Identification of Novel Peptides in Distillers’ Grains as Antioxidants, α-Glucosidase Inhibitors, and Insulin Sensitizers: In Silico and In Vitro Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Sample Preparation
2.3. Composition Analysis of Peptide by UHPLC-ESI-HRMS/MS
2.4. Determination of α-Glucosidase Inhibitory Effect
2.5. Assessment of Scavenging Capacity against DPPH and ABTS Radicals
2.6. In Silico Screening of Main Bioactive Peptides in Distillers’ Grains Protein Hydrolysates
2.7. Peptides Synthesis and Activity Verification
2.8. Determination of Insulin-Resistance HepG2 Cells on Bioactive Peptides
2.9. Evaluation of Scavenging Capacity against Intracellular ROS
2.10. Molecular Docking to Probe Mechanism of Action
2.11. Statistical Analysis
3. Results and Discussion
3.1. Identification of the Peptides in Distillers’ Grains Protein Hydrolysates
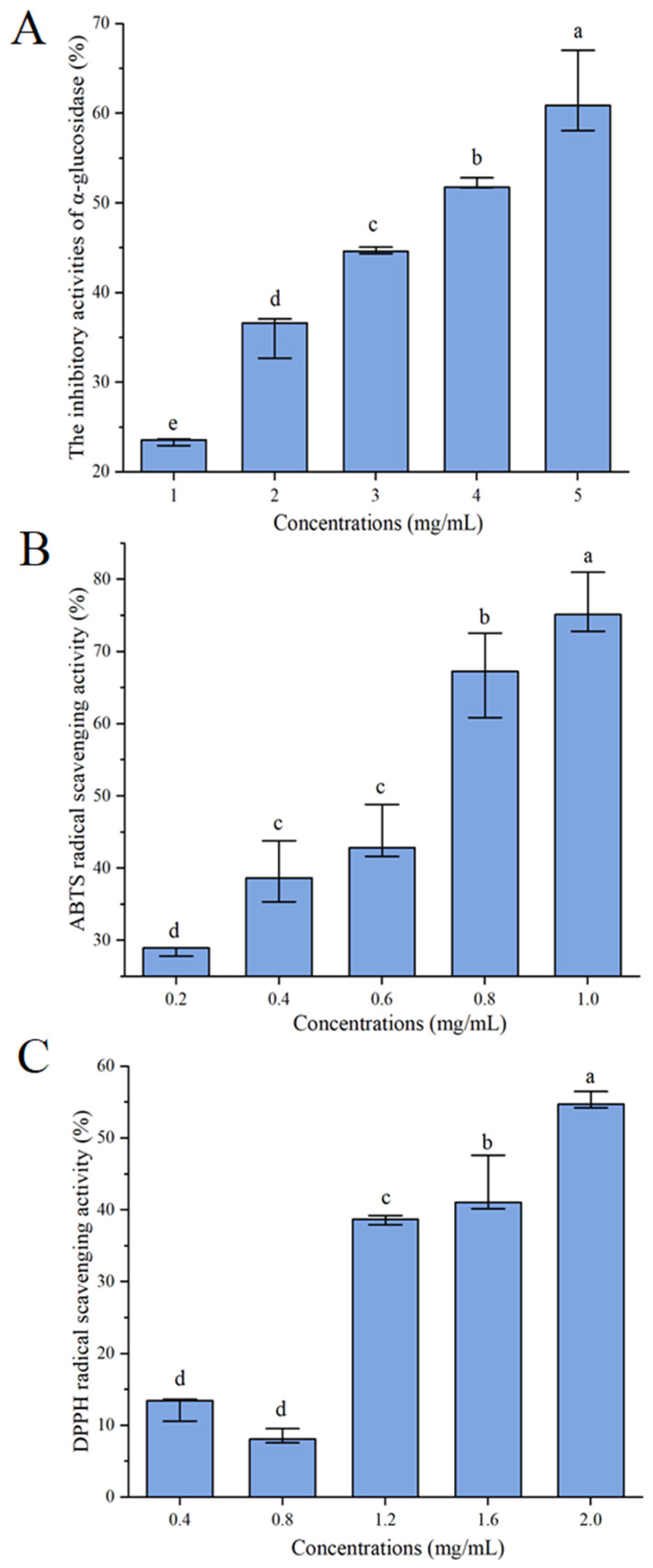
3.2. α-Glucosidase Inhibitory Effect of Distillers’ Grains Protein Hydrolysates
3.3. Scavenging Capacity against DPPH and ABTS Radicals of Distillers’ Grains Protein Hydrolysates
3.4. Screening of Main Bioactive Peptides in Distillers’ Grains Protein Hydrolysates
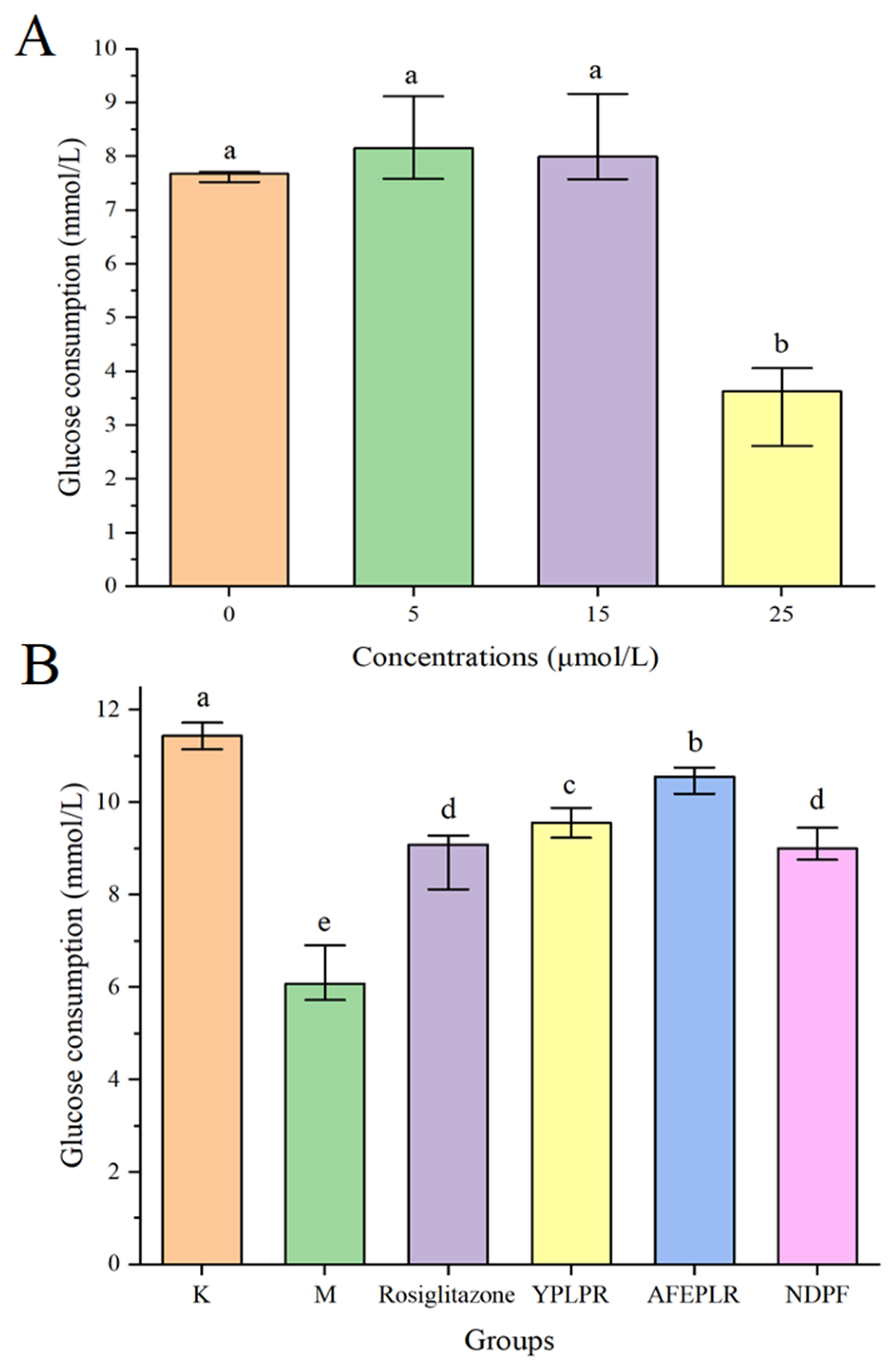
3.5. Activity Verification of Synthetic Novel Peptides
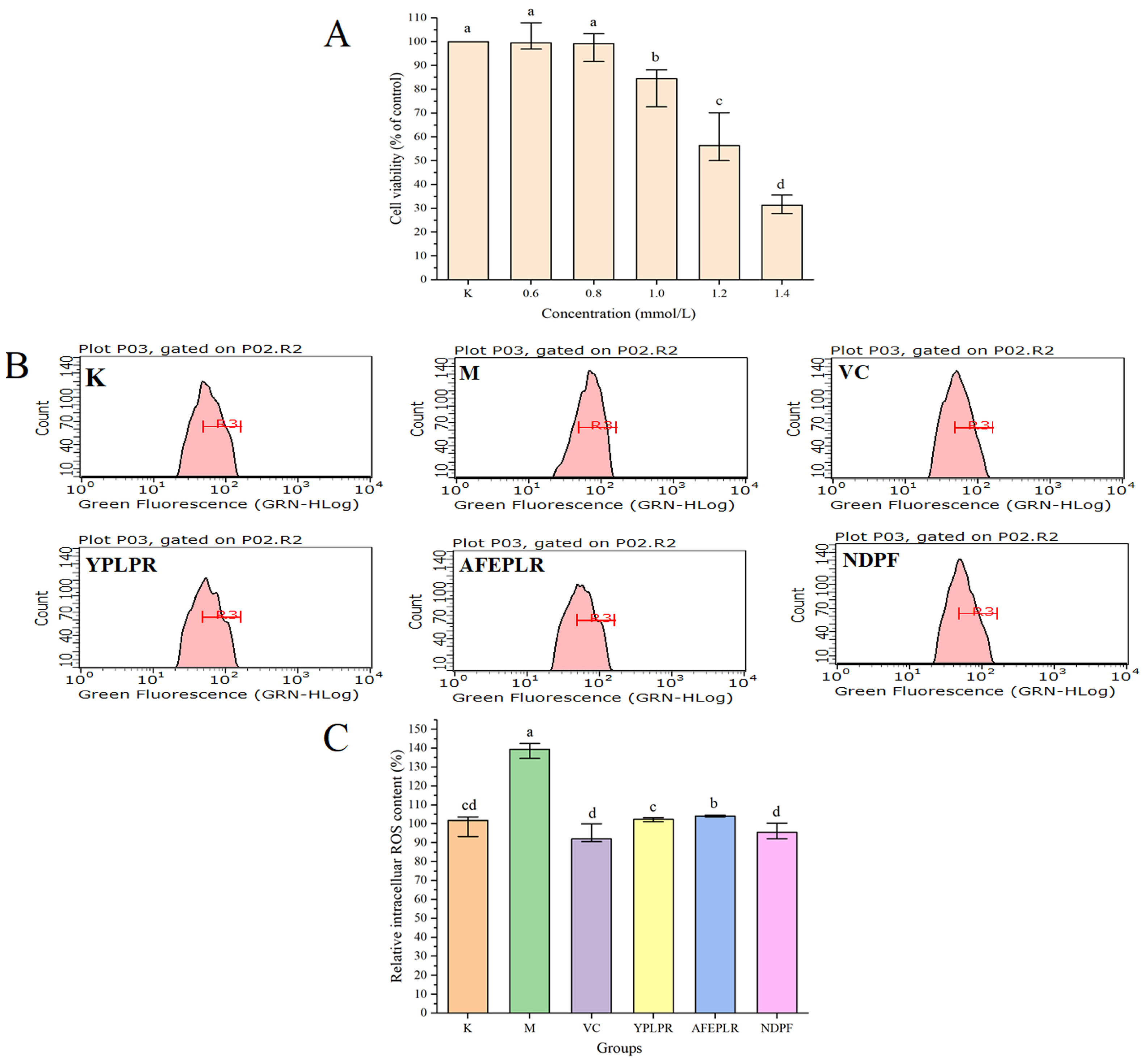
3.6. Improvement of Bioactive Peptides on Insulin Resistance in HepG2 Cells
3.7. Intracellular ROS Scavenging Activity by Bioactive Peptides
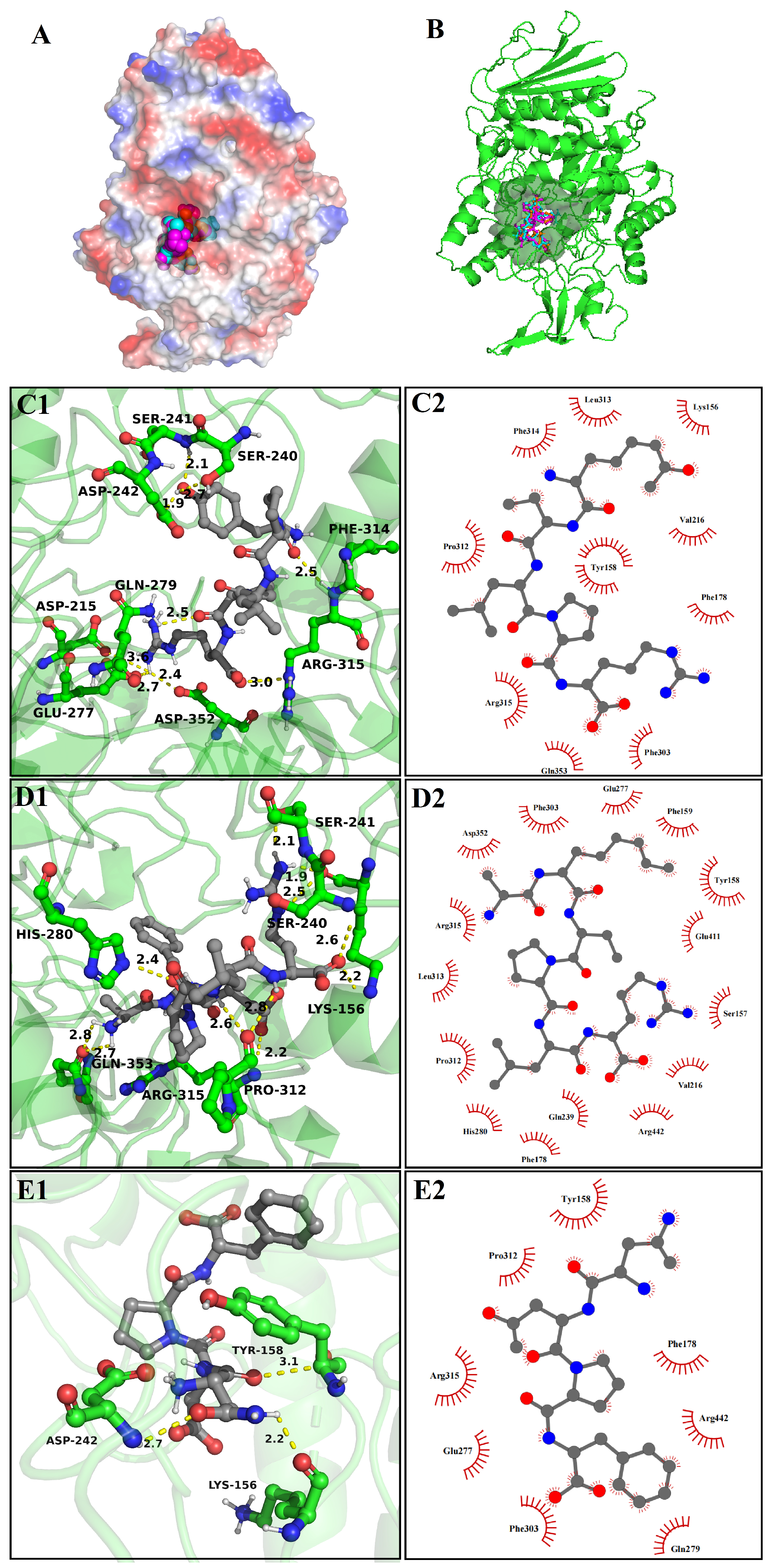
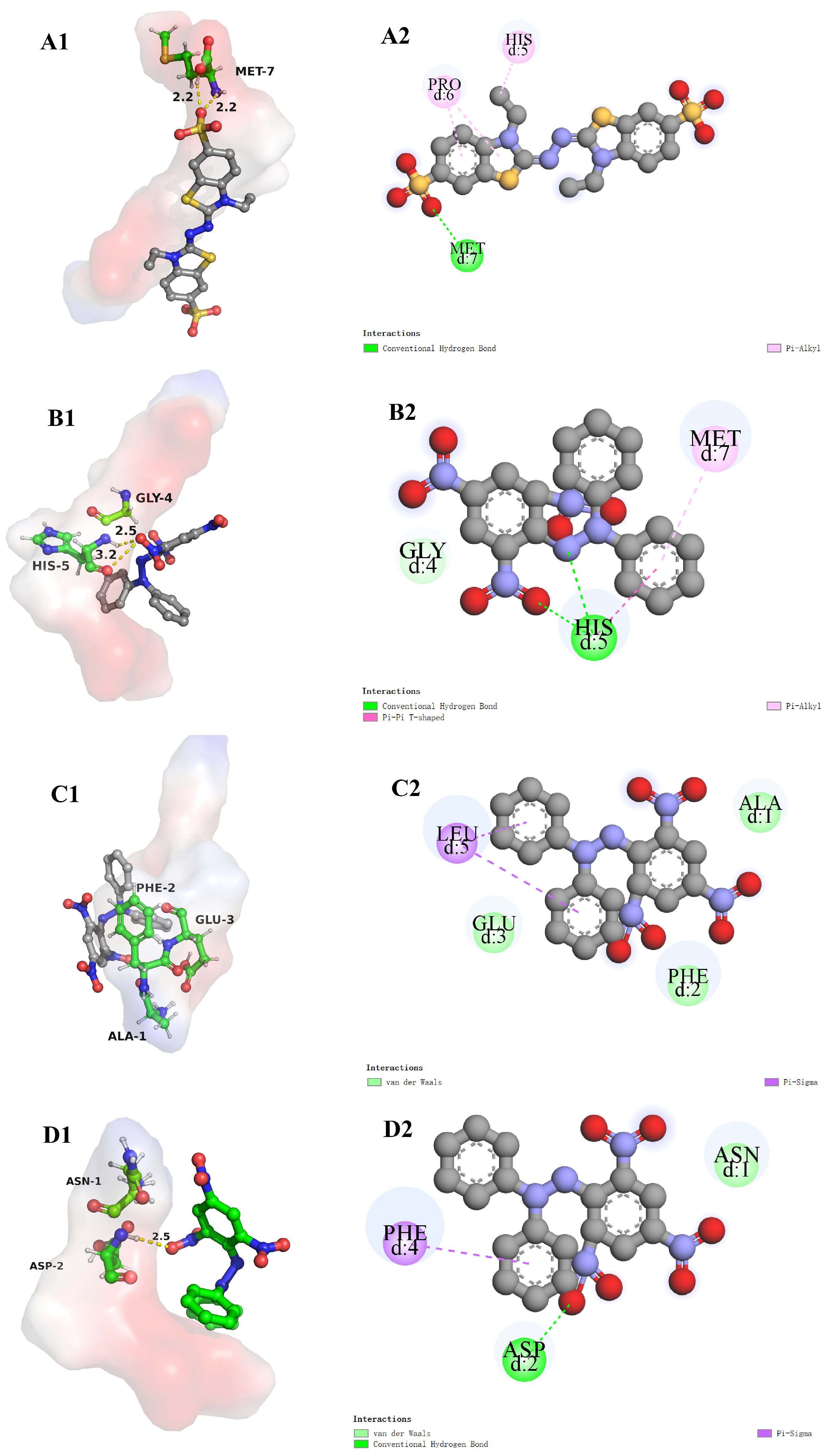
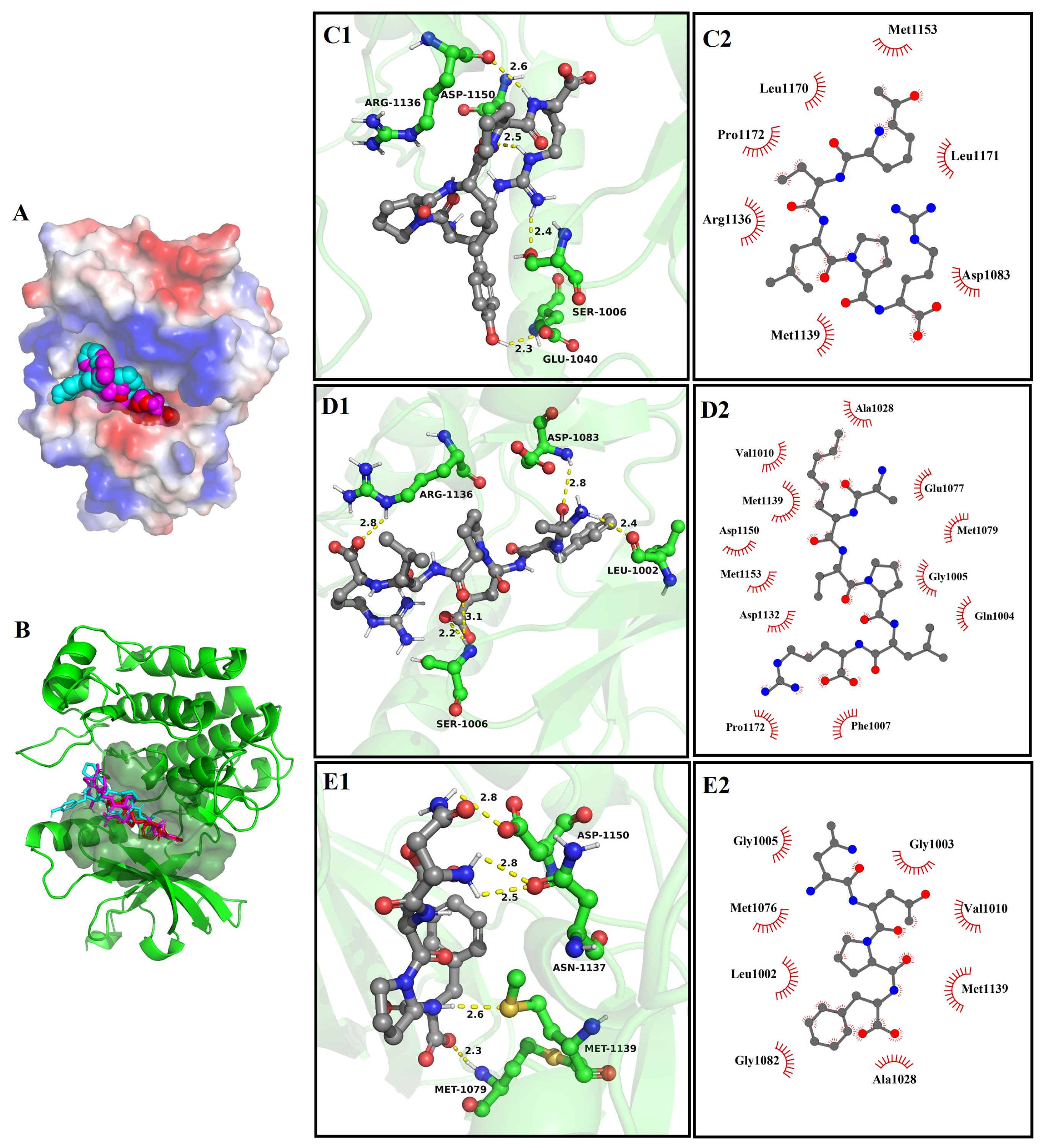
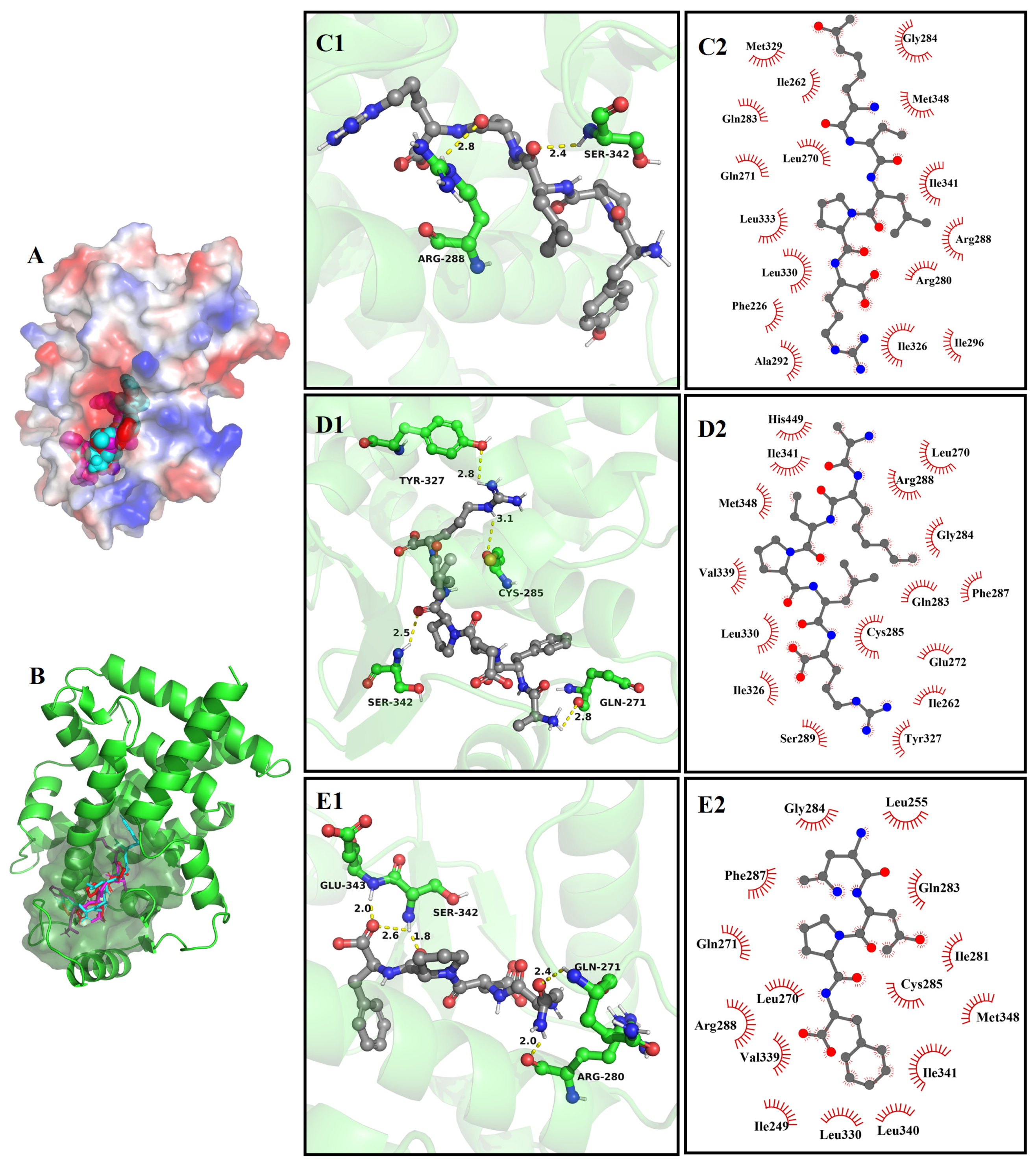
3.8. Molecular Docking
3.8.1. The Molecular Docking of Bioactive Peptides with α-Glucosidase
3.8.2. The Molecular Docking of Bioactive Peptides with ABTS and DPPH
