Effect of Sustainably Sourced Protein Consumption on Nutrient Intake and Gut Health in Older Adults: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.1.1. Population
2.1.2. Interventions
2.1.3. Comparators
2.1.4. Outcomes
2.1.5. Settings
2.1.6. Years, Language, and Publication Status
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Outcomes
2.6.1. Outcomes
- Dietary adherence or intervention: Score and adherence to a particular diet
- Nutrient intake: Energy, fat, carbohydrates, total protein, vegetable protein, animal protein and fibre.
- Food Groups: Meats, fish and poultry; fruit and vegetables; legumes; and breads, grains and cereals.
- Gut microbiome: Measurement of microbiota and inflammation status including but not limited to inflammatory markers, α-diversity, taxonomies, phenolic profiles, microbial levels or short chain fatty acids
- Health: Body mass index (BMI), self-rated health, non-communicable diseases, cholesterol, muscle mass and grip strength.
2.6.2. Other Variables
- Author, year, and country
- Study design, sample size, mean age, gender, dietary exposure, duration of follow up.
- Dietary measurement tool, dietary pattern assessment, adherence to diet.
2.7. Study Risk of Bias Assessment
2.8. Effect Measures
3. Results
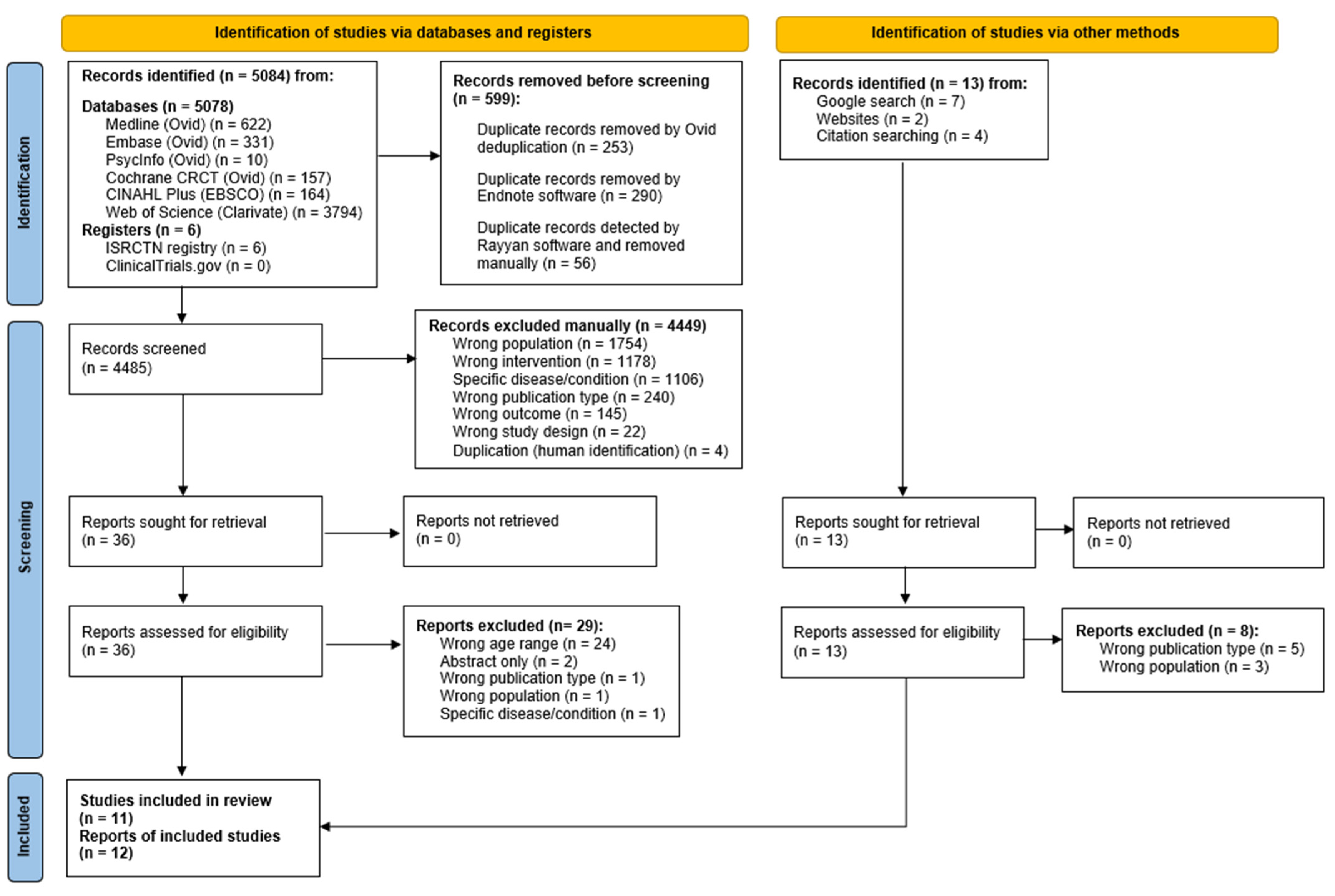
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Population and Settings
3.2.2. Study Design and Intervention
3.3. Risk of Bias in Studies
3.3.1. Bias Due to Confounding
3.3.2. Bias Arising from Measurement of the Exposure
3.3.3. Bias in Selection of Participants into Study
3.3.4. Bias Due to Post-Exposure Interventions/Missing Data/Measurement of the Outcome/Selection of the Reported Result
3.4. Results of Individual Studies
3.4.1. Adherence to Diet
3.4.2. Microbiota/Inflammatory Outcomes
3.4.3. Food Group Intake
3.4.4. Nutrient Intake
3.4.5. Health Status
4. Discussion
Limitations of Evidence and the Review Process
5. Implications for Practice, Policy and Future Research
6. Registration and Protocol
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shannon, O.M.; Ashor, A.W.; Scialo, F.; Saretzki, G.; Martin-Ruiz, C.; Lara, J.; Matu, J.; Griffiths, A.; Robinson, N.; Lilla, L.; et al. Mediterranean diet and the hallmarks of ageing. Eur. J. Clin. Nutr. 2021, 75, 1176–1192. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Ageing and Health. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health#:~:text=At%20the%20biological%20level%2C%20ageing,of%20disease%20and%20ultimately%20death (accessed on 19 July 2023).
- Office for National Statistics. Profile of the Older Population Living in England and Wales in 2021 and Changes since 2011. 2023. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/articles/profileoftheolderpopulationlivinginenglandandwalesin2021andchangessince2011/2023-04-03#:~:text=2.-,Population%20ageing,from%2016.4%25%20to%2018.6%25 (accessed on 19 July 2023).
- Whitty, C.J.M. Chief Medical Officer’s Annual Report 2023: Health in an Ageing Society. In Department of Health and Social Care 2023. Available online: https://www.gov.uk/government/publications/chief-medical-officers-annual-report-2023-health-in-an-ageing-society (accessed on 23 January 2024).
