Carbohydrate-Mediated Pregnancy Gut Microbiota and Neonatal Low Birth Weight
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Collection and Comparison Groups
2.3. Fetal Outcomes and Maternal Characteristics
2.4. DNA Extraction and 16S rRNA Amplicon Sequencing
2.5. Statistical Analyses
3. Results
3.1. Maternal Macronutrient Pattern during Pregnancy
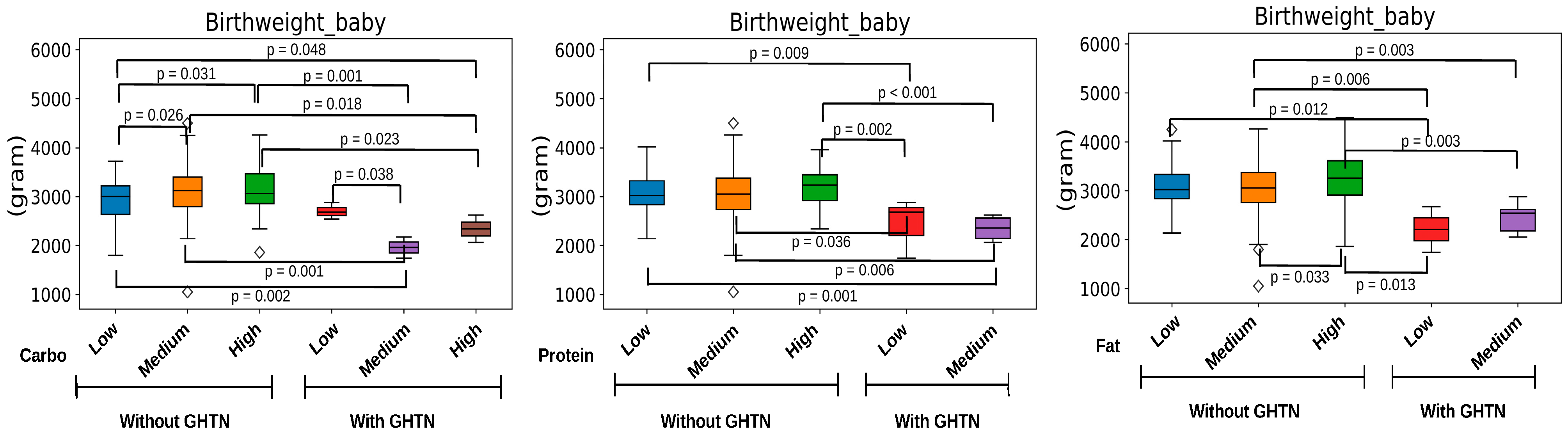
3.2. Pregnancy Carbohydrate Intake and Fetal Outcomes
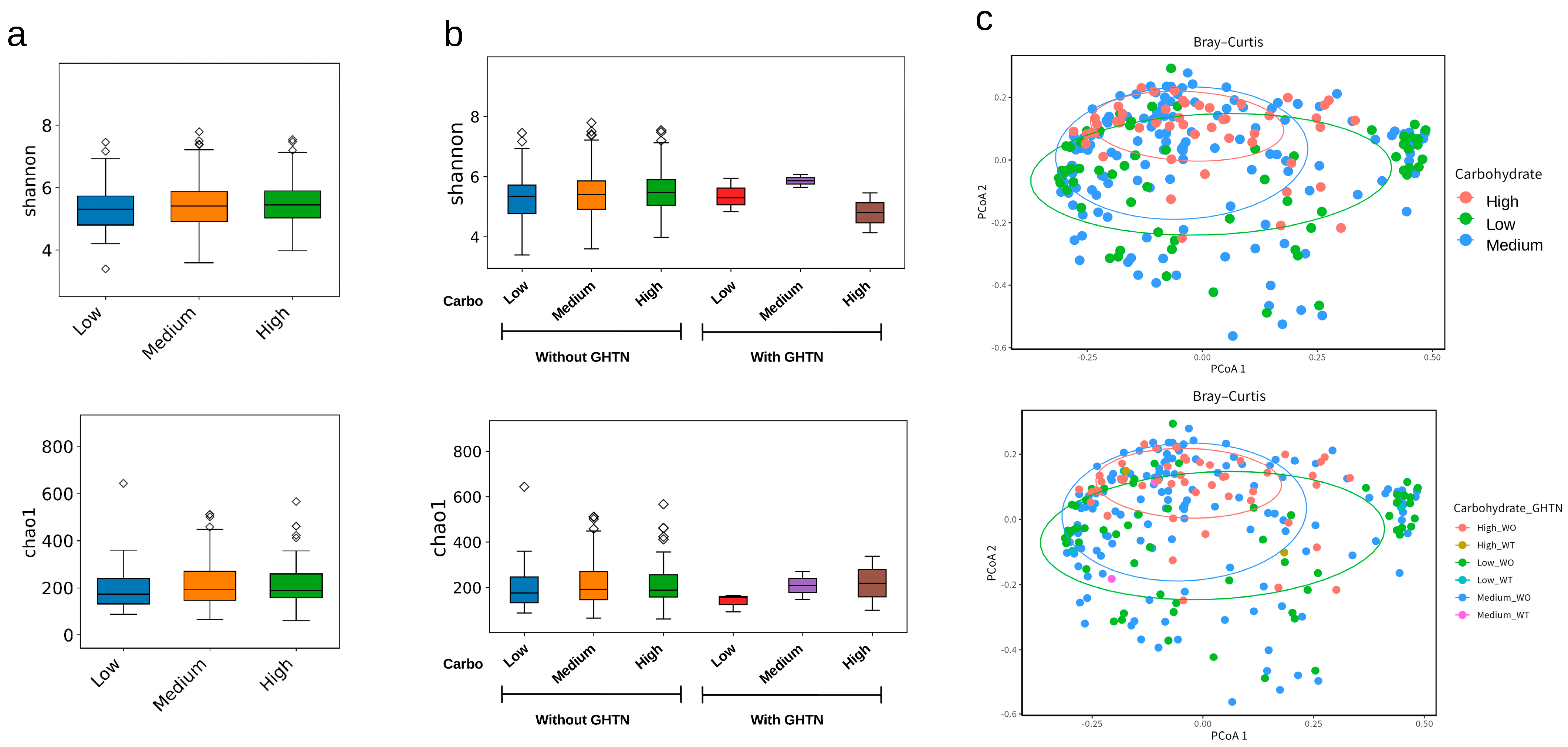
3.3. Maternal Pregnancy Gut Microbiota and Carbohydrate Intake
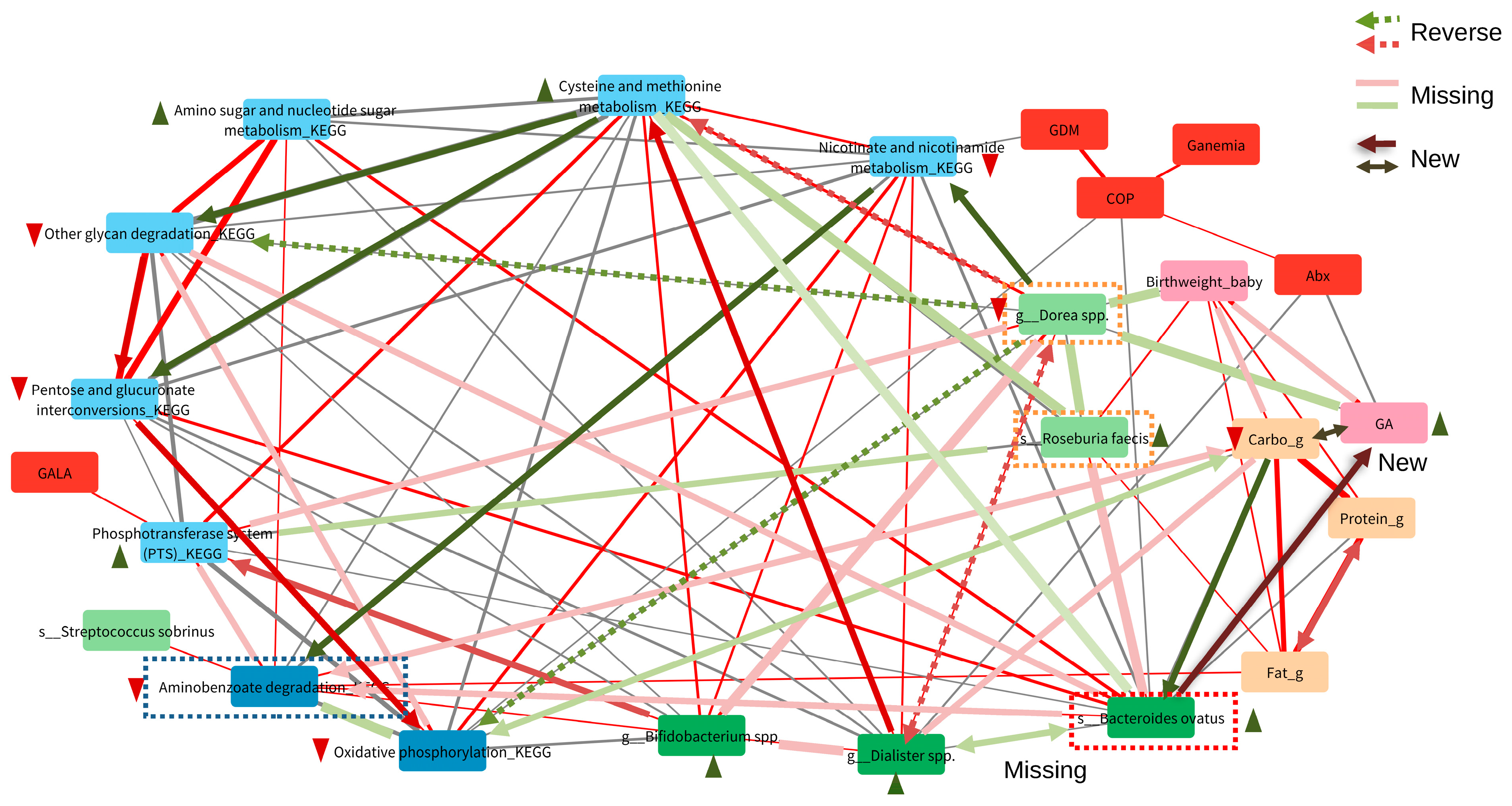
3.4. Predicted KEGG Pathway and Carbohydrate-Mediated Gut Microbiota
3.5. Important Maternal Gut Microbiota Compositions Affected by Carbohydrate Nutrition and Maternal Hypertension during Pregnancy
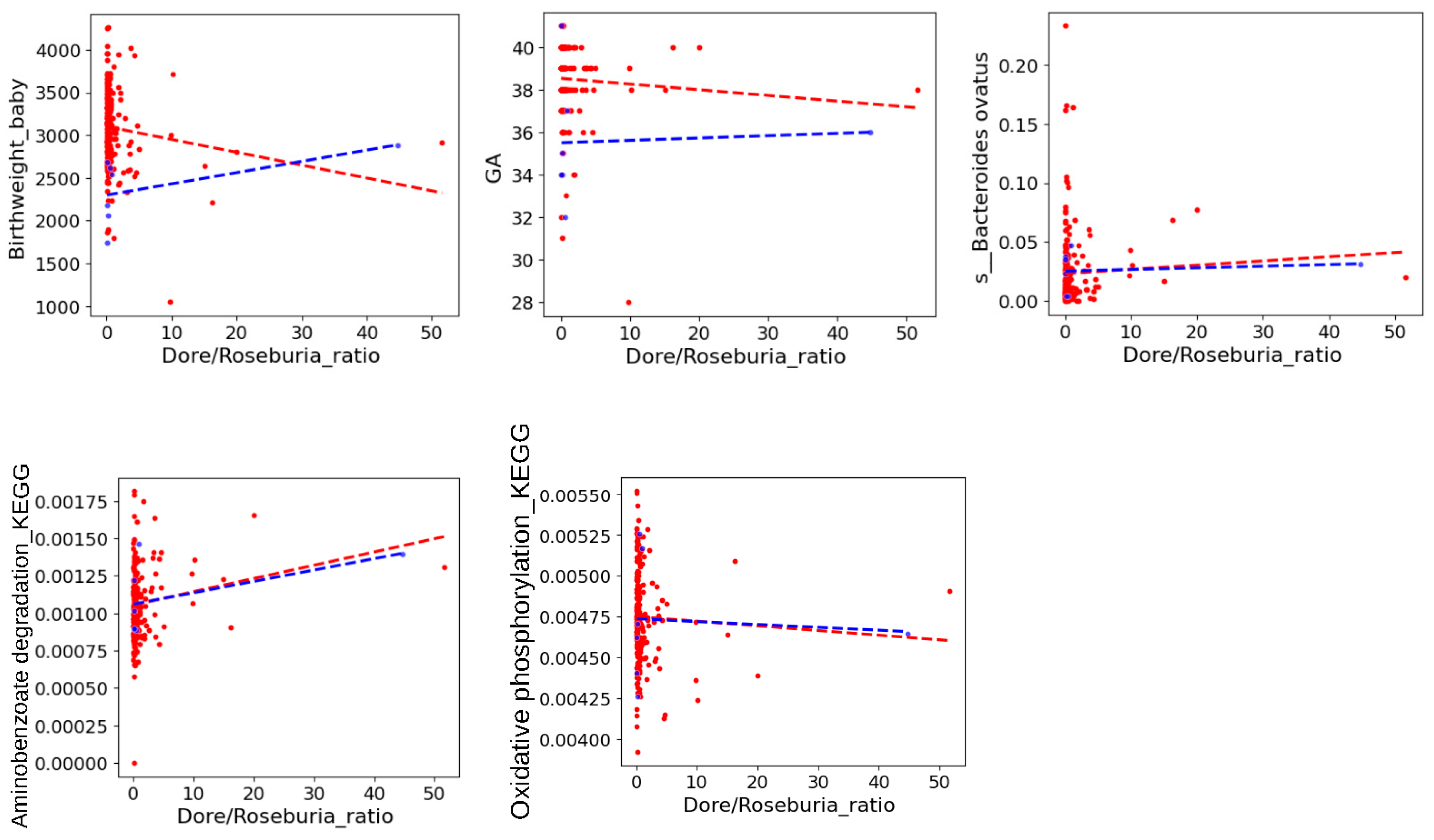
3.6. Key Carbohydrate-Mediated Pregnancy Gut Microbiota and Its Association with Fetal Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blencowe, H.; Krasevec, J.; de Onis, M.; Black, R.E.; An, X.; Stevens, G.A.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef] [PubMed]
- Lawn, J.E.; Blencowe, H.; Oza, S. Every Newborn: Progress, priorities, and potential beyond survival. Lancet 2014, 384, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Visentin, S.; Grumolato, F.; Nardelli, G.B.; Di Camillo, B.; Grisan, E.; Cosmi, E. Early origins of adult disease: Low birth weight and vascular remodeling. Atherosclerosis 2014, 237, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. The developing world of DOHaD. J. Dev. Orig. Health Dis. 2018, 9, 266–269. [Google Scholar] [CrossRef]