3.8.3. The Molecular Docking of Bioactive Peptides with Insulin Signaling Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef]
- Zhao, C.-C.; Zhang, X.-H.; Chen, J.; Shao, J.-H.; Zhao, Z.-Y.; Tang, Y.-Y. Lignans with α-Glucosidase, Protein Tyrosine Phosphatase 1B, and Aldose Reductase Inhibitory Activities from the Fruits of Viburnum Cylindricum. Ind. Crops Prod. 2022, 178, 114601. [Google Scholar] [CrossRef]
- Kazmi, M.; Zaib, S.; Ibrar, A.; Amjad, S.T.; Shafique, Z.; Mehsud, S.; Saeed, A.; Iqbal, J.; Khan, I. A New Entry into the Portfolio of α-Glucosidase Inhibitors as Potent Therapeutics for Type 2 Diabetes: Design, Bioevaluation and One-Pot Multi-Component Synthesis of Diamine-Bridged Coumarinyl Oxadiazole Conjugates. Bioorg. Chem. 2018, 77, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Oseguera-Toledo, M.E.; Gonzalez de Mejia, E.; Amaya-Llano, S.L. Hard-to-Cook Bean (Phaseolus vulgaris L.) Proteins Hydrolyzed by Alcalase and Bromelain Produced Bioactive Peptide Fractions That Inhibit Targets of Type-2 Diabetes and Oxidative Stress. Food Res. Int. 2015, 76, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and Vascular Disease: Pathophysiology, Clinical Consequences, and Medical Therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations When Using Alpha-Glucosidase Inhibitors in the Treatment of Type 2 Diabetes. Expert. Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef]
- Dash, R.P.; Babu, R.J.; Srinivas, N.R. Reappraisal and Perspectives of Clinical Drug–Drug Interaction Potential of α-Glucosidase Inhibitors Such as Acarbose, Voglibose and Miglitol in the Treatment of Type 2 Diabetes Mellitus. Xenobiotica 2018, 48, 89–108. [Google Scholar] [CrossRef]
