Abstract
Vitamin D3 (VD3) is a steroid hormone that plays pivotal roles in pathophysiology, and 1,25(OH)2D3 is the most active form of VD3. In the current study, the crucial role of VD3 in maintaining energy homeostasis under short-term fasting conditions was investigated. Our results confirmed that glucose-depriving pathways were inhibited while glucose-producing pathways were strengthened in zebrafish after fasting for 24 or 48 h. Moreover, VD3 anabolism in zebrafish was significantly suppressed in a time-dependent manner under short-fasting conditions. After fasting for 24 or 48 h, zebrafish fed with VD3 displayed a higher gluconeogenesis level and lower glycolysis level in the liver, and the serum glucose was maintained at higher levels, compared to those fed without VD3. Additionally, VD3 augmented the expression of fatty acids (FAs) transporter cd36 and lipogenesis in the liver, while enhancing lipolysis in the dorsal muscle. Similar results were obtained in cyp2r1−/− zebrafish, in which VD3 metabolism is obstructed. Importantly, it was observed that VD3 induced the production of gut GLP-1, which is considered to possess a potent gluconeogenic function in zebrafish. Meanwhile, the gene expression of proprotein convertase subtilisin/kexin type 1 (pcsk1), a GLP-1 processing enzyme, was also induced in the intestine of short-term fasted zebrafish. Notably, gut microbiota and its metabolite acetate were involved in VD3-regulated pcsk1 expression and GLP-1 production under short-term fasting conditions. In summary, our study demonstrated that VD3 regulated GLP-1 production in zebrafish by influencing gut microbiota and its metabolite, contributing to energy homeostasis and ameliorating hypoglycemia under short-term fasting conditions.
1. Introduction
All animals obtain energy from food for growth, metabolism, reproduction and physical activities. When food is unavailable due to reasons such as population competition, seasonal alternation or reproductive behavior, animals have to use their internal energy reserves to survive [1]. Fasting is a metabolic state in which the body undergoes a period without food intake. Generally, fasting is divided into three phases according to the main energy substrates, and the energy metabolism strategies through the period of each phase vary among species. In mammals, serum glucose and glycogen storage are first utilized during phase 1. As fasting time increases, lipid reserves in adipose tissues commence lipolysis and release free fatty acids (FFAs), which are absorbed by the liver and used for energy supply during phase 2. The absorption process is associated with a variety of proteins named FA translocase and transport proteins, such as CD36 and solute carrier family 27A [2]. When lipid reserves are exhausted after prolonged fasting, phase 3 occurs and the protein reserves will be utilized for energy production [3]. In fish, the utilization of main nutrients under a fasting condition resembles that of mammals [4,5], although some fish species are able to endure longer fasting periods [6,7].
During short-term fasting, several hormones, including insulin, glucagon and GLP-1, play an important role in maintaining the homeostasis of glucose and lipid metabolism [8]. It is known that the serum concentration of insulin decreases while that of glucagon increases when mammals undergo fasting, leading to a high G/I (glucagon/insulin) molar ratio. In contrast to mammals, the G/I molar ratio in fasted fish remains at a low level even after long-term fasting [7], although the general functions of insulin and glucagon in fish resemble other vertebrates [9]. Additionally, GLP-1 is co-encoded with glucagon in the pro-glucagon gene [10]. Furthermore, pro-glucagon is processed by PC1/3 (encoded by pcsk1) to produce GLP-1 in intestinal L-cells, and by PC2 (encoded by pcsk2) to produce glucagon in pancreatic α-cells [10,11]. In mammals, GLP-1 increases insulin and decreases glucagon secretion in a glucose-dependent manner, thus improving glucose homeostasis and ameliorating metabolic diseases, such as diabetes and obesity [12,13,14,15]. Notably, it was proven that GLP-1 in fish exerted different functions in regulating glucose homeostasis from that in mammals [16,17].
VD3 is a steroid hormone that plays important roles in mineral homeostasis, metabolism, immunity, inflammation, and gut microbiota [18]. To be converted to its active form 1,25(OH)2D3, VD3 must undergo two-step hydroxylation by the enzymes 25-hydroxylase and 1-alpha-hydroxylase that are encoded by cyp2r1 and cyp27b1, respectively [19]. 1,25(OH)2D3 acts primarily with the help of the nuclear vitamin D receptor (VDR) and can be deactivated by 24-hydroxylase (encoded by cyp24a1) [20,21]. Interestingly, it was reported that VD3 metabolism was influenced in fasted animals. For example, the expression level of cyp2r1 and the enzyme activity of 25-hydroxylase in mice was significantly reduced, while the expression of cyp24a1 was up-regulated after fasting for 24 h, suggesting short-term fasting suppressed 1,25(OH)2D3 biosynthesis and accelerated 1,25(OH)2D3 deactivation [22]. Similar results were reported in humans after 8 days of fasting [23].
The previous results from our research group demonstrated that VD3 lowered postprandial blood glucose levels in zebrafish under a hyperglycemia condition [24,25]. Moreover, Peng et al. showed that VD3 promoted FA oxidation in zebrafish adipose tissue [26]. Interestingly, it was proposed that cyp2r1−/− zebrafish, in which VD3 metabolism is obstructed, failed to utilize lipid reserves to provide energy after long-term fasting [26]. Nonetheless, little has been known about the function of VD3 under short-term fasting conditions. Therefore, in the present study, we attempted to investigate the effect of the function of VD3 on the regulation of glucose and lipid metabolism under short-term fasting conditions by using zebrafish as an animal model.
2. Materials and Methods
2.1. Zebrafish Maintenance
The zebrafish were maintained at 28.5 °C with a 14 h light/10 h dark rhythm in a circulating aquarium system located in Fisheries College, Ocean University of China. The fish were fed twice daily with newly hatched brine shrimp (Artemia franciscana). Embryos were obtained by natural spawning and kept in egg water at 28.5 °C after 24 h incubation with 1% methylene blue. The generation of cyp2r1−/− zebrafish has been described in a previous report [26]. According to standard methods, the developmental stages were defined by day post-fertilization (dpf) or month post-fertilization (mpf).
The husbandry and handling of the fish were performed based on the Management Rule of Laboratory Animals (Chinese order no. 676 of the State Council, revised 1 March 2017). All animal procedures in the present study were approved by the Experimental Animal Ethics Committee at Ocean University of China.