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, H.; Jung, K.; Yoon, J.; Yoo, M.; Kim, K.; Yu, B. The inflammatory process in aging. Rev. Clin. Gerontol. 2000, 10, 207–222. [Google Scholar] [CrossRef]
- Prokopidis, K.; Chambers, E.; Ni Lochlainn, M.; Witard, O.C. Mechanisms Linking the Gut-Muscle Axis with Muscle Protein Metabolism and Anabolic Resistance: Implications for Older Adults at Risk of Sarcopenia. Front. Physiol. 2021, 12, 770455. [Google Scholar] [CrossRef]
- Cappola, A.R.; Auchus, R.J.; El-Hajj Fuleihan, G.; Handelsman, D.J.; Kalyani, R.R.; McClung, M.; Stuenkel, C.A.; Thorner, M.O.; Verbalis, J.G. Hormones and Aging: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2023, 108, 1835–1874. [Google Scholar] [CrossRef]
- Nass, R.; Farhy, L.S.; Liu, J.; Pezzoli, S.S.; Johnson, M.L.; Gaylinn, B.D.; Thorner, M.O. Age-dependent decline in acyl-ghrelin concentrations and reduced association of acyl-ghrelin and growth hormone in healthy older adults. J. Clin. Endocrinol. Metab. 2014, 99, 602–608. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Turroni, S.; Garagnani, P.; Franceschi, C.; Brigidi, P. Ageing and gut microbes: Perspectives for health maintenance and longevity. Pharmacol. Res. 2013, 69, 11–20. [Google Scholar] [CrossRef]
- Zapata, H.J.; Quagliarello, V.J. The microbiota and microbiome in aging: Potential implications in health and age-related diseases. J. Am. Geriatr. Soc. 2015, 63, 776–781. [Google Scholar] [CrossRef]
- Ni Lochlainn, M.; Bowyer, R.C.E.; Steves, C.J. Dietary Protein and Muscle in Aging People: The Potential Role of the Gut Microbiome. Nutrients 2018, 10, 929. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Prokopidis, K.; Cervo, M.M.; Gandham, A.; Scott, D. Impact of Protein Intake in Older Adults with Sarcopenia and Obesity: A Gut Microbiota Perspective. Nutrients 2020, 12, 2285. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jonsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; Schwarz, Y.; Song, X.; Wang, C.Y.; Chen, C.; Trudo, S.P.; Kristal, A.R.; Kratz, M.; Eaton, D.L.; Lampe, J.W. Cruciferous vegetables have variable effects on biomarkers of systemic inflammation in a randomized controlled trial in healthy young adults. J. Nutr. 2014, 144, 1850–1857. [Google Scholar] [CrossRef]
- King, D.E.; Egan, B.M.; Woolson, R.F.; Mainous, A.G., 3rd; Al-Solaiman, Y.; Jesri, A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch. Intern. Med. 2007, 167, 502–506. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef]
- Saraf-Bank, S.; Esmaillzadeh, A.; Faghihimani, E.; Azadbakht, L. Effect of non-soy legume consumption on inflammation and serum adiponectin levels among first-degree relatives of patients with diabetes: A randomized, crossover study. Nutrition 2015, 31, 459–465. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Krok-Schoen, J.L.; Archdeacon Price, A.; Luo, M.; Kelly, O.J.; Taylor, C.A. Low Dietary Protein Intakes and Associated Dietary Patterns and Functional Limitations in an Aging Population: A NHANES analysis. J. Nutr. Health Aging. 2019, 23, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, R.; Biggs, M.L.; Djousse, L.; Durda, P.J.; Kizer, J.R.; Psaty, B.; Reiner, A.P.; Tracy, R.P.; Siscovick, D.; Mukamal, K.J. Intake and Sources of Dietary Fiber, Inflammation, and Cardiovascular Disease in Older US Adults. JAMA Netw. Open. 2022, 5, e225012. [Google Scholar] [CrossRef] [PubMed]
- Reid-McCann, R.J.; Brennan, S.F.; McKinley, M.C.; McEvoy, C.T. The effect of animal versus plant protein on muscle mass, muscle strength, physical performance and sarcopenia in adults: Protocol for a systematic review. Syst. Rev. 2022, 11, 64. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Loon, L.J.C. Plant versus animal based-proteins to support muscle conditioning. Sports Sci. Exch. 2021, 29, 1–7. Available online: https://www.gssiweb.org/docs/default-source/sse-docs/sse220_vanloon_oct_a02.pdf?sfvrsn=2 (accessed on 29 August 2023).
- Kouw, I.W.K.; Pinckaers, P.J.M.; Le Bourgot, C.; van Kranenburg, J.M.X.; Zorenc, A.H.; de Groot, L.; Verdijk, L.; Snijders, T.; van Loon, L.J.C. Ingestion of an ample amount of meat substitute based on a lysine-enriched, plant-based protein blend stimulates postprandial muscle protein synthesis to a similar extent as an isonitrogenous amount of chicken in healthy, young men. Br. J. Nutr 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef]
- Hawkes, N. Cutting Europe’s meat and dairy consumption would benefit health and environment, says report. BMJ 2014, 348, g2949. [Google Scholar] [CrossRef]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Khan, M.S. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- FAO The Future of Food and Agriculture—Trends and Challenges. Rome 2017. Available online: https://www.fao.org/3/i6583e/i6583e.pdf (accessed on 3 May 2024).
- Springmann, M.; Clark, M.A.; Rayner, M.; Scarborough, P.; Webb, P. The global and regional costs of healthy and sustainable dietary patterns: A modelling study. Lancet Planet. Health 2021, 5, e797–e807. [Google Scholar] [CrossRef]
- WHO Fa. Sustainable Healthy Diets—Guiding Principles. Rome 2019. Available online: https://www.who.int/publications/i/item/9789241516648 (accessed on 27 February 2024).