- Khalid, Z.; Shafqat, S.S.; Ahmad, H.A.; Rehman, H.M.; Munawar, M.A.; Ahmad, M.; Asiri, A.M.; Ashraf, M. Synthesis of 1,2,3-Benzotriazin-4(3H)-One Derivatives as α-Glucosidase Inhibitor and Their in-Silico Study. Med. Chem. Res. 2022, 31, 819–831. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A Novel Antioxidant and ACE Inhibitory Peptide from Rice Bran Protein: Biochemical Characterization and Molecular Docking Study. LWT 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Zheng, X.; Chi, H.; Ma, S.; Zhao, L.; Cai, S. Identification of Novel α-Glucosidase Inhibitory Peptides in Rice Wine and Their Antioxidant Activities Using in Silico and in Vitro Analyses. LWT 2023, 178, 114629. [Google Scholar] [CrossRef]
- Wei, D.; Fan, W.; Xu, Y. Identification of Water-Soluble Peptides in Distilled Spent Grain and Its Angiotensin Converting Enzyme (ACE) Inhibitory Activity Based on UPLC-Q-TOF-MS and Proteomics Analysis. Food Chem. 2021, 353, 129521. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Fujita, H.; Matoba, N.; Takenaka, Y.; Yamamoto, T.; Yamauchi, R.; Tsuruki, H.; Takahata, K. Bioactive Peptides Derived from Food Proteins Preventing Lifestyle-related Diseases. BioFactors 2000, 12, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; González-Rogel, D.; Heres, A.; Toldrá, F. Iberian Dry-Cured Ham as a Potential Source of α-Glucosidase-Inhibitory Peptides. J. Funct. Foods 2020, 67, 103840. [Google Scholar] [CrossRef]
- Hu, S.; Fan, X.; Qi, P.; Zhang, X. Identification of Anti-Diabetes Peptides from Spirulina Platensis. J. Funct. Foods 2019, 56, 333–341. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of Bioactive Peptides with Antidiabetic, Antihypertensive, and Antioxidant Activities and Identification of A-glucosidase Inhibitory Peptides from Soy Protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, J.; Li, Z.; Luan, Y.; Li, M.; Peng, X.; Xiao, S.; Zhang, S. Damage Prevention Effect of Milk-Derived Peptides on UVB Irradiated Human Foreskin Fibroblasts and Regulation of Photoaging Related Indicators. Food Res. Int. 2022, 161, 111798. [Google Scholar] [CrossRef] [PubMed]