2.2. Experimental Diets and Feeding Trial
Two experimental diets with supplementation of 800 or 0 IU VD3/kg were designed. The composition of the two diets is listed in Table 1. Before the formal feeding trial, wild-type zebrafish at 2 mpf were kept in eight tanks (5 L, 16 fish/tank), and fed the diet containing 0 IU/kg VD3 diet three times daily for one week. Thereafter, the fish were separated into two groups fed the VD3-containing or non-VD3 diet with four replicates for each group. In addition, the female-to-male ratio was adjusted to approximately 1:2 in each tank. All fish were fed 3 times at 8:30, 14:30, and 20:30 for three weeks. The waste and remaining feed were cleaned up and half of the water was renewed daily.

Table 1.
Dietary formulation of experimental diets (g/kg).
2.3. Antibiotic Treatment
The method of antibiotic treatment has been described previously [27]. In brief, wild-type zebrafish at 2 mpf were randomly allocated into 2 groups, and fed with the VD3 or non-VD3 diet for one month. In the meantime, four antibiotics (100 μg/mL ampicillin, 10 μg/mL kanamycin, 0.5 μg/mL amphotericin B, and 50 μg/mL gentamycin) were added to the water. The water containing antibiotics was renewed daily.
2.4. Sample Collection
Corresponding to the fasting period, the VD3 and non-VD3 groups were fasted for 0 h to 80 h after the last feeding before sampling. The fish were euthanized with 0.1% MS-222 (E10505, Sigma, Livonia, MI, USA). The blood sample was drawn from the caudal vessels of zebrafish according to a protocol previously described [28]. The blood samples from 3~4 fish were combined and centrifuged at 1000× g for 20 min. The supernatants were collected and immediately saved at −80 °C. In addition, the liver and gut from each fish were rapidly isolated and stored at −80 °C for further analysis.
2.5. RNA Extraction and Quantitative Real-Time PCR
The RNA of the liver and gut was extracted using the RNAeasyTM Animal RNA isolation kit (Beyotime, Shanghai, China) following the manufacturers’ instructions. The quantity and quality of total RNA were determined by using NanoDrop® One spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA (1 μg) was used for reverse transcription with the HiScript III 1st Strand cDNA Synthesis Kit (R323-01, Vazyme, Nanjing, China). Quantitative RT–PCR was performed using the ChamQ Universal SYBR qPCR Master Mix kit on a quantitative thermal cycler CFX96TM Real-Time System (Bio-Rad, Hercules, CA, USA). The mRNA levels were calculated by the 2−ΔΔCt method as the fold expression relative to the housekeeping gene, actb2. All primer sequences are listed in Table 2.

Table 2.
Primer sequences for qRT-PCR.
2.6. Measurement of Glucose, GLP-1 and 1,25(OH)2D3 Contents
The contents of glucose, GLP-1 and 1,25(OH)2D3 in zebrafish serum, gut or dorsal muscle were assessed by using the ELISA assay kits from Applygen Technologies (Cat. No. E1011, Beijing, China) and Hengyuan Biological Technology (Cat. No. HB299-QT and HB274-QT, Shanghai, China), according to the manufacturer’s instructions.
2.7. GF Zebrafish Generation and SCFA Treatment
GF zebrafish were obtained following an established method with some adjustments [29]. In brief, natural spawning zebrafish embryos were collected and incubated in GZM with antibiotics (100 μg/mL ampicillin, 10 μg/mL kanamycin, 0.5 μg/mL amphotericin B, 50 μg/mL gentamycin) at 28.5 °C for 6–8 h. Then, the embryos were treated with 0.1% PVP-I for 90 s, 0.003% bleach solution for 20 min, and rinsed twice with GZM. Tryptic soy agar plate, nutrient broth, brain–heart infusion broth, and Sab-Dex broth were used for sterility verification.
At 3 dpf, gnotobiotic zebrafish larvae were incubated with 30 mM sodium acetate, sodium propionate, or sodium butyrate for 2 days under sterile environments. Afterward, zebrafish were collected for further analysis.
2.8. Calculations and Statistical Analysis
The results are presented as means ± SEM. Raw data were analyzed by one-way or two-way ANOVA followed by Tukey’s multiple comparisons test after normality and homogeneity of variance were verified. Statistical analysis was performed using GraphPad Prism 9.5 (GraphPad Software, Inc., La Jolla, CA, USA), and p < 0.05 was considered as statistical significance.
3. Results
3.1. Short-Term Fasting Influences Glucose and Lipid Metabolism in Zebrafish
After the last feeding, zebrafish were fasted for a desired time from 0 h to 80 h before being euthanized, and the serum glucose level and total lipid content were measured. As the results showed, the postprandial serum glucose levels increased, peaked around one hour, and gradually decreased to the initial level after six hours (Figure 1A). In contrast, the lipid content showed no significant changes during the first six hours (Figure 1B). Furthermore, the postprandial serum glucose levels declined mildly between 6 h and 36 h, when the lipid content started to decrease (Figure 1A,B). Interestingly, the postprandial serum glucose rose again between 36 h and 72 h, while it declined again after 72 h when the lipid content stopped decreasing (Figure 1A,B). Compared to the gene expression in the control group (one hour after feeding), the expression levels of glycolysis-related genes (gck, pklr) in zebrafish liver significantly decreased at 24 h and 48 h after feeding (Figure 1C), while gluconeogenesis-related genes (pck1, fbp1a) increased at 48 h after feeding (Figure 1D). In addition, the expression levels of lipolysis-related genes (ppara, pgc1a) were enhanced at 48 h (Figure 1E), and those of lipogenesis-related genes (pparg, fasn) were suppressed at 24 h and 48 h (Figure 1F).