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Polleau, A.; Biermann, G. Eat local to save the planet? Contrasting scientific evidence and consumers’ perceptions of healthy and environmentally friendly diets. Curr. Res. Environ. Sustain. 2021, 3, 100054. [Google Scholar] [CrossRef]
- Langer, H.; Carlsohn, A. Effects of Different Dietary Proteins and Amino Acids on Skeletal Muscle Hypertrophy in Young Adults After Resistance Exercise: A Systematic Review. Strength. Cond. J. 2014, 36, 33–42. [Google Scholar] [CrossRef]
- Messina, M.; Lynch, H.; Dickinson, J.M.; Reed, K.E. No Difference Between the Effects of Supplementing with Soy Protein Versus Animal Protein on Gains in Muscle Mass and Strength in Response to Resistance Exercise. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.T.; Pan, B.J.; Toh, D.W.K.; Sutanto, C.N.; Kim, J.E. Animal Protein versus Plant Protein in Supporting Lean Mass and Muscle Strength: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 661. [Google Scholar] [CrossRef]
- Tieland, M.; Franssen, R.; Dullemeijer, C.; van Dronkelaar, C.; Kyung Kim, H.; Ispoglou, T.; Zhu, K.; Prince, R.L.; van Loon, L.J.C.; de Groot, L. The Impact of Dietary Protein or Amino Acid Supplementation on Muscle Mass and Strength in Elderly People: Individual Participant Data and Meta-Analysis of RCT’s. J. Nutr. Health Aging. 2017, 21, 994–1001. [Google Scholar] [CrossRef]
- Gielen, E.; Beckwee, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr. Rev. 2021, 79, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Hanach, N.I.; McCullough, F.; Avery, A. The Impact of Dairy Protein Intake on Muscle Mass, Muscle Strength, and Physical Performance in Middle-Aged to Older Adults with or without Existing Sarcopenia: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Trefflich, I.; Jabakhanji, A.; Menzel, J.; Blaut, M.; Michalsen, A.; Lampen, A.; Abraham, K.; Weikert, C. Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2990–3004. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Islam, T.; Johnson, P.; Moustaid-Moussa, N. Systematic Review of Beef Protein Effects on Gut Microbiota: Implications for Health. Adv. Nutr. 2021, 12, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Uffelman, C.N.; Bergia, R.E.; Clark, C.M.; Reed, J.B.; Cross, T.L.; Lindemann, S.R.; Tang, M.; Campbell, W.W. Meat Consumption and Gut Microbiota: A Scoping Review of Literature and Systematic Review of Randomized Controlled Trials in Adults. Adv. Nutr. 2023, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Kong, J.; Underwood, C.; Petocz, P.; Hirani, V.; Dawson, B.; O’Leary, F. Systematic review and meta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions. Br. J. Nutr. 2018, 119, 527–542. [Google Scholar] [CrossRef]
- The European Food Inofmration Council (EUFIC). Sustainable Protein: Meeting Future Needs. 2017. Available online: https://www.eufic.org/en/food-production/article/sustainable-protein-meeting-future-needs (accessed on 27 July 2023).
- Cancello, R.; Turroni, S.; Rampelli, S.; Cattaldo, S.; Candela, M.; Cattani, L.; Mai, S.; Vietti, R.; Scacchi, M.; Brigidi, P.; et al. Effect of Short-Term Dietary Intervention and Probiotic Mix Supplementation on the Gut Microbiota of Elderly Obese Women. Nutrients 2019, 11, 3011. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.M.R.; Rooney, A.; Taylor, K.; Thayer, K.; Silva, R.; Lemeris, C.; Akl, A.; Arroyave, W.; Bateson, T.; Berkman, N.; et al. Risk of Bias in Non-randomized Studies—Of Exposure (ROBINS-E). Launch version. 20 June 2023. Available online: https://www.riskofbias.info/welcome/robins-e-tool (accessed on 24 January 2024).
- Andre, P.; Pais de Barros, J.-P.; Mj Merle, B.; Samieri, C.; Helmer, C.; Delcourt, C.; Feart, C. Mediterranean diet and prudent diet are both associated with low circulating esterified 3-hydroxy fatty acids, a proxy of LPS burden, among older adults. Am. J. Clin. Nutr. 2021, 114, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, A.A.M.; van de Rest, O.; Feskens, E.J.M.; Santoro, A.; Ostan, R.; Pietruszka, B.; Brzozowska, A.; Stelmaszczyk-Kusz, A.; Jennings, A.; Gillings, R.; et al. Changes in Dietary Intake and Adherence to the NU-AGE Diet Following a One-Year Dietary Intervention among European Older Adults-Results of the NU-AGE Randomized Trial. Nutrients 2018, 10, 1905. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Cauley, J.A.; Peddada, S.D.; Langsetmo, L.; Shikany, J.M.; Orwoll, E.S.; Ensrud, K.E.; Cawthon, P.M.; Newman, A.B. Relation Between Dietary Protein Intake and Gut Microbiome Composition in Community-Dwelling Older Men: Findings from the Osteoporotic Fractures in Men Study (MrOS). J. Nutr. 2022, 152, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Diaz, I.; Fernandez-Navarro, T.; Salazar, N.; Bartolome, B.; Moreno-Arribas, M.V.; de Andres-Galiana, E.J.; Fernandez-Martinez, J.L.; de Los Reyes-Gavilan, C.G.; Gueimonde, M.; Gonzalez, S. Adherence to a Mediterranean Diet Influences the Fecal Metabolic Profile of Microbial-Derived Phenolics in a Spanish Cohort of Middle-Age and Older People. J. Agric. Food Chem. 2017, 65, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.D.; Satija, A.; Ivey, K.L.; Li, J.; Wilkinson, J.E.; Li, R.; Baden, M.; Chan, A.T.; Huttenhower, C.; et al. Plant-Based Diet Index and Metabolic Risk in Men: Exploring the Role of the Gut Microbiome. J. Nutr. 2021, 151, 2780–2789. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Rodriguez, J.; Delgado-Velandia, M.; Ortola, R.; Carballo-Casla, A.; Garcia-Esquinas, E.; Rodriguez-Artalejo, F.; Sotos-Prieto, M. Plant-based diets and risk of frailty in community-dwelling older adults: The Seniors-ENRICA-1 cohort. Geroscience 2023, 45, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Hullar, M.A.J.; Monroe, K.R.; Shepherd, J.A.; Hunt, J.; Randolph, T.W.; Wilkens, L.R.; Boushey, C.J.; Le Marchand, L.; Lim, U.; et al. Fecal Microbial Diversity and Structure Are Associated with Diet Quality in the Multiethnic Cohort Adiposity Phenotype Study. J. Nutr. 2019, 149, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Saavedra, S.; Salazar, N.; Suarez, A.; de Los Reyes-Gavilan, C.G.; Gueimonde, M.; Gonzalez, S. Comparison of Different Dietary Indices as Predictors of Inflammation, Oxidative Stress and Intestinal Microbiota in Middle-Aged and Elderly Subjects. Nutrients 2020, 12, 3828. [Google Scholar] [CrossRef]