- Wenhui, T.; Shumin, H.; Yongliang, Z.; Liping, S.; Hua, Y. Identification of in Vitro Angiotensin-converting Enzyme and Dipeptidyl Peptidase IV Inhibitory Peptides from Draft Beer by Virtual Screening and Molecular Docking. J. Sci. Food Agric. 2022, 102, 1085–1094. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Y.; Ma, Y.; Cheng, G.; Cai, S. Phenolic Composition, Antioxidant Properties, and Inhibition toward Digestive Enzymes with Molecular Docking Analysis of Different Fractions from Prinsepia Utilis Royle Fruits. Molecules 2018, 23, 3373. [Google Scholar] [CrossRef]
- Sun, D.; Huang, S.; Cai, S.; Cao, J.; Han, P. Digestion Property and Synergistic Effect on Biological Activity of Purple Rice (Oryza sativa L.) Anthocyanins Subjected to a Simulated Gastrointestinal Digestion in Vitro. Food Res. Int. 2015, 78, 114–123. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Mora, L.; Hayes, M. In Vitro and In Silico Approaches to Generating and Identifying Angiotensin-Converting Enzyme I Inhibitory Peptides from Green Macroalga Ulva Lactuca. Mar. Drugs 2019, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Ferysiuk, K.; Wójciak, K.M.; Kęska, P. Effect of Willow Herb (Epilobium angustifolium L.) Extract Addition to Canned Meat with Reduced Amount. of Nitrite on the Antioxidant and Other Activities of Peptides. Food Funct. 2022, 13, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Bu, T.; Zheng, J.; Liu, L.; Yu, S.; Li, S.; Wu, J. Peptides in Brewed Wines: Formation, Structure, and Function. J. Agric. Food Chem. 2021, 69, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, Y.; Du, Q.; Liu, Z.; Liu, X. Cichoric Acid Reverses Insulin Resistance and Suppresses Inflammatory Responses in the Glucosamine-Induced HepG2 Cells. J. Agric. Food Chem. 2015, 63, 10903–10913. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, O.; Zhou, J.; Cai, S. Different Phenolic Extracts of Oil Palm Fruits and Caffeic Acid Prevent Palmitic Acid-Induced Lipotoxicity in HepG2 Cells via Improving Mitochondrial Function. J. Food Qual. 2020, 2020, 8827707. [Google Scholar] [CrossRef]
- Chettri, S.; Tamang, S.; Rai, P.; Rai, Y.; Singha, U.K.; Pradhan, K.; Brahman, D.; Sinha, B. Synthesis, Physico-chemical Characterization and Theoretical Exploration of Some 2,4,5-triaryl Imidazole Derivatives. J. Heterocycl. Chem. 2023, 60, 1394–1415. [Google Scholar] [CrossRef]
- da Costa Leite, L.F.C.; Veras Mourão, R.H.; de Lima, M.d.C.A.; Galdino, S.L.; Hernandes, M.Z.; de Assis Rocha Neves, F.; Vidal, S.; Barbe, J.; da Rocha Pitta, I. Synthesis, Biological Evaluation and Molecular Modeling Studies of Arylidene-Thiazolidinediones with Potential Hypoglycemic and Hypolipidemic Activities. Eur. J. Med. Chem. 2007, 42, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; de Mejía, E.G. Optimization of Enzymatic Production of Anti-Diabetic Peptides from Black Bean (Phaseolus vulgaris L.) Proteins, Their Characterization and Biological Potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
- Ying, F.; Lin, S.; Li, J.; Zhang, X.; Chen, G. Identification of Monoamine Oxidases Inhibitory Peptides from Soybean Protein Hydrolysate through Ultrafiltration Purification and in Silico Studies. Food Biosci. 2021, 44, 101355. [Google Scholar] [CrossRef]