- McGuire, S. World Health Organization. Comprehensive Implementation Plan on Maternal, Infant, and Young Child Nutrition. Geneva, Switzerland, 2014. Adv. Nutr. 2015, 6, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Mullany, L.C.; Hurley, K.M.; Katz, J.; Black, R.E. Nutrition and maternal, neonatal, and child health. Semin. Perinatol. 2015, 39, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E.; Restrepo, J.M. Low Birthweight as a Risk Factor for Non-communicable Diseases in Adults. Front. Med. 2022, 8, 793990. [Google Scholar] [CrossRef] [PubMed]
- Lowensohn, R.I.; Stadler, D.D.; Naze, C. Current Concepts of Maternal Nutrition. Obstet. Gynecol. Surv. 2016, 71, 413–426. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M. Maternal dietary diversity during pregnancy and risk of low birth weight in newborns: A systematic review. Public Health Nutr. 2021, 24, 4671–4681. [Google Scholar] [CrossRef]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; O Casavale, K.; et al. Dietary patterns before and during pregnancy and birth outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109, 729S–756S. [Google Scholar] [CrossRef]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, T.L.; Mande, A.; Barbour, L.A. Nutrition therapy within and beyond gestational diabetes. Diabetes Res. Clin. Pract. 2018, 145, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Lavie, M.; Lavie, I.; Maslovitz, S. Paleolithic diet during pregnancy-A potential beneficial effect on metabolic indices and birth weight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 242, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Plows, J.F.; Ventura, E.E. Effects of Consuming Sugars and Alternative Sweeteners during Pregnancy on Maternal and Child Health: Evidence for a Secondhand Sugar Effect. Proc. Nutr. Soc. 2019, 78, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Pathirathna, M.L.; Nandasena, H.M.; Samarasekara, B.P.; Dasanayake, T.S.; Weerasekara, I.; Haruna, M. Is Maternal Carbohydrate Intake Having an Impact on Newborn Birth Weight? A Systematic Review. Nutrients 2023, 15, 1649. [Google Scholar] [CrossRef] [PubMed]