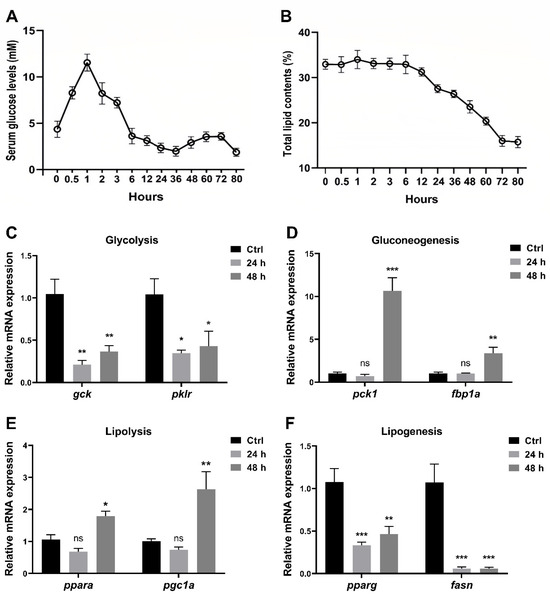
Figure 1.
Short-term fasting influences glucose and lipid metabolism in zebrafish. WT zebrafish at 3 mpf were fasted for 0 h to 80 h after the last feeding. (A,B) The serum glucose levels (A) and total lipid contents in the whole fish (B) were evaluated (n = 3 replicates, 3 fish/replicate). (C–F) Relative expression levels of glycolysis-related genes (gck and pklr) (C), gluconeogenesis-related genes (pck1 and fbp1a) (D), lipolysis-related genes (ppara and pgc1a) (E), and lipogenesis-related genes (pparg and fasn) (F) in the liver were determined (n ≥ 4/group). * p < 0.05, ** p < 0.01, *** p < 0.001, ns: no statistical significance.
3.2. 1,25(OH)2D3 Generation in Zebrafish Was Impaired under Short-Term Fasting Condition
To understand the effects of short-term fasting on VD3 metabolism in zebrafish, several key genes related to VD3 metabolism in the liver as well as 1,25(OH)2D3 concentrations in the serum were analyzed. The data showed that compared to the control group (one hour after feeding), the gene expression of cyp2r1 was suppressed after 24 h and 48 h (Figure 2A), whereas cyp27b1 showed no significant changes (Figure 2B). Meanwhile, the gene expression of cyp24a1 was significantly elevated at 24 h and 48 h after feeding (Figure 2C). Moreover, 1,25(OH)2D3 concentrations in serum displayed a decreasing trend during the 48 h fasting period (Figure 2D). Interestingly, short-term fasting inhibited the expression of vdra, whereas it enhanced the expression of vdrb in the liver (Figure 2E,F). These results suggested that short-term fasting has a remarkable impact on the VD3 metabolism and signaling in zebrafish.
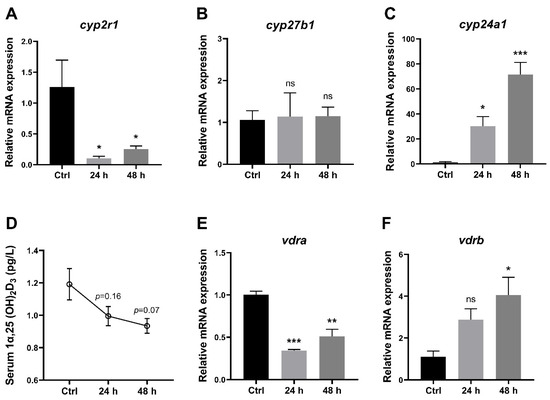
Figure 2.
1,25(OH)2D3 generation in zebrafish was impaired under short-term fasting condition. (A–C). The gene expression of cyp2r1 (A), cyp27b1 (B) and cyp24a1 (C) in zebrafish liver was assessed after fasting for 24 h or 48 h, compared to the control group (1 h postprandial) (n = 4/group). (D) The serum 1,25(OH)2D3 concentrations were determined (n = 3 replicates, 4~5 fish/replicate). (E,F) Gene expression levels of vdra (E) and vdrb (F) in the liver (n = 4/group). * p < 0.05, ** p < 0.01, *** p < 0.001, ns: no statistical significance.
3.3. VD3 Regulates Glucose Metabolism in Zebrafish under Short-Term Fasting Condition
To investigate the link between 1,25(OH)2D3 and metabolic changes under fasting conditions, WT zebrafish were fed with a VD3 diet or non-VD3 diet for one month and fasted for 24 h before sacrifice. The gene expression levels of insulin receptors (insra, insrb) in the liver were significantly enhanced in the non-VD3 group. Notably, no significant changes were detected in glucagon receptors (gcgra, gcgrb) (Figure 3A). Lack of dietary VD3 led to an increase in the gene expression of glycolysis-related genes (gck, pklr), and a decrease in gluconeogenesis-related genes (pck1, fbp1a) (Figure 3B). Moreover, the serum glucose level of the non-VD3 group zebrafish was significantly lower than that of the VD3 group under short-term fasting conditions (Figure 3C). Additionally, WT and cyp2r1−/− zebrafish at 3 mpf were fasted for 24 h. As anticipated, the gene expression of insra, insrb, gcgra, and gcgrb (Figure 3D) as well as the glycolysis-related gene (gck) and gluconeogenesis-related gene (pck1) (Figure 3E) in cyp2r1−/− zebrafish liver followed a similar pattern as those in the non-VD3 group. Moreover, the serum glucose level was significantly lower in cyp2r1−/− zebrafish after 24 h of fasting (Figure 3F). In addition, triglyceride levels in the serum and dorsal muscle were measured. Although there was an increasing trend of the triglyceride level in the dorsal muscle, the serum TG contents remained unchanged (Supplemental Figure S1).
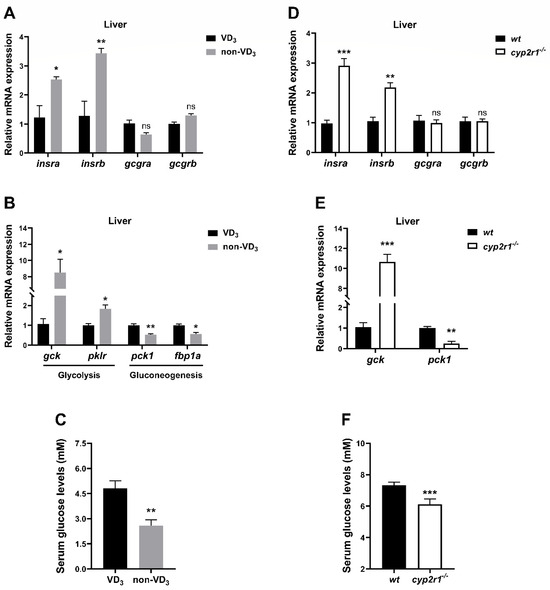
Figure 3.
