatric Surgery and Long-Term Cardiovascular Events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef]
- Adams, T.D.; Gress, R.; Smith, S.C.; Halverson, C.; Simper, S.C.; Rosamond, W.D.; LaMonte, M.J.; Stroup, A.M.; Hunt, S.C. Long-Term Mortality After Gastric Bypass Surgery. N. Engl. J. Med. 2007, 357, 753–761. [Google Scholar] [CrossRef]
- Aminian, A.; Al-Kurd, A.; Wilson, R.; Bena, J.; Fayazzadeh, H.; Singh, T.; Albaugh, V.L.; Shariff, F.U.; Rodriguez, N.A.; Jin, J.; et al. Association of Bariatric Surgery with Major Adverse Liver and Cardiovascular Outcomes in Patients with Biopsy-Proven Nonalcoholic Steatohepatitis. JAMA 2021, 326, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, G.; Caiazzo, R.; Ntandja-Wandji, L.C.; Gnemmi, V.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; et al. Bariatric Surgery Provides Long-Term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Truong, E.; Noureddin, M. Improvement in Nonalcoholic Fatty Liver Disease through Bariatric Surgery. Clin. Liver Dis. 2022, 20, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Seeberg, K.; Borgeraas, H.; Hofsø, D.; Småstuen, M.; Kvan, N.; Grimnes, J.; Lindberg, M.; Fatima, F.; Seeberg, L.; Sandbu, R.; et al. Gastric Bypass Versus Sleeve Gastrectomy in Type 2 Diabetes: Effects on Hepatic Steatosis and Fibrosis: A Randomized Controlled Trial. Ann. Intern. Med. 2022, 175, 74–83. [Google Scholar] [CrossRef]
- Verrastro, O.; Panunzi, S.; Castagneto-Gissey, L.; De Gaetano, A.; Lembo, E.; Capristo, E.; Guidone, C.; Angelini, G.; Pennestrì, F.; Sessa, L.; et al. Bariatric–Metabolic Surgery versus Lifestyle Intervention plus Best Medical Care in Non-Alcoholic Steatohepatitis (BRAVES): A Multicentre, Open-Label, Randomised Trial. Lancet 2023, 401, 1786–1797. [Google Scholar] [CrossRef]
- Buzza, A.F.B.; Machado, C.A.; Pontes, F.; Sampaio, L.G.; Contador, J.S.; Sampaio, C.L.; Radominski, R.B.; Boguszewski, C.L.; Borba, V.Z.C. Prevalence of Sarcopenia in Women at Stable Weight Phase after Roux-En-Y Gastric Bypass. Arch. Endocrinol. Metab. 2022, 66, 362–371. [Google Scholar] [CrossRef]
- Molero, J.; Olbeyra, R.; Flores, L.; Jiménez, A.; de Hollanda, A.; Andreu, A.; Ibarzabal, A.; Moizé, V.; Cañizares, S.; Balibrea, J.M.; et al. Prevalence of Low Skeletal Muscle Mass Following Bariatric Surgery. Clin. Nutr. ESPEN 2022, 49, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Voican, C.S.; Lebrun, A.; Maitre, S.; Lainas, P.; Lamouri, K.; Njike-Nakseu, M.; Gaillard, M.; Tranchart, H.; Balian, A.; Dagher, I.; et al. Predictive Score of Sarcopenia Occurrence One Year after Bariatric Surgery in Severely Obese Patients. PLoS ONE 2018, 13, e0197248. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, M.; Tranchart, H.; Maitre, S.; Perlemuter, G.; Lainas, P.; Dagher, I. Preoperative Detection of Sarcopenic Obesity Helps to Predict the Occurrence of Gastric Leak After Sleeve Gastrectomy. Obes. Surg. 2018, 28, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving Healthy Muscle during Weight Loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367.e5. [Google Scholar] [CrossRef]
- Cook, N.S.; Nagar, S.H.; Jain, A.; Balp, M.M.; Mayländer, M.; Weiss, O.; Chatterjee, S. Understanding Patient Preferences and Unmet Needs in Non-alcoholic Steatohepatitis (NASH): Insights from a Qualitative Online Bulletin Board Study. Adv. Ther. 2019, 36, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, R. Health action process approach (HAPA) as a theoretical framework to understand behavior change. Actual. En Psicol. 2016, 30, 119–130. [Google Scholar] [CrossRef]