- Bayar, E.; Bennett, P.R.; Chan, D.; Sykes, L.; MacIntyre, D.A. The pregnancy microbiome and preterm birth. Semin. Immunopathol. 2020, 42, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Lee, M.S.; Pan, W.H.; Wahlqvist, M.L. Validation of a simplified food frequency questionnaire as used in the Nutrition and Health Survey in Taiwan (NAHSIT) for the elderly. Asia Pac. J. Clin. Nutr. 2011, 20, 134–140. [Google Scholar] [PubMed]
- Department of Health. Nutrient Composition Data Bank for Foods in Taiwan Area; Department of Health, Republic of China: Taipei, Taiwan, 1998. (In Chinese)
- Hsieh, W.S.; Wu, H.C.; Jeng, S.F.; Liao, H.F.; Su, Y.N.; Lin, S.J.; Hsieh, C.J.; Chen, P.C. Nationwide singleton birth weight percentiles by gestational age in Taiwan, 1998-2002. Acta Paediatr. Taiwan 2006, 47, 25–33. [Google Scholar]
- Yu, H.R.; Tsai, C.C.; Chan, J.Y.H.; Lee, W.C.; Wu, K.L.H.; Tain, Y.L.; Hsu, T.-Y.; Cheng, H.-H.; Huang, H.-C.; Huang, C.-H.; et al. A Higher Abundance of Actinomyces spp. In the Gut Is Associated with Spontaneous Preterm Birth. Microorganisms 2023, 11, 1171. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Il-lumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Ruebel, M.L.; Gilley, S.P.; Sims, C.R.; Zhong, Y.; Turner, D.; Chintapalli, S.V.; Piccolo, B.D.; Andres, A.; Shankar, K. Associations between Maternal Diet, Body Composition and Gut Microbial Ecology in Pregnancy. Nutrients 2021, 13, 3295. [Google Scholar] [CrossRef] [PubMed]