VD regulates glucose metabolism in zebrafish under short-term fasting conditions. (A–C) After the feeding trial, zebrafish fed with VD3 or non-VD3 diet were fasted for 24 h before sampling. The gene expression of insra, insrb, gcgra, gcgrb (A) and gck, pklr, pck1, fbp1a (B) in the liver was measured (n = 4/group). Serum glucose levels were determined (C) (n = 3~4 replicates, 4~5 fish/replicate). (D–F) WT and cyp2r1−/− zebrafish at 3 mpf were fasted for 24 h before sampling. The gene expression of insra, insrb, gcgra, gcgrb (D) and gck, pck1 (E) in the liver was analyzed (n = 4/genotype). Serum glucose levels were determined (F) (n = 4 replicates, 3 fish/replicate). * p < 0.05, ** p < 0.01, *** p < 0.001, ns: no statistical significance.
3.4. VD3 Regulates Lipid Metabolism in Zebrafish under Short-Term Fasting Condition
Compared to the VD3 group, the expression levels of lipolysis-related genes (ppara and pgc1a) were significantly elevated in the non-VD3 group, while the lipogenesis-related genes (pparg and fasn) decreased (Figure 4A). The expression of the FA absorption-related gene cd36 in the liver was significantly suppressed, while the gene expression of slc27a2a showed no significant changes (Figure 4B). In contrast, the gene expression of ppara and pgc1a in the dorsal muscle was inhibited in the non-VD3 group, while fasn showed no significant changes (Figure 4C). In addition, WT and cyp2r1−/− zebrafish at 3 mpf were fasted for 24 h. Compared to WT zebrafish, the expression levels of lipolysis-related genes (ppara and pgc1a) were enhanced while those of lipogenesis-related genes (pparg and fasn) were suppressed after 24 h of fasting (Figure 4D). Meanwhile, the gene expression of cd36 was significantly reduced, though slc27a2a was increased in cyp2r1−/− zebrafish after 24 h fasting (Figure 4E). Similarly, the expression of the lipolysis-related gene ppara was decreased in the dorsal muscle, while that of pgc1a and fasn showed no significant changes (Figure 4F). These results further confirmed that VD3 plays a crucial role in regulating lipid metabolism in zebrafish under short-term fasting.
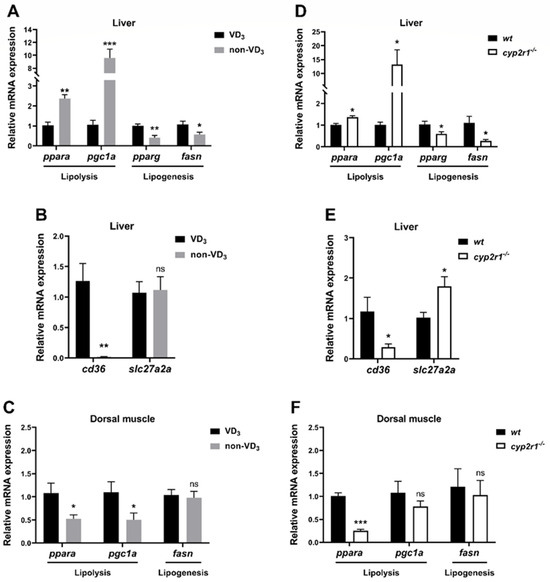
Figure 4.
VD regulates lipid metabolism in zebrafish under short-term fasting conditions. (A–C) After the last feeding, zebrafish fed with VD3 or non-VD3 diet were fasted for 24 h before sampling. The gene expression of ppara, pgc1a, pparg, fasn (A) (n = 4/group), and FFA absorption-related genes cd36, slc27a2a (B) (n = 5/group) in the liver were determined. The gene expressions of ppara, pgc1a, fasn in the dorsal muscle were analyzed (C) (n = 6/group). (D–F) WT and cyp2r1−/− zebrafish at 3 mpf were fasted for 24 h before sampling. The gene expressions of ppara, pgc1a, pparg, fasn (D) (n = 4/genotype), and cd36, slc27a2a (E) (n = 4/genotype) in the liver were determined. The gene expressions of ppara, pgc1a, fasn in the dorsal muscle were determined (F) (n = 4/genotype). * p < 0.05, ** p < 0.01, *** p < 0.001, ns: no statistical significance.
3.5. VD3 Promotes the Synthesis and Processing of GLP-1 in the Gut under Short-Term Fasting Condition
Considering the significant impact of gastrointestinal tract-derived hormone peptides on lipid and carbohydrate metabolism [30], we further verified whether these hormones participated in regulating energy metabolism by VD3 during short-term fasting. The results showed no significant differences in the expression levels of gip, gipr, pyya, pyyb, and sglt between VD3 and non-VD3 groups (Figure 5A). However, the gene expression of gcga was significantly suppressed when zebrafish were fed with a non-VD3 diet, and the gene expression of pcsk1 in zebrafish intestine was significantly reduced in the non-VD3 group compared to the VD3 group, while gcgb and pcsk2 displayed no significant changes (Figure 5B). As expected, the level of GLP-1, the product of the gcg gene, was lower in the non-VD3 group compared to the VD3 group, both in fish serum (Figure 5C) and the gut (Figure 5D). Moreover, similar results were obtained in the cyp2r1−/− zebrafish compared to WT zebrafish, except that no significant changes were detected in the serum GLP-1 level (Figure 5E) or the gene expression of pcsk1 in the gut (Figure 5H).
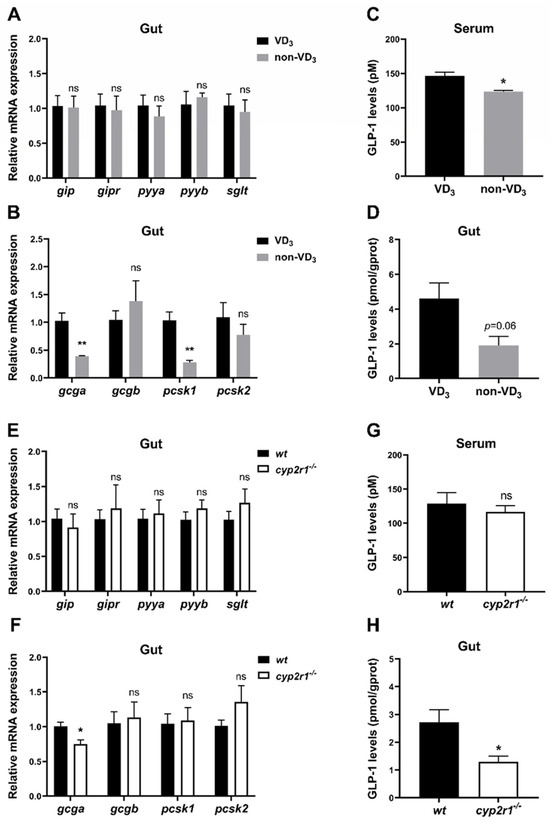
Figure 5.