- Dai, L.; Kong, L.; Cai, X.; Jiang, P.; Liu, N.; Zhang, D.; Li, Z. Analysis of the Structure and Activity of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Oligopeptides from Sorghum Kafirin. J. Agric. Food Chem. 2022, 70, 2010–2017. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Wen, Y.; Liu, Y.; Wang, J.; Sun, B. Molecular Mechanism for the α-Glucosidase Inhibitory Effect of Wheat Germ Peptides. J. Agric. Food Chem. 2021, 69, 15231–15239. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Sommella, E.; Ventre, G.; Scala, M.C.; Adesso, S.; Ostacolo, C.; Marzocco, S.; Novellino, E.; Campiglia, P. Antioxidant Peptides Released from Gastrointestinal Digestion of “Stracchino” Soft Cheese: Characterization, in Vitro Intestinal Protection and Bioavailability. J. Funct. Foods 2016, 26, 494–505. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, L.; Liu, C.; Liu, X.; Li, H.; Shi, J.; Li, M.; Min, W. Peptides from Walnut (Juglans Mandshurica Maxim.) Protect Hepatic HepG2 Cells from High Glucose-Induced Insulin Resistance and Oxidative Stress. Food Funct. 2020, 11, 8112–8121. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Gao, J.; Wang, Y.; Luo, Q.W.; Guo, K.R.; Ren, F.Z.; Mao, X.Y. Identification of Novel Peptides from Goat Milk Casein That Ameliorate High-Glucose-Induced Insulin Resistance in HepG2 Cells. J. Dairy. Sci. 2020, 103, 4907–4918. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Du, B.; Zhang, C.; Zaman, F.; Huang, Y. Isolation and Identification of an Antioxidant Collagen Peptide from Skipjack Tuna (Katsuwonus pelamis) Bone. RSC Adv. 2019, 9, 27032–27041. [Google Scholar] [CrossRef]
- Mi, S.; Liu, J.; Liu, X.; Fu, Y.; Yi, J.; Cai, S. Inhibitory Effects of Myricetrin and Dihydromyricetin toward α-Glucosidase and Pancreatic Lipase with Molecular Docking Analyses and Their Interaction. J. Food Qual. 2021, 2021, 9943537. [Google Scholar] [CrossRef]
| Peptides | ALC (%) | RT (min) | Mass (Da) | Local Confidence (%) |