- Selma-Royo, M.; Garcia-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martinez-Costa, C.; Collado, M.C. Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur. J. Nutr. 2021, 60, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.K.; Edens, T.J.; Geum, H.M.; Baharmand, I.; Gill, S.K.; Robertson, R.C.; Mutasa, K.; Ntozini, R.; Smith, L.E.; Chasekwa, B.; et al. Maternal fecal microbiome predicts gestational age, birth weight and neonatal growth in rural Zimbabwe. EBioMedicine 2021, 68, 103421. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Chiang Chiau, J.S.; Chang, J.H.; Hsu, C.H.; Lin, C.Y.; Ko, M.H.J.; Lee, H.C. Characteristics of Gut Microbiota in Small for Gestational Age Infants with Very Low Birth Weight. Nutrients 2022, 14, 5158. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, H.; Collado, M.C.; Ollila, H.; Kolari, T.; Tölkkö, S.; Isolauri, E.; Salminen, S.; Rautava, S. Spontaneous preterm delivery is reflected in both early neonatal and maternal gut microbiota. Pediatr Res. 2022, 91, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Mora-Flores, L.P.; Moreno-Terrazas Casildo, R.; Fuentes-Cabrera, J.; Pérez-Vicente, H.A.; de Anda-Jáuregui, G.; Neri-Torres, E.E. The Role of Carbohydrate Intake on the Gut Microbiome: A Weight of Evidence Systematic Review. Microorganisms 2023, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guo, R.; Li, S.; Liang, F.; Tian, C.; Zhao, X.; Long, Y.; Liu, F.; Jiang, M.; Zhang, Y.; et al. Systematic analysis of gut microbiota in pregnant women and its correlations with individual heterogeneity. NPJ Biofilms Microbiomes 2020, 6, 32. [Google Scholar] [CrossRef]
- Palmu, J.; Lahti, L.; Niiranen, T. Targeting Gut Microbiota to Treat Hypertension: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1248. [Google Scholar] [CrossRef]