VD3 promotes the synthesis and processing of GLP-1 in the gut under short-term fasting conditions. (A–D) Zebrafish were fed with or without VD3 for a month and fasted for 24 h. The gene expression of gip, gipr, pyya, pyyb, sglt (A) and gcga, gcgb, pcsk1, pcsk2 (B) in the gut was analyzed (n = 4/group). GLP-1 levels in the serum (C) and the intestine (D) were measured (n = 3 replicates, 4~5 fish/replicate). (E–H) WT and cyp2r1−/− zebrafish at 3 mpf fasted for 24 h. The gene expression of gip, gipr, pyya, pyyb, sglt (E), and gcga, gcgb, pcsk1, pcsk2 (F) in the intestine was analyzed (n = 5/genotype). GLP-1 levels in the serum (G) (n = 4 replicates, 3 fish/replicate) and the intestine (H) (n = 3/ genotype) were measured. * p < 0.05, ** p < 0.01, ns: no statistical significance.
3.6. Interaction between VD3 and Gut Microbiota under Short-Term Fasting Condition
Our recent study provided evidence that VD3 could exert its physiological functions by influencing gut microbiota [31]. Interestingly, the serum glucose level exhibited no significant increment in the zebrafish fed with a VD3 diet after fasting for 24 h when the gut microbiota was removed by an antibiotic cocktail treatment (Figure 6A). The serum GLP-1 was decreased by the depletion of microbiota, and the effects of VD3 on GLP-1 diminished without the microbiota under short-term fasting conditions (Figure 6B). Notably, VD3 deficiency still caused a significant reduction in the expression of gcga when the microbiota was depleted (Figure 6C). In contrast, VD3 deficiency strongly restrained the gene expression of gcgb in the gut of zebrafish under short-term fasting, although the regulation of gcgb by VD3 could not be detected without the depletion of gut microbiota (Figure 6D). Interestingly, the inhibition of pcsk1 gene expression in the non-VD3 group was abolished after the depletion of microbiota (Figure 6E). The expression level of pcsk2 showed no evident response to both VD3 and microbiota (Figure 6F). Importantly, germ free (GF) zebrafish were incubated with short-chain fatty acids, including acetate, propionate and butyrate, which are crucial metabolites of gut microbiota [32]. The result showed that the gene expression of pcsk1 in zebrafish was significantly enhanced by acetate treatment (Figure 6G). Altogether, our data confirmed that the microbiota is required in the regulation of GLP-1 production by VD3 under short-term fasting conditions.
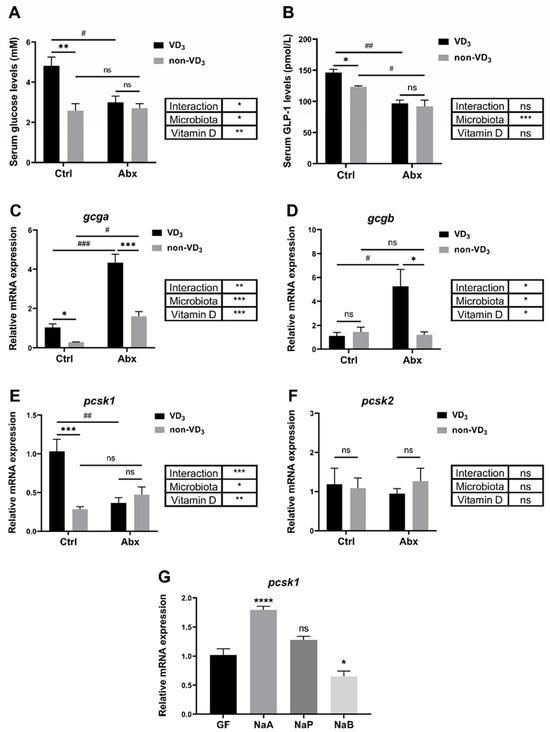
Figure 6.
Interaction between VD3 and gut microbiota under short-term fasting conditions. (A–F) Zebrafish were treated with an antibiotic cocktail (Abx) while fed a VD3 or non-VD3 diet for one month. The serum glucose levels (A) and serum GLP-1 levels (B) were assayed (n = 3 replicates, 4~5 fish/replicate). The expression levels of gcga (C), gcgb (D), pcsk1 (E) and pcsk2 (F) in the intestine were analyzed (n = 4/group). (G) GF zebrafish at 3 dpf were incubated with NaA, NaP, or NaB (30 mM) for two days. The gene expression of pcsk1 was determined (n = 5 replicates, 10 larvae/replicate). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, # p < 0.05, ## p < 0.01, ### p < 0.001, ns: no statistical significance.
4. Discussion
In the present study, we have demonstrated that VD contributes to global changes in glucose and lipid metabolism and ameliorates energy homeostasis under short-term fasting conditions. On one hand, VD promotes GLP-1 production that is helped by gut microbiota, resulting in higher gluconeogenesis and lower glycolysis levels in the liver, which maintains higher serum glucose levels in short-term fasted zebrafish. On the other hand, VD seems to enhance lipid mobilization for energy supply under short-term fasting conditions.
During fasting, the energy for animal survival comes from the reserved nutrients including glycogens, lipids and proteins. Though influenced by many factors, glucose is regarded as the primary energy substance during early fasting [8]. It has been known that major glucose-depriving pathways are glycolysis, glycogenesis and lipogenesis, whereas glucose-producing pathways are glycogenolysis and gluconeogenesis [33]. Our data confirmed that glucose-depriving pathways were suppressed while glucose-producing pathways were promoted after fasting for 48 h, which is associated with the second wave of the serum glucose level. Interestingly, our results demonstrated that VD contributed to maintaining the blood glucose concentrations at appropriate levels by further down-regulating glucose-depriving pathways and enhancing glucose-producing pathways under short-term fasting conditions. Notably, 1,25(OH)2D3 generation in zebrafish was impaired under short-term fasting conditions, which could have an adverse influence on maintaining glucose homeostasis.