- Dan, A.A.; Kallman, J.B.; Wheeler, A.; Younoszai, Z.; Collantes, R.; Bondini, S.; Gerber, L.; Younossi, Z.M. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2007, 26, 815–820. [Google Scholar] [CrossRef]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, S.; Calvani, R.; Marzetti, E.; Picca, A.; Coelho-Júnior, H.J.; Martone, A.M.; Massaro, C.; Tosato, M.; Landi, F. Low Adherence to Mediterranean Diet Is Associated with Probable Sarcopenia in Community-Dwelling Older Adults: Results from the Longevity Check-Up (Lookup) 7+ Project. Nutrients 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]

| Medication/Approach | Mechanism of Action | Potential Benefits | Limitations | Supporting Studies |
|---|---|---|---|---|
| Mitochondrial Uncouplers | Target mitochondria to enhance electron flow and ATP synthesis | Reduce adiposity while preserving lean mass; enhance energy expenditure | Efficacy in larger mammals and humans unknown; structural optimization needed | Studies on mitochondrial uncouplers such as BAM15 and SHC517 [62,63,64] suggest their potential to reverse adiposity while preserving lean mass |
| S1P Receptor Agonists | Downregulate S1P receptors and degrade sphingolipids | Increased lean mass and strength in animal models; reduced ceramide accumulation | Uncertain efficacy in aged individuals; potential inflammatory responses | Studies on Fingolimod showed increased lean mass and strength in mice with obesity [65] |
| AMPK Agonists | Stimulate AMPK, promote mitochondrial function and antioxidant capacity | Potential improvement in muscle function, reduced insulin resistance | Efficacy and side effects in humans still under research | Studies involving resveratrol activation of the AMPK pathway showed benefits in muscle function and mass in animal models [66] |
| Glutathione Agonists (GlyNAC) | Increase glutathione, an endogenous antioxidant protecting against oxidative stress | Improved muscle function and mitochondrial function in animal models and clinical trials | Long-term effects and larger population studies needed | GlyNAC supplementation demonstrated significant improvements in muscle function, gait speed, and strength in clinical trials [67] |
| Pathway | Outcome | Pathophysiology | Impact on Sarcopenia |
|---|---|---|---|
| Mechanical | Weight-loss | ↓ mechanical stress on joints and muscles | Facilitates mobility, physical function, and rehabilitation regimens |
| Metabolic | Improved insulin resistance | ↓ intramuscular insulin resistance ↓ myosteatosis ↑ protein synthesis | ↑ muscle function and strength, irrespective of muscle mass |
| Inflammation | Resolution of low-grade pro-inflammatory status | ↓ adipokines ↓ inflammatory markers ↓ inflammation-induced muscular catabolism ↑ protein synthesis | ↑ muscle mass, contractile function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crişan, D.; Avram, L.; Morariu-Barb, A.; Grapa, C.; Hirişcau, I.; Crăciun, R.; Donca, V.; Nemeş, A. Sarcopenia in MASLD—Eat to Beat Steatosis, Move to Prove Strength. Nutrients 2025, 17, 178. https://doi.org/10.3390/nu17010178
Crişan D, Avram L, Morariu-Barb A, Grapa C, Hirişcau I, Crăciun R, Donca V, Nemeş A. Sarcopenia in MASLD—Eat to Beat Steatosis, Move to Prove Strength. Nutrients. 2025; 17(1):178. https://doi.org/10.3390/nu17010178
Chicago/Turabian StyleCrişan, Dana, Lucreţia Avram, Andreea Morariu-Barb, Cristiana Grapa, Ioana Hirişcau, Rareş Crăciun, Valer Donca, and Andrada Nemeş. 2025. "Sarcopenia in MASLD—Eat to Beat Steatosis, Move to Prove Strength" Nutrients 17, no. 1: 178. https://doi.org/10.3390/nu17010178
APA StyleCrişan, D., Avram, L., Morariu-Barb, A., Grapa, C., Hirişcau, I., Crăciun, R., Donca, V., & Nemeş, A. (2025). Sarcopenia in MASLD—Eat to Beat Steatosis, Move to Prove Strength. Nutrients, 17(1), 178. https://doi.org/10.3390/nu17010178