- Lv, L.J.; Li, S.H.; Li, S.C.; Zhong, Z.C.; Duan, H.L.; Tian, C.; Li, H.; He, W.; Chen, M.C.; He, T.W.; et al. Early-Onset Preeclampsia Is Associated With Gut Microbial Alterations in Antepartum and Postpartum Women. Front. Cell Infect. Microbiol. 2019, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F., 3rd. Maternal carbohydrate intake and pregnancy outcome. Proc. Nutr. Soc. 2002, 61, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Scholl, T.O.; Chen, X.; Khoo, C.S.; Lenders, C. The dietary glycemic index during pregnancy: Influence on infant birth weight, fetal growth, and biomarkers of carbohydrate metabolism. Am. J. Epidemiol. 2004, 159, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Coughlin, K.B.; Frederick, I.O.; Sorensen, T.K.; Williams, M.A. Dietary fiber intake in early pregnancy and risk of subsequent preeclampsia. Am. J. Hypertens. 2008, 21, 903–909. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Solomon, C.G.; Hu, F.B. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef]
- Godfrey, K.; Robinson, S.; Barker, D.J.; Osmond, C.; Cox, V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ 1996, 312, 410. [Google Scholar] [CrossRef]
- Chia, A.R.; Chen, L.W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Gershuni, V.; Li, Y.; Elovitz, M.; Li, H.; Wu, G.D.; Compher, C.W. Maternal gut microbiota reflecting poor diet quality is associated with spontaneous preterm birth in a prospective cohort study. Am J Clin Nutr. 2021, 113, 602–611. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Fultz, R.; Ticer, T.; Ihekweazu, F.D.; Horvath, T.D.; Haidacher, S.J.; Hoch, K.M.; Bajaj, M.; Spinler, J.K.; Haag, A.M.; Buffington, S.A.; et al. Unraveling the Metabolic Requirements of the Gut Commensal Bacteroides ovatus. Front. Microbiol. 2021, 12, 745469. [Google Scholar] [CrossRef] [PubMed]