- Shikany, J.M.; Demmer, R.T.; Johnson, A.J.; Fino, N.F.; Meyer, K.; Ensrud, K.E.; Lane, N.E.; Orwoll, E.S.; Kado, D.M.; Zmuda, J.M.; et al. Association of dietary patterns with the gut microbiota in older, community-dwelling men. Am. J. Clin. Nutr. 2019, 110, 1003–1014. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Lo, H.-C.; Yang, F.L.; Liu, Y.-F.; Wu, W.-M.; Chou, C.-C. Plant-Based, Antioxidant-Rich Snacks Elevate Plasma Antioxidant Ability and Alter Gut Bacterial Composition in Older Adults. Nutrients 2021, 13, 3872. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public. Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Smidowicz, A.; Regula, J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv. Nutr. 2015, 6, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, C.A.; van de Put, M.; Bisschops, M.; Walrabenstein, W.; de Jonge, C.S.; Herrema, H.; van Schaardenburg, D. The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review. Nutrients 2021, 13, 3208. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108 (Suppl. 2), S183–S211. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Li, C.Y.; Fang, A.P.; Ma, W.J.; Wu, S.L.; Li, C.L.; Chen, Y.M.; Zhu, H.L. Amount Rather than Animal vs Plant Protein Intake Is Associated with Skeletal Muscle Mass in Community-Dwelling Middle-Aged and Older Chinese Adults: Results from the Guangzhou Nutrition and Health Study. J. Acad. Nutr. Diet. 2019, 119, 1501–1510. [Google Scholar] [CrossRef]
- Carbone, J.W.; Pasiakos, S.M. The role of dietary plant and animal protein intakes on mitigating sarcopenia risk. Curr. Opin. Clin. Nutr. Metab. Care. 2022, 25, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.A.; Roberts, K.M.; Gundersen, A.; Farris, Y.; Zwickey, H.; Bradley, R.; Weir, T.L. Relationships between Habitual Polyphenol Consumption and Gut Microbiota in the INCLD Health Cohort. Nutrients 2024, 16, 773. [Google Scholar] [CrossRef] [PubMed]
- Zhubi-Bakija, F.; Bajraktari, G.; Bytyci, I.; Mikhailidis, D.P.; Henein, M.Y.; Latkovskis, G.; Rexhaj, Z.; Zhubi, E.; Banach, M. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: A position paper from the International Lipid Expert Panel (ILEP). Clin. Nutr. 2021, 40, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of Animal and Plant Protein Intake with All-Cause and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, J.I.; Kyle, J.; Horgan, G.W.; Loe, J.; Fyfe, C.; Johnstone, A.; McNeill, G. Sustainable diets for the future: Can we contribute to reducing greenhouse gas emissions by eating a healthy diet? Am. J. Clin. Nutr. 2012, 96, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.; Ramos, O.L.; Vicente, A.A. Emergent food proteins—Towards sustainability, health and innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Belobrajdic, D.; Hamzelou, S.; Jones, D.; Leifert, W.; Ponce-Reyes, R.; Terefe, N.S.; Williams, G.; Colgrave, M. How Healthy Are Non-Traditional Dietary Proteins? The Effect of Diverse Protein Foods on Biomarkers of Human Health. Foods 2022, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, R. Protein and older adults. J. Am. Coll. Nutr. 2004, 23, 627S–630S. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef]
- Leonard, U.M.; Leydon, C.L.; Arranz, E.; Kiely, M.E. Impact of consuming an environmentally protective diet on micronutrients: A systematic literature review. Am. J. Clin. Nutr. 2024, 119, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Fouillet, H.; Juillet, B.; Gaudichon, C.; Mariotti, F.; Tome, D.; Bos, C. Absorption kinetics are a key factor regulating postprandial protein metabolism in response to qualitative and quantitative variations in protein intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1691–R1705. [Google Scholar] [CrossRef] [PubMed]
- Markofski, M.M.; Volpi, E. Protein metabolism in women and men: Similarities and disparities. Curr. Opin. Clin. Nutr. Metab. Care. 2011, 14, 93–97. [Google Scholar] [CrossRef] [PubMed]
| Author, Year (Country) | Study Design | Sample Size (n) | Age Mean (SD) Years | Female n (%) | Dietary Intervention/ Exposure | Outcomes | Duration (Months) |
|---|---|---|---|---|---|---|---|
| André, 2021 [57] (France) | Cross-sectional | 698 | 73.1 (4.4) | 432 (61.9) | Mediterranean vs. prudent vs. traditional vs. complex carbs | Circulating 3-OH FAs, a proxy of LPS-type endotoxins burden. | Dietary survey conducted after 24 months |
| Berendsen, 2018 [58] (The Netherlands) Recruited from five EU centres | Randomised multicentre, single-blind, controlled trial | 1141 | 71.0 (4.0) | 631 (55) | Mediterranean-like diet (NU-AGE diet) with counselling and dietary advice vs. control | Dietary intake | 12 months follow up |
| Farsijani, 2022 [59] (USA) | Cross-sectional | 775 | 84.2 (4.0) | 0 (0) | Usual food intake to measure total daily protein intake | Microbial DNA extraction from stool sample for gut microbiome profiling (16S rRNA gene sequencing) | Mailed FFQ to MrOS mean of 4.6 (SD 11.7) days after stool collection. |