|---|---|---|---|---|
| ELELLE | 96 | 2.31 | 744.3905 | 99 97 98 94 95 98 |
| RFDR | 96 | 2.2 | 592.3081 | 95 96 97 98 |
| PDVGHPM | 94 | 14.64 | 751.3323 | 89 97 97 91 96 94 96 |
| YPLPR | 92 | 15.28 | 644.3646 | 94 97 96 89 83 |
| VLEPR | 91 | 7.08 | 612.3595 | 88 98 97 85 90 |
| AFEPLR | 91 | 15.98 | 731.3966 | 90 97 98 85 92 87 |
| FEEL | 91 | 17.76 | 536.2482 | 86 93 96 89 |
| WNVN | 88 | 14.76 | 531.2441 | 85 79 94 94 |
| EEEF | 88 | 13.81 | 552.2067 | 72 94 98 89 |
| FEPLR | 88 | 14.89 | 660.3595 | 86 98 81 91 82 |
| WLDY | 87 | 18.69 | 595.2642 | 87 89 88 87 |
| ERR | 87 | 1.04 | 459.2554 | 96 85 81 |
| FEPL | 87 | 18.25 | 504.2584 | 86 94 79 90 |
| LDFEPR | 87 | 17.02 | 775.3864 | 88 91 90 96 76 79 |
| FDGVLRGP | 86 | 17 | 859.4551 | 89 96 90 95 89 74 79 79 |
| YAGE | 86 | 2.81 | 438.175 | 86 85 77 95 |
| NDPF | 85 | 15.77 | 491.2016 | 84 87 84 88 |
| RVLEPR | 85 | 4.98 | 768.4606 | 82 88 90 95 79 77 |
| WTVN | 85 | 15.35 | 518.2489 | 80 82 91 88 |
| WNLN | 85 | 17.09 | 545.2598 | 85 77 93 85 |
| Peptides | Tag Length | Peptide Ranker Score | Affinity (kcal/mol) | Estimated Toxicity | Estimated Solubility |
|---|---|---|---|---|---|
| RFDR | 4 | 0.6986 | −8.7 | Non-toxin | Good |
| PDVGHPM | 7 | 0.6039 | −8.6 | Non-toxin | Good |
| YPLPR | 5 | 0.7972 | −10.7 | Non-toxin | Good |
| AFEPLR | 6 | 0.7571 | −9.6 | Non-toxin | Good |
| FEPLR | 5 | 0.7106 | −9.0 | Non-toxin | Good |
| WLDY | 4 | 0.7981 | −8.6 | Non-toxin | Poor |
| FEPL | 4 | 0.7531 | −8.8 | Non-toxin | Good |
| FDGVLRGP | 8 | 0.6470 | −8.7 | Non-toxin | Good |
| NDPF | 4 | 0.8886 | −9.1 | Non-toxin | Good |
| WNLN | 4 | 0.6784 | −9.2 | Non-toxin | Poor |
| WLRL (Positive control) | 4 | 0.9210 | −9.1 | Non-toxin | Poor |
| YPLPR | AFEPLR | NDPF | |
|---|---|---|---|
| Affinity (kcal/mol) | −10.7 | −9.6 | −9.1 |