- Virwani, P.D.; Qian, G.; Hsu, M.S.S.; Pijarnvanit, T.K.K.T.S.; Cheung, C.N.; Chow, Y.H.; Tang, L.K.; Tse, Y.H.; Xian, J.W.; Lam, S.S.; et al. Sex Differences in Association Between Gut Microbiome and Essential Hypertension Based on Ambulatory Blood Pressure Monitoring. Hypertension 2023, 80, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]


| All (n = 257) | Carbohydrate Intake 1 | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (n = 65) | Medium (n = 139) | High (n = 50) | |||||||
| Maternal diet during pregnancy | |||||||||
| Total calories intake (Kcal) | 1623.87 | (494.08) | 1156.44 | (245.06) | 1583.47 | (265.27) | 2237.37 | (352.80) | <0.0001 |
| Protein (%) | 15.46 | (2.68) | 16.58 | (3.01) | 15.42 | (2.56) | 14.27 | (1.96) | 0.0069 |
| Fat (%) | 27.92 | (6.34) | 29.45 | (6.78) | 27.80 | (5.68) | 26.13 | (7.14) | <0.0001 |
| Carbohydrate (%) | 56.61 | (6.89) | 53.96 | (7.27) | 56.78 | (6.33) | 59.60 | (6.85) | <0.0001 |
| Total protein (g) | |||||||||
| Mean (SD) | 62.77 | 19.62 | 48.42 | (13.26) | 62.17 | (17.36) | 80.11 | (14.00) | <0.0001 |
| Mmedian (25h, 75th) | 60.40 | (49.10, 75.50) | 48.50 | (37.80, 55.30) | 59.60 | (49.90, 69.90) | 78.75 | (69.90, 88.30) | <0.0001 |
| Total fat (g) | |||||||||
| Mean (SD) | 51.59 | 23.02 | 39.37 | (15.51) | 50.18 | (16.07) | 67.51 | (31.34) | <0.0001 |
| Median (25h, 75th) | 48.40 | (36.40, 62.20) | 36.90 | (28.60, 46.90) | 48.60 | (39.60, 60.90) | 64.00 | (48.50, 78.50) | <0.0001 |
| Total Carbohydrate (g) | |||||||||
| Mean (SD) | 230.47 | 71.78 | 155.00 | (26.39) | 224.02 | (31.46) | 331.45 | (35.64) | <0.0001 |
| Median (25h, 75th) | 220.00 | (178.50, 263.90) | 157.00 | (139.10, 171.50) | 226.60 | (196.20, 250.40) | 325.80 | (306.50, 353.20) | <0.0001 |