| Ghosh, 2020 [60] (Ireland) Recruited from five EU study centres | Randomised multicentre, single-blind, controlled trial | 612 | 71 (range: 65–79) | 326 (53) | Mediterranean-like diet (NU-AGE diet) + counselling + dietary advice vs. control | Gut microbiome profile | 12 months follow up |
| Gutierrez-Díaz, 2016 [61] (Spain) | Cross-sectional | 74 (50 yrs and above) 50–65 yrs: n = 37 ≥65 yrs: n = 37 | 71.3 (11.2) (Mean for subgroups not reported) | Not reported for ≥65 years subset. Total sample: 54 (73) | Mediterranean diet score | Anthopometric data, microbiological and phenolic metabolite assessment of fecal specimens. | Cross sectional |
| Li, 2021 [62] (USA) | Cohort | 303 | 71 (4.0) | 0 (0) | hPDI | Gut microbiome profile | 12 months follow up |
| Maroto-Rodriguez, 2022 [63] (Spain) | Cohort | 1880 | 68.65 (6.38) | 971 (51.56) | hPDI & uPDI | Health outcomes recorded at baseline and frailty status recorded at follow up | Follow up was on average 40 months |
| Maskarinec, 2019 [64] (USA) | Cohort | 1735 | 69.2 (at stool collection) | 877 (50.5) | HEI-2010, AHEI-2010, aMED, and DASH diet | Association of diet quality with measures of stool microbial community structure. | Main analysis is cross sectional |
| Ruiz-Saavedra, 2020 [65] (Spain) | Cross-sectional | 40 (≥65 yrs) Total sample: n = 73 | 2 sub-groups: 50–65 years and 65–95 years (mean not reported) | Not reported for ≥65 subset. Total sample: 53 (73) | DII, EDII, HEI, AHEI, DQI-I, MMDS, and rMED | Major phylogenetic types of the intestinal microbiota determined by qPCR and SCFAs | Cross-sectional |
| Shikany, 2019 [66] (USA) | Cross-sectional | 517 | 84.3 (4.1) | 0 (0) | 2 dietary patterns: Western and Prudent. | Diversity of gut bacterial microbiota | Dietary assessment completed within a mean (SD) 4.6 (11.7) days of the stool sample collection |
| Trichopoulou, 2003 [67] (Greece) | Cohort | 4369 (≥65 yrs) Total sample n = 22,043 | 3 sub-groups: <55 years, 55–64 years, and ≥65 years (mean not reported) | Not reported for ≥65 subset. Total 13,143 (60) | Mediterranean diet | Mortality | 44 months follow up |
| Zhang, 2021 [68] (Taiwan) | Cohort | 59 | Female: 77.7 (7.6) Male: 85.3 (8.4) | 30 (51) | Plant-based, antioxidant-rich smoothies and sesame seed snacks | Antioxidant ability and gut microbial composition | Follow up at 2 and 4 months |
| Author, Year | Dietary Measurement Tool | Dietary Pattern Assessment | Adherence to Diet | Main Findings |
|---|---|---|---|---|
| André, 2021 [57] | FFQ (By registered dietitian) | Med diet: Score ranged from 0, low adherence to 18, high adherence. Carbs/traditional/prudent diet: Factor analysis with tertile range (upper tertile = higher adherence). | Med diet: Mean score: 10.7 (SD 2.0) n = 698 Low (<9): n = 187 (27.0%) Medium (10–12): n = 264 (38.0%) High (>12): n = 247 (35.0%) Carbs/traditional/prudent diet: Low: n = 232 (33.2%) Medium: n = 233 (33.4%) High: n = 233 (33.4%) | Plant-based dietary patterns were associated with lower 3-OH FA concentrations, and thus a lower LPS burden, which is considered a potent trigger of inflammatory response. |
| Berendsen, 2018 [58] | Self-reported 7-day records (with prior training) | NU-AGE index score, with diet compliance ranging from 0 (low) to 160 (high). | Baseline mean score (SD): Control group: 82.6 (16.5) Diet group: 82.6 (15.3) Follow-up mean score (SD): Control group: 84.6 (16.1) Diet group: 105.7 (17.6) | The NU-AGE dietary intervention may be a feasible strategy to improve dietary intake in an aging European population. |
| Farsijani, 2022 [59] | Self-reported Brief FFQ (Block 98.2 MrOS) | Total daily protein intake (g/d) was estimated from the collected FFQs. Data was recorded by quartile of energy adjusted protein intake. | Q1: ≤55.44 g/d, n = 194 (25.0%) Q2: 55.45–61.17 g/d, n = 193 (24.9%) Q3: 61.18–67.98 g/d, n = 194 (25.0%) Q4: ≥67.99 g/d, n = 194 (25.1%) | Higher protein consumptions from either animal or vegetable sources were associated with higher gut microbiome diversity. |
| Ghosh, 2020 [60] | Self-reported 7-day records (with prior training) | NU-AGE index score, with diet compliance ranging from 0 (low) to 160 (high). | Dietary variations within the intervention group were significantly different from the control group (envfit p < 0.006). Intervention group: increased intake of fibres, vitamins (C, B6, B9, thiamine) and minerals (Cu, K, Fe, Mn, Mg). Control: Increase in fat intake (saturated fats and mono-unsaturated fatty acids) relative to the intervention group. | Increasing adherence to the NU-AGE diet was associated with higher gut microbiome diversity |
| Gutierrez-Díaz, 2016 [61] | FFQ 24 h dietary intake | Med Diet Score (MDS) calculated based on eight dietary components, with a possible range of 0–8 points. Cut-off for greater adherence and health-promoting effects was 4 points. | Total sample (50 years and above) MDS < 4 points: n = 32 (43%) MDS ≥ 4 points: n = 42 (57%) Diet scores not reported for >65 years subgroup | Older subjects (>65 years), and subjects with sedentary habits exhibited higher values for the fecal content of phenylacetic, 4-hydroxyphenylacetic, and phthalic acids. |
| Li, 2021 [62] | Validated FFQ | hPDI was derived from FFQ. Scores were summed to give a hPDI score of 18 (lowest) to 90 (highest). | hPDI score, mean (SD): Q1: 46.5 (2.6) n = 59 (19.6%) Q2: 51.3 (0.9) n = 62 (20.4%) Q3: 54.4 (0.9) n = 60 (19.8%) Q4: 58.0 (1.2) n = 62 (20.4%) Q5: 64.1 (2.8) n = 60 (19.8%) | A greater adherence to a healthy plant-based diet was associated with a microbial profile characterized by a higher abundance of multiple species. |