It is well-known that glucose and lipid metabolism are correlated in every facet [34]. The association between FAs and gluconeogenesis was discovered in the 1990s [35]. Moreover, the mechanism through which insulin inhibits gluconeogenesis by suppressing adipose tissue lipolysis was confirmed recently [36]. As the pivot organ of lipid metabolism, the liver absorbs FFAs from food digestion, synthesizes triglycerides, and transports them to other target organs for lipid storage or energy supply [34]. During fasting, foodborne FFAs run out and the liver absorbs FFAs produced by lipid-storage organs, such as abdominal fat and intramuscular fat [37]. Most of the FFAs are transported into the hepatocyte with the help of FA translocase and FA transport proteins, such as CD36 and solute carrier family 27A. Our data showed that VD deficiency significantly enhanced lipolysis and suppressed lipogenesis in the liver while restraining the lipolysis pathway in the dorsal muscle during short-term fasting. Notably, the expression of cd36 was dramatically suppressed in the liver of VD-deficient zebrafish. It is known that CD36 is a critical fatty acid sensor and regulator of lipid metabolism [38], and it was reported that CD36 expression in murine liver increased during fasting possibly to enhance the hepatic uptake of FA mobilized from other tissues [39]. Hence, the suppressed CD36 expression in the liver of VD-deficient zebrafish inferred that the lipid transportation from other tissues, such as dorsal muscles, to the liver might be dampened. Interestingly, it was reported that cyp2r1−/− zebrafish failed to mobilize the fat storage in abdominal adipose tissue over 10 days or 35 days of fasting [26]. Meanwhile, serum FFA contents were higher in cyp2r1−/− zebrafish, both under postprandial and fasting conditions [26], indicating the obstruction in FFA absorption and utilization. Hence, we conjectured that VD promoted lipid mobilization and energy supplements during short-term fasting, leading to a more stable level of blood glucose.
In the present study, we demonstrated that VD3 induced GLP-1 production in zebrafish under short-term fasting conditions. GLP-1 is a hormone peptide secreted from intestinal L-cells and processed by PC1/3 (encoded by pcsk1) [10]. As previous reports showed, GLP-1 might interact with the central neuronal circuits involved in food intake control through the gut–brain axis [17,40]. Notably, GLP-1 in fish displayed opposite functions in regulating glucose metabolism, compared to those in mammals, which may be related to the genome duplication event and the depletion of the GLP-1 receptor in fish [41]. Importantly, we further identified that the gut microbiota was involved in VD-regulated pcsk1 expression and GLP-1 production, although VD-regulated gene expression of gcga was independent of gut microbiota. Hence, it was possible that the processing of proglucagon to GLP-1 was influenced by the gut microbiota. Interestingly, previous studies have reported that VD3 influenced the composition of gut microbiota in humans and mice [42,43]; however, the holistic mechanism is still a mystery. Considering that the bacteria do not have VDR, it was conjectured that VD might influence the gut microbiota in indirect manners [44,45]. Nonetheless, our recent study uncovered that VD3 promoted the in vitro growth of certain probiotics directly [31].
On the other hand, short-chain fatty acids (SCFAs), including butyrate, propionate and acetate, are the main metabolites produced by gut microbiota [46]. Sanna et al. demonstrated that the level of circulating SCFAs was related to insulin sensitivity and GLP-1 concentration in humans [32]. Moreover, Kumar et al. reported that SCFAs significantly induced the gene expression of pcsk1 in the STC-1 cells [47]. Recently, we discovered that VD significantly increased the relative abundance of Cetobacterium spp. in the gut microbiota of zebrafish, as well as the serum concentration of acetate, a major product of Cetobacterium spp. [31]. In the present study, we confirmed that acetate rather than propionate or butyrate enhanced the gene expression of pcsk1, suggesting that the up-regulation of GLP-1 production may be attributed to the VD-induced changes in the metabolite from gut microbiota.
It is noteworthy that the potential hypoglycemic role of VD3 in diabetes has attracted increasing attention in recent years [48,49]. Our research group has demonstrated that VD3 lowers postprandial blood glucose levels in zebrafish under hyperglycemia conditions [24,25]. Although diabetes patients suffer from postprandial hyperglycemia, they may encounter hypoglycemia caused by strict food control and the use of diabetes drugs, which has long been recognized as a major barrier to achieving normoglycemia for diabetic patients with intensive therapy [50,51]. Considering our results that proved VD alleviated hypoglycemia caused by short-term fasting in zebrafish, it would be very intriguing to validate the potential application of VD3 in the therapy of hypoglycemia in humans.
This study has demonstrated an unexpected role of VD3 in glucose metabolism under short-term fasting conditions, using zebrafish as a model. Considering zebrafish are a good model for physiological research, the results could shed light on both teleost and human research. Given that VD3 lowers postprandial blood glucose levels in zebrafish under hyperglycemia conditions [24,25], the current study provided clear evidence for the first time that this multifunctional hormone may exert varied effects on glucose metabolism in a different metabolic state, i.e., short-term fasting condition. However, the mechanistic link between VD3 supplement and lipid mobilization remains obscure in the present study. Further studies are warrantied to clarify this issue.