| Fibers (mg), mean (SD) | 13.25 | (5.19) | 10.57 | (4.13) | 12.92 | (4.36) | 16.92 | (5.49) | <0.0001 |
| Fetal outcomes | |||||||||
| Male infant, n (%) | 126 | (49.03) | 33 | (50.77) | 66 | (47.48) | 25 | (50.00) | 0.8930 |
| Birth weight (Kg), n (%) | |||||||||
| <2.5 | 20 | (7.78) | 8 | (12.31) | 8 | (5.76) | 4 | (8.00) | 0.0747 |
| 2.5 to <4 | 217 | (84.44) | 56 | (86.15) | 119 | (85.61) | 39 | (78.00) | . |
| ≥4 | 20 | (7.78) | 1 | (1.54) | 12 | (8.63) | 7 | (14.00) | . |
| Gestational age category, n (%) | |||||||||
| SGA | 27 | (10.51) | 11 | (16.92) | 13 | (9.35) | 3 | (6.00) | 0.0702 |
| AGA | 190 | (73.93) | 49 | (75.38) | 103 | (74.10) | 35 | (70.00) | . |
| LGA | 40 | (15.56) | 5 | (7.69) | 23 | (16.55) | 12 | (24.00) | . |
| Birth mode, n (%) | |||||||||
| Vaginal | 175 | (68.09) | 44 | (67.69) | 98 | (70.50) | 31 | (62.00) | 0.5403 |
| Caesarean | 82 | (31.91) | 21 | (32.31) | 41 | (29.50) | 19 | (38.00) | . |
| Maternal Health | |||||||||
| Change in BMI, n (%) 2 | |||||||||
| <25th | 58 | (22.57) | 19 | (29.23) | 30 | (21.58) | 7 | (14.00) | 0.2620 |
| 25th to <50th | 61 | (23.74) | 19 | (29.23) | 32 | (23.02) | 10 | (20.00) | . |
| 50th to <75th | 66 | (25.68) | 12 | (18.46) | 37 | (26.62) | 17 | (34.00) | . |
| ≥75th | 72 | (28.02) | 15 | (23.08) | 40 | (28.78) | 16 | (32.00) | . |
| Weight gain range by recommendation, n (%) 2 | |||||||||
| Below | 96 | (37.35) | 29 | (44.62) | 51 | (36.69) | 15 | (30.00) | 0.5506 |
| Within | 96 | (37.35) | 20 | (30.77) | 53 | (38.13) | 22 | (44.00) | . |
| Above | 65 | (25.29) | 16 | (24.62) | 35 | (25.18) | 13 | (26.00) | . |
| Comorbid conditions during the pregnancy, n (%) | |||||||||