| Number of hydrogen interactions | 9 | 11 | 3 |
| Amino acid residues involved in hydrogen bonds | Asp 215, Ser 240, Ser 241, Asp 242, Glu 277, Gln 279, Phe 314, Arg 315, Asp 352 | Lys 156, Ser 240, Ser 241, His 280, Pro 312, Arg 315, Gln 353 | Lys 156, Tyr 158, Asp 242 |
| Number of hydrophobic interactions | 10 | 15 | 8 |
| Amino acid residues participated in van der waals | Lys 156, Tyr 158, Phe 178, Val 216, Phe 303, Pro 312, Leu 313, Phe 314, Arg 315, Gln 353 | Ser 157, Tyr 158, Phe 159, Phe 178, Val 216, Gln 239, Glu 277, His 280, Phe 303, Pro 312, Leu 313, Arg 315, Asp 352, Glu 411, Arg 442 | Tyr 158, Phe 178, Glu277, Gln 279, Phe 303, Pro 312, Arg 315, Arg 442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Zheng, X.; Zhao, L.; Cai, S. Identification of Novel Peptides in Distillers’ Grains as Antioxidants, α-Glucosidase Inhibitors, and Insulin Sensitizers: In Silico and In Vitro Evaluation. Nutrients 2024, 16, 1279. https://doi.org/10.3390/nu16091279
Ding L, Zheng X, Zhao L, Cai S. Identification of Novel Peptides in Distillers’ Grains as Antioxidants, α-Glucosidase Inhibitors, and Insulin Sensitizers: In Silico and In Vitro Evaluation. Nutrients. 2024; 16(9):1279. https://doi.org/10.3390/nu16091279
Chicago/Turabian StyleDing, Lixin, Xiuqing Zheng, Lei Zhao, and Shengbao Cai. 2024. "Identification of Novel Peptides in Distillers’ Grains as Antioxidants, α-Glucosidase Inhibitors, and Insulin Sensitizers: In Silico and In Vitro Evaluation" Nutrients 16, no. 9: 1279. https://doi.org/10.3390/nu16091279
APA StyleDing, L., Zheng, X., Zhao, L., & Cai, S. (2024). Identification of Novel Peptides in Distillers’ Grains as Antioxidants, α-Glucosidase Inhibitors, and Insulin Sensitizers: In Silico and In Vitro Evaluation. Nutrients, 16(9), 1279. https://doi.org/10.3390/nu16091279