| Maroto-Rodriguez, 2022 [63] | A validated computerised face-to-face diet history (DH-ENRICA) developed from EPIC cohort study in Spain | hPDI was derived from FFQ. Scores were summed to give hPDI and uPDI scores of 18 (lowest) to 90 (highest) and then categorized into 3 tertiles. | hPDI, mean score (SD): Total: 59.73 (5.73) n = 1880 T1: 52.43 (2.62) n = 429 (23%) T2: 58.60 (1.71) n = 765 (41%) T3: 65.56 (3.22) n = 686 (36%) uPDI, mean score (SD): Total: 54.85 (5.32) n = 1880 T1: 50.32 (3.13) n= 879 (47%) T2: 56.83 (1.37) n = 639 (34%) T3: 62.38 (2.52) n = 362 (19%) | In older adults, the hPDI was associated with lower risk of frailty, while the opposite was found for the uPDI. |
| Maskarinec, 2019 [64] | QFFQ covering over 180 food items | Scores for 4 diets were calculated. Score ranges shown in next column. In all 4 diets the higher the score the higher the adherence to the diet. | HEI-2010, score range: T1: 35.2—68.4, n = 578 (33.3%) T2: 68.5—77.7, n = 579 (33.4%) T3: 77.8—99.1, n = 578 (33.3%) AHEI-2010, score range: T1: 35.6—64.5, n = 578 (33.3%) T2: 64.6—73.0, n = 579 (33.4%) T3: 73.1—99.4, n = 578 (33.3%) aMED, score range: T1: 0–3, n = 643 (37.1%) T2: 4–5, n = 627 (36.1%) T3: 6–9, n = 465 (26.8%) DASH, score range: T1: 9–22, n = 631 (36.4%) T2: 23–26, n = 545 (31.4%) T3: 27–38, n = 559 (32.2%) | Diet quality was strongly associated with fecal microbial alpha diversity and beta diversity and several genera previously associated with human health |
| Ruiz-Saavedra, 2020 [65] | Semi-QFFQ | Scores for 7 dietary indices were calculated for the total sample (aged 50–95 years). | Subgroup >65 years, mean score (SD) (n = 40): DII: 0.98 (2.02) EDII: 1.02 (0.69) HEI: 54.46 (10.16) AHEI: 58.39 (6.88) DQI-I: 46.60 (5.96) MMDS: 3.13 (1.49) rMED: 6.15 (2.03) | DII, HEI, DQI-I and MMDS were identified as predictors of Faecalibacterium prausnitzii levels, AHEI and MMDS were negatively associated with Lactobacillus group. HEI, AHEI and MMDS were positively associated with fecal SCFAs. |
| Shikany, 2019 [66] | Block 98.2 MrOS FFQ (NutritionQuest) | Final factor scores were calculated through analysis of the FFQs. Adherence to the dietary patterns was divided into quartiles, with Quartile 1 representing the lowest adherence and Quartile 4 representing the highest adherence. | Western diet: Q1: n = 130 (25.0%) Q2: n = 129 (25.0%) Q3: n = 129 (25.0%) Q4: n = 129 (25.0%) Prudent diet Q1: n = 130 (25.0%) Q2: n = 129 (25.0%) Q3: n = 129 (25.0%) Q4: n = 129 (25.0%) | Significant associations between measures of gut microbial composition and dietary patterns. |
| Trichopoulou, 2003 [67] | Semi-QFFQ administered by specially trained interviewers | Mediterranean-diet score ranged from 0 (minimal adherence to the traditional Mediterranean diet) to 9 (maximal adherence) | Subgroup >65 years, Med diet score range: T1: Score 0–3, n = 1598 (36.6%) T2: Score 4–5, n = 1829 (41.9%) T3: Score 6–9, n = 942 (21.5%) | Greater adherence to the traditional Mediterranean diet is associated with a significant reduction in total mortality |
| Zhang, 2021 [68] | Consumption of specified smoothies and snacks | Each serving of a plant-based smoothie contained 1 exchange of vegetables (2 kinds), 1 exchange of fruits (2 kinds), and 1 exchange of nuts. | All participants were provided with 5 servings of plant-based smoothies and 3 servings of sesame seed snacks per week. Participants received these for a 4-month period. | Consumption of Plant-based smoothies and snacks prompted significant decreases in observed bacterial species and their richness. |
| RoB 2.0 | D1 | D2 | D3 | D4 | D5 | Overall | ||
|---|---|---|---|---|---|---|---|---|
| Berendsen, 2018 [58] | Low | Low | Low | Low | Low | Low | ||
| Ghosh, 2020 [60] | Low | Low | Low | Low | Low | Low | ||
| ROBINS-E | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall |
| Andre, 2021 [57] | Low | Some concerns | Low | Low | Low | Low | Low | Some concerns |
| Farsijani, 2022 [59] | Some concerns | Some concerns | Low | Low | Low | Low | Low | Some concerns |
| Gutierrez-Diaz, 2016 [61] | Some concerns | Some concerns | Low | Low | Low | Low | Low | Some concerns |
| Li, 2021 [62] | Some concerns | Some concerns | Low | Low | Low | Low | Low | Some concerns |
| Maroto-Rodriguez, 2022 [63] | Low | Some concerns | Low | Low | Low | Low | Low | Some concerns |
| Maskarinec, 2019 [64] | Low | Some concerns | Low | Low | Low | Low | Low | Some concerns |
| Ruiz-Saavedra, 2020 [65] | Low | Some concerns | Low | Low | Low | Low | Low | Some concerns |
| Shikany, 2019 [66] | Some concerns | Some concerns | Low | Low | Low | Low | Low | Some concerns |
| Trichopoulou, 2003 [67] | Low | Some concerns | Low | Low | Low | Low | Low | Some concerns |
| Zhang, 2021 [68] | Some concerns | High | Low | Low | Low | Low | Low | High |
| Author, Year | Microbiology |
|---|---|
| André, 2021 [57] | 3-OHFAs pmol/mL (SD) Med diet adherence: Low: 276.7 (110.4), Medium: 261.8 (92.9), High: 263.7 (99.7) Comp carbs adherence: Low: 270.6 (118.7), Medium: 266.1 (87.2), High: 262.6 (92.6) Trad diet adherence: Low: 249.9 (83.4), Medium: 258.6 (91.4), High: 290.8 (118.4) Prudent diet adherence: Low: 283.0 (114.6), Medium: 261.1 (85.2), High: 255.3 (97.3) |
| Farsijani, 2022 [59] | α-diversity Higher protein intake from vegetable sources compared to lower intake from vegetable sources was associated with higher Chao1 and Shannon indices (overall p < 0.05). Higher protein intake from animal sources compared to lower intake from animal sources was associated with higher Shannon and Inverse Simpson indices (overall p < 0.05). |
| Ghosh, 2020 [60] | Taxonomies Adherence to the Mediterranean diet led to increased abundance of specific taxa that were positively associated with several markers of lower frailty and improved cognitive function, and negatively associated with inflammatory markers including C-reactive protein and interleukin-17. |