| Diabetes | 33 | (12.84) | 12 | (18.46) | 18 | (12.95) | 3 | (6.00) | 0.1435 |
| Hypertension/Pre-eclampsia | 7 | (2.72) | 3 | (4.62) | 2 | (1.44) | 2 | (4.00) | 0.3629 |
| Anemia | 12 | (4.67) | 5 | (7.69) | 5 | (3.60) | 2 | (4.00) | 0.4226 |
| Other gestational comorbidities | 110 | (38.91) | 29 | (44.62) | 52 | (37.41) | 19 | (38.00) | 0.6028 |
| Any one of the above | 118 | (45.91) | 35 | (53.85) | 62 | (44.60) | 21 | (42.00) | 0.3646 |
| Medication uses during the pregnancy, n (%) | |||||||||
| Antibacterial | 107 | (41.63) | 28 | (43.08) | 56 | (40.29) | 22 | (44.00) | 0.8723 |
| Gastric-acid-lowering 3 | 62 | (24.12) | 13 | (20.00) | 35 | (25.18) | 13 | (26.00) | 0.6752 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-R.; Yeh, Y.-T.; Tzeng, H.-T.; Dai, H.-Y.; Lee, W.-C.; Wu, K.L.H.; Chan, J.Y.H.; Tain, Y.-L.; Hsu, C.-N. Carbohydrate-Mediated Pregnancy Gut Microbiota and Neonatal Low Birth Weight. Nutrients 2024, 16, 1326. https://doi.org/10.3390/nu16091326
Yu H-R, Yeh Y-T, Tzeng H-T, Dai H-Y, Lee W-C, Wu KLH, Chan JYH, Tain Y-L, Hsu C-N. Carbohydrate-Mediated Pregnancy Gut Microbiota and Neonatal Low Birth Weight. Nutrients. 2024; 16(9):1326. https://doi.org/10.3390/nu16091326
Chicago/Turabian StyleYu, Hong-Ren, Yao-Tsung Yeh, Hong-Tai Tzeng, Hong-Ying Dai, Wei-Chia Lee, Kay L. H. Wu, Julie Y. H. Chan, You-Lin Tain, and Chien-Ning Hsu. 2024. "Carbohydrate-Mediated Pregnancy Gut Microbiota and Neonatal Low Birth Weight" Nutrients 16, no. 9: 1326. https://doi.org/10.3390/nu16091326
APA StyleYu, H.-R., Yeh, Y.-T., Tzeng, H.-T., Dai, H.-Y., Lee, W.-C., Wu, K. L. H., Chan, J. Y. H., Tain, Y.-L., & Hsu, C.-N. (2024). Carbohydrate-Mediated Pregnancy Gut Microbiota and Neonatal Low Birth Weight. Nutrients, 16(9), 1326. https://doi.org/10.3390/nu16091326