5. Conclusions
The present study uncovered the crucial role of VD3 in maintaining energy homeostasis in zebrafish under short-term fasting conditions. Importantly, VD3 promotes GLP-1 production in a gut microbiota-dependent manner, resulting in the alleviation of fasting-caused hypoglycemia. Our study emphasized the importance of sufficient VD3 in maintaining energy homeostasis, and highlighted the potential application of VD3 in the therapy of hypoglycemia and regulating glucose homeostasis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16091271/s1, Figure S1. Triglyceride levels in the serum and dorsal muscle of zebrafish. (A,B) After the feeding trial, zebrafish fed with VD3 or non-VD3 diet were fasted for 24 h before sampling. TG contents in the serum (A) were determined (n = 3~4 replicates, 4~5 fish/replicate), as well as dorsal muscle (B) (n = 4/group). (C) WT and cyp2r1−/− zebrafish at 3 mpf were fasted for 24 hours before sampling. TG contents in the dorsal muscle were determined (n = 4/genotype). ns: no statistical significance
Author Contributions
Q.D. designed and performed the experiments, analyzed the data and wrote the manuscript; R.S., W.W., H.Z., X.L. and Z.W. performed the experiments; Z.Y. generated cyp2r1−/− zebrafish and revised the manuscript; Q.A. and K.M. supervised the project; X.T. supervised the project and revised the manuscript; M.W. supervised the project, designed the experiments and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 31972802); Natural Science Foundation of Shandong Province (Grant No. ZR2019MC041); Special Foundation for Taishan Scholar of Shandong Province (Grant No. tsqn201812023).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of Ocean University of China (No. 676-20210306, approval date: 11 March 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Original data in this study are available from the corresponding author according to reasonable request.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
| WT | wild type |
| FA | fatty acid |
| FFAs | free fatty acids |
| PC1/3 | prohormone convertase 1/3 |
| PC2 | prohormone convertase 2 |
| GLP-1 | glucagon-like peptide 1 |
| GF | germ-free |
| GZM | gnotobiotic zebrafish medium |
| SCFAs | short-chain fatty acids |
| NaA | sodium acetate |
| NaP | sodium propionate |
| NaB | sodium butyrate |
| hk1 | hexokinase 1 |
| gck | glucokinase |
| pklr | pyruvate kinase L/R |
| pck1 | phosphoenolpyruvate carboxykinase 1 |
| fbp1a | fructose-1,6-bisphosphatase 1a |
| g6p1a.1 | glucose-6-phosphatase catalytic subunit 1a, tandem duplicate 1 |
| ppara | peroxisome proliferator-activated receptor alpha |
| pgc1a | peroxisome proliferator-activated receptor gamma, coactivator 1 alpha |
| pparg | peroxisome proliferator-activated receptor gamma |
| fasn | fatty acid synthase |
| cyp2r1 | cytochrome P450, family 2, subfamily R, polypeptide 1 |
| cyp27b1 | cytochrome P450, family 27, subfamily A, polypeptide 1 |
| cyp24a1 | cytochrome P450, family 24, subfamily A, polypeptide 1 |
| vdra | vitamin D receptor a |
| vdrb | vitamin D receptor b |
| insra | insulin receptor a |
| insrb | insulin receptor b |
| gcgra | glucagon receptor a |
| gcgrb | glucagon receptor b |
| gip | gastric inhibitory polypeptide |
| gipr | gastric inhibitory polypeptide receptor |
| pyya | peptide YY a |
| pyyb | peptide YY b |
| sglt | sodium-glucose cotransporter 1 |
| cd36 | CD36 molecule |
| slc27a2a | solute carrier family 27 member 2a |
| gcga | glucagon a |
| gcgb | glucagon b |
| pcsk1 | proprotein convertase subtilisin/kexin type 1 |
| pcsk2 | proprotein convertase subtilisin/kexin type 2 |
| actb2 | actin, beta 2 |
References
- Wang, T.; Hung, C.C.Y.; Randall, D.J. The Comparative Physiology of Food Deprivation: From Feast to Famine. Annu. Rev. Physiol. 2006, 68, 223–251. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1. [Google Scholar] [CrossRef]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef]
- Gou, N.; Wang, K.; Jin, T.; Yang, B. Effects of Starvation and Refeeding on Growth, Digestion, Nonspecific Immunity and Lipid-Metabolism-Related Genes in Onychostoma macrolepis. Animals 2023, 13, 1168. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, W.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Liu, H.; Xie, S. Effects of starvation on glucose and lipid metabolism in gibel carp (Carassius auratus gibelio var. CAS III). Aquaculture 2018, 496, 166–175. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, W.; Kenzior, A.; Olsen, L.; Krishnan, J.; Persons, J.; Medley, K.; Peuß, R.; Wang, Y.; Chen, S.; et al. Enhanced lipogenesis through Pparγ helps cavefish adapt to food scarcity. Curr. Biol. 2022, 32, 2272–2280.e2276. [Google Scholar] [CrossRef] [PubMed]
- Navarro, I.; Gutiérrez, J. Chapter 17 Fasting and starvation. In Biochemistry and Molecular Biology of Fishes; Hochachka, P.W., Mommsen, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 4, pp. 393–434. [Google Scholar]
- Secor, S.M.; Carey, H.V. Integrative Physiology of Fasting. Compr. Physiol. 2016, 6, 773–825. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 1015–1045. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Metabolic Messengers: Glucagon-like peptide 1. Nat. Metab. 2021, 3, 142–148. [Google Scholar] [CrossRef]
- Plisetskaya, E.M.; Mommsen, T.P. Glucagon and Glucagon-like Peptides in Fishes. Int. Rev. Cytol. 1996, 168, 187–257. [Google Scholar]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-h.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.N.; Luo, J.N.; Harris, D.A.; Aliakbarian, H.; Yao, L.; Paik, D.; Subramaniam, R.; Adhikari, A.A.; Vernon, A.H.; Kiliç, A.; et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe 2021, 29, 408–424.e407. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, D.; Ding, C.Z.; Guo, F.; Wu, L.N.; Huang, F.J.; Liu, Y.L.; Zhao, S.Y.; Xin, Y.; Ma, S.N.; et al. MicroRNA-194: A novel regulator of glucagon-like peptide-1 synthesis in intestinal L cells. Cell Death Dis. 2021, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cheng, D.; Wang, L.; Chen, F.; Chen, H.; Ma, H.; Yang, Y.; Yuan, X. GLP-1 responds to postprandial hyperglycemia by reducing transcription level in grass carp (Ctenopharyngodon idella). Aquac. Rep. 2022, 23, 101045. [Google Scholar] [CrossRef]