| Gutierrez-Díaz, 2016 [61] | Phenolic profiles (μg/mL) Subgroup age ≥ 65 yrs (n = 37): Phenylacetic acid: 16.56 (20.38) Phenylpropionic acid: 10.03 (9.82) Benzoic acid: 0.54 (0.97) 3-hydroxyphenylacetic acid: 0.22 (0.28) |
| Li, 2021 [62] | Taxonomies A higher hPDI score was significantly associated with 7 microbial species, with: Higher relative abundance (%) of: Bacteroides cellulosilyticus (2.58%; 95% CI: 1.39, 3.77) and Eubacterium eligens (1.37%; 95% CI: 0.55, 2.20) Lower abundance (%) of: Ruminococcus torques (−1.09%; 95% CI: −1.67, −0.50), Ruminococcus gnavus (−1.10%; 95% CI: −1.69, −0.52), Clostridium leptum (−0.66%; 95% CI: −1.03, −0.30), Lachnospiraceae bacterium 1_4_56faa (−0.29%; 95% CI: −0.45, −0.12), and Erysipelotrichaceae bacterium 21_3 (−0.12%; 95% CI: −0.18, −0.05) |
| Maskarinec, 2019 [64] | α-diversity (Shannon), mean (95% CI) HEI-2010, T1: 6.03 (5.89, 6.17), T2: 6.15 (6.01, 6.28), T3: 6.15 (6.01, 6.29) AHEI-2010, T1: 6.02 (5.88, 6.15), T2: 6.13 (6.00, 6.27), T3: 6.14 (6.00, 6.28) aMED, T1: 6.05 (5.91, 6.18), T2: 6.11 (5.97, 6.24), T3: 6.16 (6.02, 6.31) DASH, T1: 6.07 (5.94, 6.21), T2: 6.11 (5.98, 6.25), T3: 6.17 (6.03, 6.31) Phylum Actinobacterium, mean (95% CI) HEI-2010, T1: 2.01 (1.80, 2.23), T2: 1.69 (1.47, 1.90), T3: 1.65 (1.43, 1.87) AHEI-2010, T1: 1.91 (1.69, 2.12), T2: 1.78 (1.56, 1.99), T3: 1.67 (1.45, 1.88) aMED, T1: 1.98 (1.76, 2.19), T2: 1.70 (1.49, 1.91), T3: 1.60 (1.37, 1.82) DASH, T1: 1.94, (1.72, 2.16), T2: 1.73 (1.52, 1.95), T3: 1.63 (1.41, 1.86) |
| Ruiz-Saavedra, 2020 [65] | Microbiological parameters: Significant differences were observed in most of the microbiological parameters analyzed according to age. Subjects over 65 years of age presented lower fecal levels of Bacteroides-Prevotella-Porphyromonas group, Clostridia cluster XIVa and Faecalibacterium, as well as all the short chain fatty acids determined. Blood parameters are within the normal physiological ranges and were similar between the groups evaluated except for MDA, IL-8, IL-12 and TNF-α, whose concentration is higher in subjects over 65 years of age. Microbial levels for sub group >65 years: Bacteroides-Prevotella-Porphyromonas (Log10 n ◦ cells/g feces): 8.79 (0.69) Clostridia cluster XIVa (Log10 n ◦ cells/g feces): 6.45 (1.54) Faecalibacterium prausnitzii (Log10 n ◦ cells/g feces): 6.42 (1.31) Acetic acid (mM): 23.18 (14.45) Propionic acid (mM): 9.50 (7.46) Butyric acid (mM): 8.44 (7.94) |
| Shikany, 2019 [66] | α-diversity, mean (SD) Western diet: Shannon: Total sample: 3.39 (0.61), Q1: 3.42 (0.66), Q2: 3.43 (0.62), Q3: 3.38 (0.60), Q4: 3.32 (0.57) Inverse Simpson: Total sample: 15.9 (9.8), Q1: 16.9 (10.2), Q2: 16.6 (10.8), Q3: 15.9 (9.8), Q4: 14.3 (8.3) Prudent diet: Shannon: Total sample: 3.39 (0.61), Q1: 3.38 (0.63), Q2: 3.44 (0.59), Q3: 3.36 (0.61), Q4: 3.38 (0.62) Inverse Simpson: Total sample: 15.9 (9.8), Q1: 15.9 (9.8), Q2: 16.8 (10.3), Q3: 15.4 (10.0), Q4: 15.6 (9.4) Beta-diversity In multivariable-adjusted models, greater adherence to the Western pattern was positively associated with families Mogibacteriaceae and Veillonellaceae and genera Alistipes, Anaerotruncus, CC115, Collinsella, Coprobacillus, Desulfovibrio, Dorea, Eubacterium, and Ruminococcus, while greater adherence to the prudent pattern was positively associated with order Streptophyta, family Victivallaceae, and genera Cetobacterium, Clostridium, Faecalibacterium, Lachnospira, Paraprevotella, and Veillonella. Beta diversity measures were significantly associated with both Western and prudent patterns in multivariable-adjusted analyses. |
| Zhang, 2021 [68] | α-diversity and SCFAs, at baseline, month 2 and month 4, mean (SD) Acetic acid (µmol/g): 40.98 (16.83), 38.59 (17.86), 30.10 (17.26) Propionic acid (µmol/g) 40.88 (19.21), 42.26 (20.48), 38.89 (20.85) Butyric acid (µmol/g) 36.06 (18.20), 31.61 (16.76), 34.21 (19.06) Chao1: 391.1 (112.5), 301.2 (85.4), 310.2 (77.9) ACE: 387.9 (111.4), 294.6 (78.9), 308.8 (77.9) Shannon 5.09 (0.74), 4.98 (0.68), 5.00 (0.66) Simpson 0.92 (0.06), 0.92 (0.05), 0.93 (0.04) Changes in SCFA content in the feces were not significantly different after 2 and 4 months of intervention Gut microbiota, at baseline, month 2 and month 4, mean (SD) After adjusting for age, gender, and intervention compliance, the older adults were found to have significantly decreased levels of the following bacterial taxa: class Bacilli, genus Streptococcus, genus Ruminiclostridium_5, class Deltaproteobacteria, phylum Actinobacteria, class Bifidobacteriales, and phylum Patescibacteria and increased levels of genus Lactobacillus after 2 and 4 months relative to the baseline. There were significant increases in the phylum Bacteroidetes and species Bacteroides thetaiotaomicron after 2 months and in the genus Agathobacter after 4 months relative to the baseline. There were no appreciable differences in the ratios of Firmicutes and Bacteroidetes—an indicator that is strongly associated with several diseases—at the baseline and 2 and 4 months after the commencement of the intervention (6.6, 4.0, and 6.85, respectively). |
| Author, Year | Measure of Food Group | Fruit, Mean (SD) | Veg, Mean (SD) | Legumes, Mean (SD) | Poultry, Mean (SD) | Meat, Mean (SD) | Fish, Mean (SD) | Wholegrains/Bread/Cereal, Mean (SD) |
|---|---|---|---|---|---|---|---|---|
| André, 2021 [57] | adherence to Med diet, servings/ week adherence to Comp carbs diet, servings/ week adherence to Trad diet, servings/ week adherence to Prudent diet, servings/week | Low: 9.1 (6.6) Medium: 13.4 (6.6) High: 15.6 (5.4) Low: 12.9 (7.3) Medium: 13.3 (6.8) High: 13.0 (6.1) Low: 14.6 (7.1) Medium: 13.2 (6.2) High: 11.5 (6.5) Low: 10.2 (6.1) Medium: 12.6 (5.9) High: 16.3 (6.8) | Low: 8.4 (4.0) Medium: 9.9 (4.1) High: 11.8 (4.0) Low: 9.4 (4.0) Medium: 10.0 (4.0) High: 11.1 (4.5) Low: 10.2 (4.3) Medium: 10.5 (4.2) High: 9.8 (4.1) Low: 7.3 (3.5) Medium: 9.8 (2.9) High: 13.4 (3.6) | Low: 0.5 (0.5) Medium: 0.6 (0.7) High: 0.7 (0.6) Low: 0.4 (0.4) Medium: 0.6 (0.6) High: 0.8 (0.7) Low: 0.3 (0.4) Medium: 0.6 (0.5) High: 0.9 (0.8) Low: 0.6 (0.7) Medium: 0.6 (0.5) High: 0.6 (0.6) | Low: 1.6 (1.1) Medium: 1.8 (1.4) High: 1.9 (1.1) Low: 1.3 (0.9) Medium: 1.7 (1.0) High: 2.5 (1.5) Low: 1.9 (1.4) Medium: 1.8 (1.2) High: 1.7 (1.1) Low: 1.6 (1.1) Medium: 1.8 (1.2) High: 2.1 (1.4) | Low: 5.8 (3.0) Medium: 4.4 (2.4) High: 4.4 (1.9) Low: 4.8 (2.8) Medium: 5.0 (2.3) High: 4.5 (2.3) Low: 3.4 (2.0) Medium: 4.6 (1.9) High: 6.3 (2.6) Low: 5.0 (2.7) Medium: 5.0 (2.5) High: 4.3 (2.3) | Low: 1.9 (1.3) Medium: 2.9 (1.7) High: 3.6 (1.6) Low: 2.2 (1.4) Medium: 3.0 (1.5) High: 3.5 (1.8) Low: 2.8 (1.8) Medium: 2.9 (1.6) High: 2.9 (1.6) Low: 2.3 (1.3) Medium: 2.9 (1.7) High: 3.5 (1.7) | Low: 17.1 (6.0) Medium: 18.6 (5.0) High: 19.4 (4.3) Low: 16.5 (6.0) Medium: 19.1 (4.6) High: 19.9 (4.1) Low: 15.7 (6.2) Medium: 19.4 (4.2) High: 20.4 (3.3) Low: 16.7 (5.6) Medium: 19.0 (4.6) High: 19.8 (4.7) |
| Berendsen, 2018 [58] | Control group, g/day Diet group (Med style diet), g/day | Baseline: 260.0 (158.7) Follow up: 255.7 (154.0) Baseline: 248.2 (140.2) Follow up: 268.2 (140.0) | Baseline: 221.4 (120.7) Follow up: 213.2 (125.7) Baseline: 214.5 (110.8) Follow up: 234.2 (103.7) | Baseline: 11.1 (20.0) Follow up: 10.8 (19.2) Baseline: 10.4 (20.9) Follow up: 17.6 (21.9) | Baseline: 41.2 (33.4) Follow up: 40.5 (31.4) Baseline: 40.5 (31.6) Follow up: 38.5 (27.9) | N/A | Baseline: 28.4 (29.3) Follow up: 24.9 (23.2) Baseline: 28.4 (25.3) Follow up: 37.1 (28.1) | Baseline: 54.4 (53.9) Follow up: 62.6 (60.7) Baseline: 55.7 (58.3) Follow up: 107.2 (66.4) |
| Li, 2021 [62] | adherence to hPDI, servings/ day | Q1: 1.3 (0.8) Q2: 1.6 (0.6) Q3: 1.7 (0.8) Q4: 1.9 (1.1) Q5: 2.6 (1.4) | Q1: 3.2 (1.0) Q2: 3.4 (1.4) Q3: 3.5 (1.7) Q4: 3.9 (1.7) Q5: 4.6 (1.6) | Q1: 0.4 (0.2) Q2: 0.5 (0.2) Q3: 0.4 (0.2) Q4: 0.4 (0.2) Q5: 0.7 (0.5) | Not reported | Q1: 1.6 (0.5) Q2: 1.4 (0.5) Q3: 1.3 (0.6) Q4: 1.1 (0.4) Q5: 0.7 (0.4) | Q1: 0.3 (0.1) Q2: 0.4 (0.2) Q3: 0.3 (0.2) Q4: 0.4 (0.2) Q5:0.4 (0.2) | Q1: 1.6 (0.7) Q2: 1.7 (0.9) Q3: 2.1 (1.2) Q4: 1.9 (0.9) Q5:2.5 (1.7) |
| Author, Year | Measure of Nutrient Group | Energy, Mean (SD) (kcal/d) | Fat, Mean (SD) (% of kcal/d) | Carbohydrate, Mean (SD) (g/d) | Total Protein, Mean (SD) (g/d) | Veg/Plant Protein, Mean (SD) (g/d) | Animal Protein, Mean (SD) (g/d) | Fibre, Mean (SD) (g/d) |
|---|---|---|---|---|---|---|---|---|
| Farsijani, 2022 [59] | Total daily protein intake by quartile | Q1: 1710 (695) Q2: 1372 (568) Q3: 1355 (482) Q4: 1700 (622) Total sample: 1534 (620) | Q1: 41.8 (7.1) Q2: 40.2 (6.8) Q3: 41.3 (7.2) Q4: 40.0 (6.9) | % of kcal/d: Q1: 48.1 (7.5) Q2: 47.4 (7.0) Q3: 44.3 (7.4) Q4: 43.0 (6.9) | Q1: 55.3 (23.5) Q2: 52.3 (21.2) Q3: 57.4 (17.9) Q4: 81.8 (26.1) Total sample, 62.0 (10.8) | Q1: 25.7 (12.5) Q2: 22.1 (10.3) Q3: 21.8 (10.5) Q4: 28.8 (13.4) | Q1: 29.6 (15.1) Q2: 30.2 (13.8) Q3: 35.6 (11.8) Q4: 53.0 (18.4) | Q1: 16.1 (7.9) Q2: 14.9 (6.7) Q3: 14.7 (7.1) Q4: 18.8 (9.0) |
| Li, 2021 [62] | Adherence to a high protein diet by quintile | Q1: 2353 (411) Q2: 2254 (512) Q3: 2069 (481) Q4: 1987 (510) Q5: 1921 (459) | Not reported | Q1: 275.0 (56.8) Q2: 273.0 (70.7) Q3: 251.0 (64.5) Q4: 241.0 (69.2) Q5: 254.0 (76.7) | Q1: 96.0 (17.5) Q2: 92.7 (23.9) Q3: 90.2 (23.7) Q4: 85.5 (20.8) Q5: 81.0 (18.7) | Q1: 28.6 (6.1) Q2: 30.3 (8.6) Q3: 30.1 (8.9) Q4: 30.0 (8.9) Q5: 35.0 (12.7) | Q1: 67.5 (14.5) Q2: 62.3 (17.4) Q3: 60.0 (17.3) Q4: 55.5 (14.1) Q5: 46.0 (16.2) | Q1: 21.8 (5.5) Q2: 24.5 (7.4) Q3: 24.1 (7.4) Q4: 25.2 (7.9) Q5: 30.4 (10.6) |
| Maroto-Rodriguez, 2022 [63] | Adherence to a high protein diet by tertile | T1: 2335 (550) T2: 2053 (554) T3: 1815 (499) Total sample, 2031 (569) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Maskarinec, 2019 [64] | Adherence to HEI, AHEI, aMED and DASH by tertile | HEI-2010: T1: 1957 (1050) T3: 1779 (766) AHEI-2010: T1: 1760 (887) T3: 1967 (833) aMED: T1: 1463 (615) T3: 2450 (1136) DASH: T1: 1618 (699) T3: 2109 (1082) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, D.; Celis-Morales, C.; Gray, S.R.; Morrison, D.J.; Ozanne, S.E.; Jain, M.; Mattin, L.R.; Burden, S. Effect of Sustainably Sourced Protein Consumption on Nutrient Intake and Gut Health in Older Adults: A Systematic Review. Nutrients 2024, 16, 1398. https://doi.org/10.3390/nu16091398
Jones D, Celis-Morales C, Gray SR, Morrison DJ, Ozanne SE, Jain M, Mattin LR, Burden S. Effect of Sustainably Sourced Protein Consumption on Nutrient Intake and Gut Health in Older Adults: A Systematic Review. Nutrients. 2024; 16(9):1398. https://doi.org/10.3390/nu16091398
Chicago/Turabian StyleJones, Debra, Carlos Celis-Morales, Stuart R. Gray, Douglas J. Morrison, Susan E. Ozanne, Mahek Jain, Lewis R. Mattin, and Sorrel Burden. 2024. "Effect of Sustainably Sourced Protein Consumption on Nutrient Intake and Gut Health in Older Adults: A Systematic Review" Nutrients 16, no. 9: 1398. https://doi.org/10.3390/nu16091398
APA StyleJones, D., Celis-Morales, C., Gray, S. R., Morrison, D. J., Ozanne, S. E., Jain, M., Mattin, L. R., & Burden, S. (2024). Effect of Sustainably Sourced Protein Consumption on Nutrient Intake and Gut Health in Older Adults: A Systematic Review. Nutrients, 16(9), 1398. https://doi.org/10.3390/nu16091398