- Chivite, M.; Naderi, F.; Conde-Sieira, M.; Soengas, J.L.; Lopez-Patiño, M.A.; Míguez, J.M. Central serotonin participates in the anorexigenic effect of GLP-1 in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2021, 304, 113716. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Aatsinki, S.M.; Elkhwanky, M.S.; Kummu, O.; Karpale, M.; Buler, M.; Viitala, P.; Rinne, V.; Mutikainen, M.; Tavi, P.; Franko, A.; et al. Fasting-Induced Transcription Factors Repress Vitamin D Bioactivation, a Mechanism for Vitamin D Deficiency in Diabetes. Diabetes 2019, 68, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Żychowska, M.; Rola, R.; Borkowska, A.; Tomczyk, M.; Kortas, J.; Anczykowska, K.; Pilis, K.; Kowalski, K.; Pilch, W.; Antosiewicz, J. Fasting and Exercise Induce Changes in Serum Vitamin D Metabolites in Healthy Men. Nutrients 2021, 13, 1963. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Liao, X.; Lan, Y.; Zhang, H.; Jiao, L.; Du, Q.; Han, D.; Ai, Q.; Mai, K.; Wan, M. Vitamin D regulates insulin pathway and glucose metabolism in zebrafish (Danio rerio). FASEB J. 2022, 36, e22330. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Liao, X.; Wang, W.; Lan, Y.; Zhang, H.; Du, Q.; Jiao, L.; Yin, Z.; Ai, Q.; Mai, K.; et al. Vitamin D Regulates Glucose Metabolism in Zebrafish (Danio rerio) by Maintaining Intestinal Homeostasis. J. Nutr. Biochem. 2023, 123, 109473. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shang, G.; Wang, W.; Chen, X.; Lou, Q.; Zhai, G.; Li, D.; Du, Z.; Ye, Y.; Jin, X.; et al. Fatty Acid Oxidation in Zebrafish Adipose Tissue Is Promoted by 1α,25(OH)(2)D(3). Cell Rep. 2017, 19, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lan, Y.; Shao, R.; Liu, J.; Liang, S.; Yin, Z.; Gudmundsson, G.H.; Bergman, P.; Wan, M. Vitamin D Enhances Neutrophil Generation and Function in Zebrafish (Danio rerio). J. Innate Immun. 2021, 14, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Shimada, Y.; Nishimura, Y.; Tanaka, T.; Nishimura, N. A novel, reliable method for repeated blood collection from aquarium fish. Zebrafish 2013, 10, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.N.; Kanther, M.; Semova, I.; Rawls, J.F. Methods for generating and colonizing gnotobiotic zebrafish. Nat. Protoc. 2008, 3, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Coate, K.C.; Kliewer, S.A.; Mangelsdorf, D.J. SnapShot: Hormones of the gastrointestinal tract. Cell 2014, 159, 1478.e1471. [Google Scholar] [CrossRef]
- Liao, X.; Lan, Y.; Wang, W.; Zhang, J.; Shao, R.; Yin, Z.; Gudmundsson, G.H.; Bergman, P.; Mai, K.; Ai, Q.; et al. Vitamin D influences gut microbiota and acetate production in zebrafish (Danio rerio) to promote intestinal immunity against invading pathogens. Gut Microbes 2023, 15, 2187575. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef]
- Jones, J.G. Hepatic glucose and lipid metabolism. Diabetologia 2016, 59, 1098–1103. [Google Scholar] [CrossRef]
- Roden, M.; Stingl, H.; Chandramouli, V.; Schumann, W.C.; Hofer, A.; Landau, B.R.; Nowotny, P.; Waldhäusl, W.; Shulman, G.I. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 2000, 49, 701–707. [Google Scholar] [CrossRef]
- Perry Rachel, J.; Camporez, J.-P.G.; Kursawe, R.; Titchenell Paul, M.; Zhang, D.; Perry Curtis, J.; Jurczak Michael, J.; Abudukadier, A.; Han Myoung, S.; Zhang, X.-M.; et al. Hepatic Acetyl CoA Links Adipose Tissue Inflammation to Hepatic Insulin Resistance and Type 2 Diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef]
- van Ginneken, V.; Verhey, E.; Poelmann, R.; Ramakers, R.; van Dijk, K.W.; Ham, L.; Voshol, P.; Havekes, L.; Van Eck, M.; van der Greef, J. Metabolomics (liver and blood profiling) in a mouse model in response to fasting: A study of hepatic steatosis. Biochim. Et. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2007, 1771, 1263–1270. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Yang, G.; Xu, K.; Yin, Y.; Brecchia, G.; Yin, J. CD36 favours fat sensing and transport to govern lipid metabolism. Prog. Lipid Res. 2022, 88, 101193. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef]
- Polakof, S.; Míguez, J.M.; Soengas, J.L. Evidence for a gut-brain axis used by glucagon-like peptide-1 to elicit hyperglycaemia in fish. J. Neuroendocrinol. 2011, 23, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.M.; Mojsov, S. Diversification of the functions of proglucagon and glucagon receptor genes in fish. Gen. Comp. Endocrinol. 2018, 261, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the gut microbiome: A systematic review of in vivo studies. Eur. J. Nutr. 2019, 58, 2895–2910. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef]
- Vanherwegen, A.S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094. [Google Scholar] [CrossRef]
- Del Pinto, R.; Ferri, C.; Cominelli, F. Vitamin D Axis in Inflammatory Bowel Diseases: Role, Current Uses and Future Perspectives. Int. J. Mol. Sci. 2017, 18, 2360. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Kumar, V.; Khare, P.; Devi, K.; Kaur, J.; Kumar, V.; Kiran Kondepudi, K.; Chopra, K.; Bishnoi, M. Short-chain fatty acids increase intracellular calcium levels and enhance gut hormone release from STC-1 cells via transient receptor potential Ankyrin1. Fundam. Clin. Pharmacol. 2021, 35, 1004–1017. [Google Scholar] [CrossRef]
- Leung, P.S. The Potential Protective Action of Vitamin D in Hepatic Insulin Resistance and Pancreatic Islet Dysfunction in Type 2 Diabetes Mellitus. Nutrients 2016, 8, 147. [Google Scholar] [CrossRef]
- Viloria, K.; Hewison, M.; Hodson, D.J. Vitamin D binding protein/GC-globulin: A novel regulator of alpha cell function and glucagon secretion. J. Physiol. 2022, 600, 1119–1133. [Google Scholar] [CrossRef]
- Cryer, P.E. The Barrier of Hypoglycemia in Diabetes. Diabetes 2008, 57, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Desouza, C.V.; Bolli, G.B.; Fonseca, V. Hypoglycemia, Diabetes, and Cardiovascular Events. Diabetes Care 2010, 33, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
